Abstract
Patients with chronic heart failure (HF) experience muscle atrophy during the course of the disease. The mechanisms underlying muscle atrophy in HF, however, are not understood. Thus, we evaluated leg phenylalanine balance and kinetics in HF patients and controls following a brief fast (24 hr) and under euglycemic-hyperinsulinemic-hyperaminoacidemic conditions to determine if HF increases muscle protein catabolism in response to nutritional deprivation and/or diminishes the anabolic response to meal-related stimuli (insulin and amino acids) and whether alterations in protein metabolism correlate to circulating cytokine levels. No differences in phenylalanine balance, rate of appearance or rate of disappearance were found between patients and controls under fasting conditions. However, the anabolic response to hyperinsulinemia-hyperaminoacidemia was reduced by more than 50% in patients compared to controls. The diminished anabolic response was due to reduced suppression of leg phenylalanine appearance rate, an index of protein breakdown, in HF patients; whereas no group difference was found in the increase in leg phenylalanine disappearance rate, an index of protein synthesis. The diminished responses of both phenylalanine balance and appearance rate to hyperinsulinemia-hyperaminoacidemia were related to greater circulating interleukin-6 levels. Our results suggest that, following a brief period of nutritional deprivation, HF patients demonstrate an impaired muscle protein anabolic response to meal-related stimuli due to an inability to suppress muscle proteolysis, and that this diminished protein anabolic response correlates with markers of immune activation. The inability to stimulate muscle protein anabolism following periods of nutritional deficiency may contribute to muscle wasting in HF patients.
Keywords: heart failure, cachexia, sarcopenia, inflammation, proteolysis
INTRODUCTION
Patients with chronic heart failure (HF) lose skeletal muscle tissue during the course of the disease. Muscle atrophy can lead to functional disability, as it is a primary determinant of both muscle strength [1] and endurance [1, 2], and may contribute to poor prognosis [3]. Despite these important clinical consequences, the mechanisms underlying atrophy in HF patients are not well understood.
Because protein is the primary structural and functional macromolecular component of skeletal muscle, tissue size and functionality is dictated by the balance between protein synthesis and breakdown. Muscle protein is catabolized during periods of nutrient deficiency (ie, fasting) to provide energy and gluconeogenic substrates for other tissues, and is repleted following subsequent nutrient intake [4]. In this context, the response of muscle protein metabolism to periods of nutrient deficiency and intake is a primary determinant of muscle size and alterations in these normal responses could promote muscle atrophy. That an impaired muscle protein metabolic response to nutritional stimuli might contribute to muscle atrophy in HF is made more likely by the fact that patients frequently experience weight loss during the course of the disease [3]. During these periods of energy imbalance, an increased protein catabolic response to energy deficiency, an impaired anabolic response to subsequent nutrient intake or a combination of these two factors could promote muscle atrophy. To our knowledge, however, no study has examined how HF affects the muscle protein metabolic response to nutrient deficiency or the anabolic response upon re-feeding.
Inflammatory cytokines are elevated in HF patients [5] and several correlative studies have suggested that cytokine levels are related to skeletal muscle atrophy [6, 7]. Although the ability of cytokines to promote muscle atrophy has been firmly established in animal models [8], there is question whether they have similar catabolic effects on skeletal muscle protein metabolic processes in humans [9, 10]. In heart failure patients, cytokines and inflammatory markers are negatively related to myofibrillar gene expression and synthesis under postabsorptive conditions [11, 12]. Whether inflammatory cytokines relate to the protein catabolic response to fasting or the anabolic response to meal-related stimuli, however, has not been examined.
The primary objective of the present study was to evaluate the muscle protein metabolic response to nutritional deprivation and repletion in HF patients and controls. To accomplish this goal, leg phenylalanine balance and kinetics were measured using a combination of arteriovenous balance and stable isotope tracer methodologies following a brief (24 hr) fast and during simulated feeding conditions. For the feeding condition, we chose to infuse insulin and amino acids intravenously because, compared to oral feeding, this approach affords greater control over hormone and substrate levels important for postprandial protein anabolism. A secondary goal of these studies was to evaluate whether the protein metabolic response to fasting or to hyperinsulinemia-hyperaminoacidemia was related to circulating levels of proinflammatory cytokines, their soluble receptors or inflammatory markers. Additionally, because HF is an insulin resistant syndrome [13], we also evaluated the relationship of insulin-stimulated glucose disposal, an index of tissue insulin sensitivity, to the anabolic response to insulin-amino acid infusion. We hypothesized that HF patients would demonstrate a more pronounced catabolic response to fasting and diminished anabolic response to insulin/amino acid infusion and that both defects would be related to greater circulating levels of inflammatory cytokines/markers.
METHODS
Subjects
A total of 9 volunteers were studied. Four male patients with chronic HF were recruited from the Heart Failure Clinic of the Cardiology Unit at the University of Vermont. The population consisted of patients with systolic dysfunction (left ventricular ejection fraction <40%). The average (mean ± SE) New York Heart Association functional class was 2.25 ± 0.25, with 3 class II patients and 1 class III patient. The etiology of HF was ischemic in 3 patients, as defined by history of myocardial infarction and/or multi-vessel coronary obstructions upon cardiac catheterization, and idiopathic dilated cardiomyopathy in one patient. Two patients had Type II diabetes mellitus and were being treated with oral hypoglycemic agents. All patients were non-smokers, clinically stable and had not been hospitalized for 6 months prior to testing. None had signs or symptoms of severe hepatic (ie, cirrhosis) or renal disease (ie, plasma creatinine >3), peripheral vascular disease or an active neoplastic process and none were smokers. Upon physical exam, there was no evidence of peripheral edema during screening or at the time of testing. All patients were on stable doses of HF medications, including angiotensin-converting enzyme inhibitors/receptor blockers (100%), β-blockers (100%) and diuretics (100%) and none were taking testosterone replacement therapy.
Controls (5 men) were recruited who were non-smokers and had a stable body weight (±2 kg) for 6 months prior to testing. They had no signs or symptoms of HF, coronary heart disease or diabetes (fasting blood glucose <126 mg/dL and 2 hr glucose following 75 g oral glucose load of <140 mg/dL), normal blood counts and biochemistry values and were not taking testosterone replacement therapy. Written consent was obtained from each volunteer and the protocol was approved by the Committees on Human Research at the University of Vermont.
Experimental protocol
Volunteers were tested during outpatient and inpatient visits to the research center. Following an outpatient screening visit to determine eligibility and perform aerobic capacity measurements, patients were studied during an overnight visit. For 3 days prior to admission, all subjects were provided with a weight maintenance diet (60% carbohydrate, 25% fat, 15% protein). The volunteer was admitted to the research center in the early morning and the last meal of the standardized diet was consumed by 1000h. Thereafter, all volunteers were fasted and only allowed water and medications until completion of testing the following day. After completion of the meal, body composition measurements were performed and a catheter was placed in the antecubital vein for infusions. Upon waking the following morning, the volunteer was allowed to void, catheters were placed in the radial artery of the arm contralateral to the infusion catheter and in the femoral vein of the right leg for sampling of arterial and venous blood, respectively. Leg phenylalanine balance and kinetics were measured first over a 180 min period that represented leg protein metabolism under fasting conditions and during a second 180 min period of infusion with insulin, glucose and amino acids meant to simulate meal-related anabolic stimuli (ie, hyperinsulinemia-hyperaminoacidemia). Following collection of baseline blood samples for background isotope levels and inflammatory cytokines/markers, a primed (37.5 μmol/kg), constant (37.5 μmol/kg • hr−1) infusion of [ring-2H5]phenylalanine was started (time 0 min) and maintained throughout the study. Blood for arterial and venous phenylalanine concentration and enrichment were drawn at 135, 150, 165 and 180 min into tubes containing perchloric acid (1:1). Directly following each blood draw, leg blood flow was measured by venous occlusion plethysmography, as previously described [14]. Five separate measures were taken at each time point and were averaged together. At 180 min, a euglycemic-hyperinsulinemic clamp was begun, as described [15], and an infusion of 10% Aminosyn II (Hospira Inc; Lake Forest, IL; 66 mg/kg • hr−1) was started. [ring-2H5]Phenylalanine was added to the amino acid infusate to minimize alterations in steady state levels of the tracer. Blood for arterial and venous phenylalanine concentration and enrichment were drawn at 315, 330, 345 and 360 min into tubes containing perchloric acid (1:1), followed by measurement of leg blood flow. All infusions were stopped at 360 min except for the dextrose infusion, which was continued and tapered until no longer required to maintain normal glycemia.
Total and regional body composition
Body mass was measured on a digital scale (ScaleTronix, Wheaton, IL). Total and regional fat mass and fat-free mass were measured by dual energy x-ray absorptiometry using a GE Lunar Prodigy densitometer (GE Lunar, Madison, WI). Appendicular skeletal muscle mass was measured as described by Heymsfield et al. [16].
Peak oxygen consumption (peak VO2)
Peak VO2 was determined as described previously [11] and was defined as the highest 30 sec average VO2 value during the final 2 minutes of the test.
Hormone and substrate measurements
Serum insulin was measured by enzyme-linked immunosorbent assay (ELISA; ALPCO Inc; Salem, NH). Plasma glucose concentrations were measured by a glucose analyzer (Yellow Springs Instruments; Yellow Springs, OH). Plasma amino acid levels were measured by cation exchange high-performance liquid chromatography with fluorescent detection (Dionex; Sunnyvale, CA) by the Yale General Clinical Research Center core laboratory, with interassay coefficient of variations (CV) of between 5 and 10%. C-reactive protein (CRP) levels were measured by ELISA [17] with an interassay CV of 2 to 4%. Tumor necrosis factor-α (TNF-α) and interleukin-6 levels and their soluble receptors (TNF-RII and IL-6 sR, respectively) were measured by ultra-sensitive ELISA (R&D Systems, Minneapolis, MN) with interassay CVs from 6 to 16%.
Mass spectrometry measurements
Arterial and venous [ring-2H5]phenylalanine enrichment and concentration were measured by positive chemical ionization gas chromatography mass spectrometry (GCMS), as previously described [18]. Prior to measurement, an aliquot of [2H2]phenyalanine was added to each blood sample to serve as an internal standard, amino acids were isolated and then converted to their N-heptafluorobutryl, n-propyl (HFBP) derivative [19]. HFBP-amino acid derviatives were injected into the GCMS (model 5973, Hewlett-Packard, Palo Alto, CA) and ions at a mass to charge ratio of 404, 406 and 409 were monitored for unlabeled, [2H2]- and [ring-2H5]phenylalanine, respectively. Ion current ratios derived from these species were used to calculate enrichment and concentration values, as described previously [20].
Calculations
The net balance of phenylalanine across the leg was calculated from arterial (A) and venous (V) whole blood phenylalanine concentrations and leg blood flow (F) as:
| (Eq 1) |
Whole blood data were used to avoid assumptions regarding the equilibration of amino acids between plasma and red blood cells [21] in the event that red blood cells participate in the inter-organ exchange of phenylalanine. The leg tissue disposal rate (Rd) of phenylalanine was calculated as:
| (Eq 2) |
where fphe is the fractional extraction of the phenyalanine tracer, calculated as the arteriovenous difference in tracer enrichment divided by arterial enrichment. Rate of appearance (Ra) of phenyalalnine from leg tissues was calculated as:
| (Eq 3) |
where Ev and Ea are the enrichment of the phenylalanine tracer in venous and arterial blood, respectively. Because phenylalanine is neither synthesized nor metabolized in muscle [22], the kinetic processes defined by Rd and Ra should reflect the rate of incorporation (ie, synthesis) and release (ie, breakdown) of phenylalanine, respectively, from tissue protein. The rate of whole body phenylalanine flux was calculated from arterial phenylalanine enrichment under fasting conditions using standard equations [19] and under hyperinsulinemic-hyperaminoacidemic conditions using equations that consider the input of tracer from two exogenous sources (ie, tracer infusion and amino acid infusion), as previously described [15].
Euglycemic-hyperinsulinemic clamp
Insulin-stimulated glucose disposal, an index of tissue insulin sensitivity, was derived as described [15]. Briefly, during the constant infusion of insulin (40 mU/m2•min−1), euglycemia was maintained by a continuous infusion of 20% dextrose. Plasma glucose was monitored every 5 min and the dextrose infusion was adjusted to maintain euglycemia [23]. The average rate of glucose infusion during the last 30 min of the clamp, expressed relative to fat-free tissue mass (ie, ml/kg fat-free mass • min−1), was used as an estimate of insulin-stimulated glucose disposal, assuming complete suppression of endogenous glucose production. This assumption is reasonable in HF patients given the level of hyperinsulinemia achieved with our protocol and the fact that all patients were receiving β blocker medication, the latter of which has been shown to enhance the suppression of endogenous glucose production in patients to a level similar to non-diseased controls [24, 25].
Statistics
All data are reported as mean ± SEM. Unpaired Student t tests were used to compare groups for those measurements performed under fasting conditions. Examination of the response of phenylalanine balance to insulin and amino acid infusions was made using a 2 × 2 repeated measures analysis of variance with group (control vs. HF) as the between-subject factor and infusion (no infusion (fasting) vs. insulin/amino acid infusion) as the within-subject factor. Relationships between variables were determined using Pearson correlation coefficients. If a variable was not normally distributed (Shapiro-Wilk test), log10 transformation was performed. Distributional assumptions of all variables were confirmed prior to analysis. All analyses were conducted with SPSS software version 15 (SPSS Inc; Chicago, IL).
RESULTS
Physical characteristics of HF patients and controls are shown in Table 1. Groups were similar for age, body mass and total and regional body composition, although peak oxygen consumption was lower (P<0.01) in heart failure patients, as expected. Although not significant (P=0.31 and 0.25, respectively), the greater body mass and fat mass in controls was due to one control volunteer with a body mass of 125 kg and fat mass of 43.4 kg (height=1.94 m; BMI=33.2).
Table 1.
Physical characteristics of controls and HF patients.
| Controls | Heart failure | |
|---|---|---|
| Age (yrs) | 69.8 ± 7.5 | 61.5 ± 6.6 |
| Height (cm) | 175.9 ± 4.7 | 177.0 ± 2.1 |
| Body mass (kg) | 84.7 ± 10.2 | 71.3 ± 4.9 |
| Fat mass (kg) | 21.3 ± 5.6 | 12.8± 2.7 |
| Fat-free mass (kg) | 60.4 ± 4.4 | 56.2 ± 2.6 |
| Leg fat mass (kg) | 6.74 ± 1.98 | 4.05 ± 0.77 |
| Leg fat-free mass (kg) | 20.2 ± 1.4 | 19.0 ± 1.1 |
| Peak oxygen consumption (ml/kg FFM*min−1) | 41.5 ± 1.1 | 25.6 ± 3.9 * |
Data are mean ± SE for n=5 controls and n=4 HF patients. FFM, fat-free mass.
P<0.01 control vs. HF.
Insulin and amino acid levels under fasting and hyperinsulinemic-hyperaminoacidemic conditions are shown in Table 2. There were no differences between patients and controls in fasting insulin (P=0.51) or amino acid levels (range of P-values: 0.13 to 0.98). The insulin infusion increased plasma insulin levels in both groups similarly (ie, P<0.01 infusion effect, P=0.98 group × infusion interaction effect). Levels of most amino acids were increased (P<0.05) by the infusion, with the exception of asparagine, glutamine and tyrosine, which were decreased (P<0.05), and methionine, valine and cysteine, which did not change (P=0.13, 0.69 and 0.64, respectively). There were no differences noted between groups in the increase, decrease or lack of change in amino acid levels with the infusion (ie, no group × infusion interaction effects).
Table 2.
Serum insulin and plasma amino acid levels in controls and HF patients under fasting conditions and in response to insulin and amino acid infusions (Ins/AA).
| Controls | Heart Failure | |||
|---|---|---|---|---|
| Fasting | Ins/AA | Fasting | Ins/AA | |
| Insulin (μU/ml) | 6.3 ± 1.8 | 70.4 ± 7.5 | 4.4 ± 2.2 | 68.8 ± 9.5 * |
| Non-essential amino acids (μM) | ||||
| Alanine | 191.7 ± 31.3 | 346.6 ± 32.1 | 162.5 ± 5.4 | 259.9 ± 16.6 * |
| Arginine | 70.2 ± 4.9 | 129.5 ± 8.0 | 72.4 ± 4.9 | 125.9 ± 5.2 * |
| Asparagine | 48.8 ± 4.4 | 28.6 ± 3.0 | 43.8 ± 3.4 | 28.2 ± 1.2 * |
| Aspartate | 3.5 ± 0.8 | 21.1 ± 1.4 | 3.8 ± 0.2 | 22.4 ± 0.6 * |
| Cysteine | 32.4 ± 3.0 | 29.8 ± 5.5 | 35.9 ± 2.8 | 34.8 ± 5.9 |
| Gluatmate | 48.5 ± 7.4 | 73.0 ± 7.5 | 69.0 ± 12.0 | 100.9 ± 9.1 * |
| Gluatmine | 404.5 ± 30.3 | 361.8 ± 23.1 | 433.5 ± 41.0 | 365.9 ± 20.7 * |
| Glycine | 168.5 ± 21.4 | 224.8 ± 21.1 | 167.7 ± 28.7 | 217.8 ± 24.4 * |
| Histidine | 65.6 ± 3.3 | 77.8 ± 2.6 | 77.2 ± 6.6 | 86.8 ± 3.9 * |
| Serine | 74.9 ± 5.7 | 95.9 ± 3.8 | 79.2 ± 4.0 | 99.7 ± 2.7 * |
| Tyrosine | 47.7 ± 5.2 | 33.2 ± 2.3 | 48.2 ± 2.1 | 39.4 ± 2.3 * |
| Essential amino acids (μM) | ||||
| Isoleucine | 63.2 ± 6.8 | 88.2 ± 6.3 | 61.8 ± 3.8 | 93.9 ± 8.1 * |
| Leucine | 134.7 ± 6.7 | 149.8 ± 9.8 | 139.3 ± 4.5 | 164.8 ± 13.4 * |
| Lysine | 132.3 ± 7.0 | 220.3 ± 8.5 | 152.0 ± 8.0 | 229.8 ± 11.7* |
| Methionine | 21.3 ± 2.3 | 24.3 ± 1.7 | 20.6 ± 0.8 | 22.3 ± 1.6 |
| Phenylalanine | 60.0 ± 5.2 | 68.5 ± 2.8 | 63.2 ± 9.9 | 70.5 ± 8.8 * |
| Threonine | 78.4 ± 4.5 | 91.3 ± 3.7 | 66.2 ± 6.0 | 80.6 ± 3.8 * |
| Tryptophan | 30.7 ± 5.6 | 64.1 ± 5.8 | 36.4 ± 3.2 | 57.2 ± 2.5 * |
| Valine | 212.9 ± 12.5 | 207.0 ± 12.4 | 220.0 ± 10.0 | 229.9 ± 16.2 |
Data are mean ± SE.
P<0.05 significant effect of infusion.
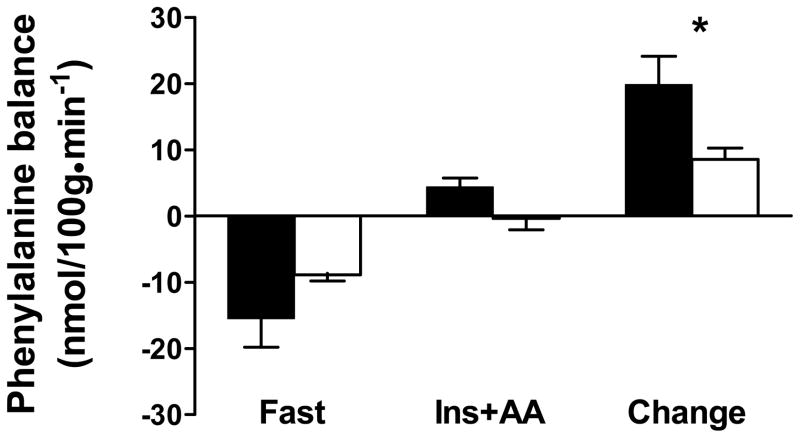
Both arterial and venous phenylalanine concentrations were in steady state during the final 45 minutes of both fasting and hyperinsulinemic-hyperaminoacidemic conditions (ie, slope between phenylalanine concentration and time is not different from zero). Leg phenylalanine balance under fasting and hyperinsulinemic-hyperaminoacidemic conditions and its change between these two conditions are shown in Figure 1. There was no difference between groups in phenylalanine balance in the fasting condition(C: −15.46 ± 4.36 vs. HF: −8.89 ± 0.92 nmol/100g • min−1; P=0.23). As expected, the insulin/amino acid infusion promoted net protein anabolism, as indicated by a more positive leg phenylalanine balance (C: 4.36 ± 1.37 vs. HF: −0.36 ± 1.73 nmol/100g • min−1; P<0.01 infusion effect). The response of phenylalanine balance to insulin/amino acid infusion showed a strong trend (P=0.06 group ×time interaction effect) toward a greater anabolic response in controls (change in Phe balance: C: 19.82 ± 4.29 vs. 8.53 ± 1.71 nmol/100g • min−1).
Figure 1.
Leg phenylalanine net balance in controls (filled bars) and HF patients (open bars) under fasting (fast) and hyperinsulinemic-hyperaminoacidemic (Ins+AA) conditions and the change between these two conditions. Values are mean ± SE. *, P=0.06.
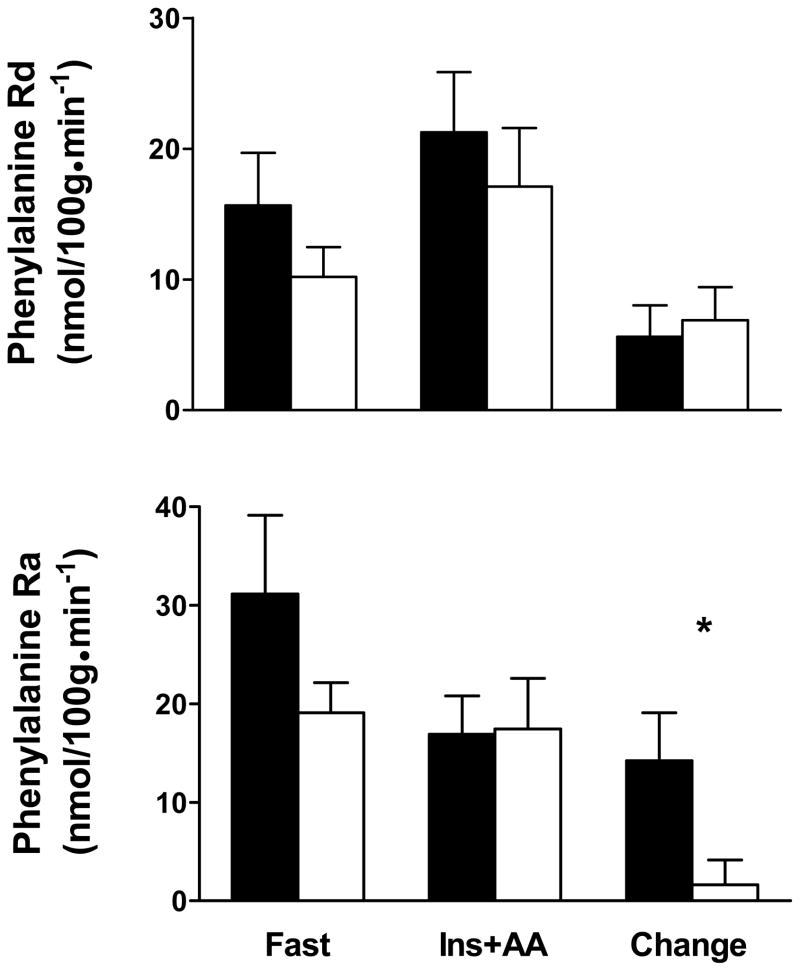
To evaluate the basis for the diminished anabolic response to insulin/amino acid infusion in HF patients, leg phenylalanine Ra and Rd were measured using [2H5]phenylalanine kinetics (Figure 2). Both arterial and venous phenylalanine enrichments were in steady state during the final 45 minutes of both fasting and hyperinsulinemic-hyperaminoacidemic conditions (ie, slope between phenylalanine enrichment and time is not different from zero; data not shown). No group differences were found in phenylalanine Rd in the fasting condition (C: 15.67 ± 4.02 vs. HF: 10.20 ± 2.28 nmol/100g • min−31). As expected, insulin/amino acid infusion increased phenylalanine Rd (C: 21.27 ± 4.61 vs. HF: 17.09 ± 4.50 nmol/100g • min−1; P<0.01 infusion effect). This increase in phenylalanine Rd did not differ between groups (change in Rd: C: 5.6 ± 2.41 vs. HF: 6.89 ± 2.51 nmol/100g • min−1; P=0.72). The Ra of phenylalanine did not differ between groups in the fasting condition (C: 31.13 ± 8.01 vs. HF: 19.09 ± 3.04 nmol/100g • min−1; P=0.24). As expected, the insulin/amino acid infusions promoted a reduction in phenylalanine Ra (C: 16.91 ± 3.88 vs. HF: 17.45 ± 5.15 nmol/100g • min−1; P<0.05 infusion effect). This effect of insulin/amino acid infusion to diminish phenylalanine Ra was primarily driven by the response in controls as there was a strong trend (P=0.07 group ×time interaction effect) toward a greater reduction in phenylalanine Ra in controls compared to HF patients (change in Ra: C: 14.22 ± 4.88 vs. HF: 1.64 ± 2.51 nmol/100g • min−1). Finally, no group differences in leg blood flow were noted under fasting conditions (P=0.26), in the response to insulin/amino acid infusion (P=0.70 infusion effect) or any difference between groups in the response to infusion (P=0.66 group ×infusion interaction effect; data not shown in Figure).
Figure 2.
Leg phenylalanine rate of disappearance (Rd) and appearance (Ra) in controls (filled bars) and HF patients (open bars) under fasting (fast) and hyperinsulinemic-hyperaminoacidemic (Ins+AA) conditions and the change between these two conditions. Values are mean ± SE. *, P=0.07.
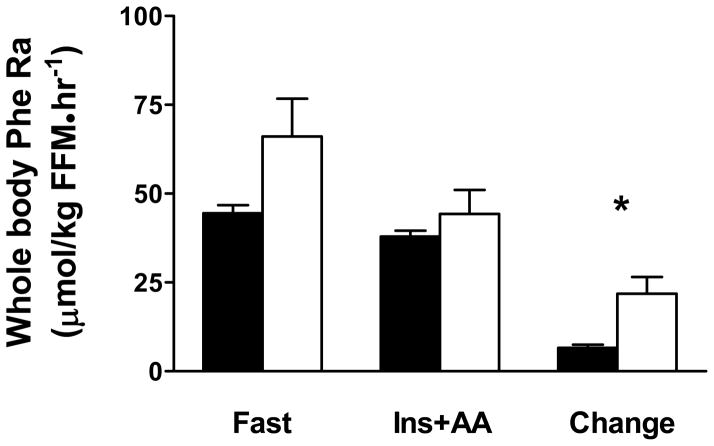
Whole body phenylalanine appearance rate data are shown in Figure 3. Whole body phenylalanine rate of appearance did not differ between patients and controls in the fasting condition (C: 44.5 ± 2.2 vs. HF: 62.7 ± 11.3 μmol/kg FFM • hr−1; P=0.12). As expected, the insulin/amino acid infusion reduced (P<0.01 infusion effect) whole body phenylalanine appearance rate (C: 38.0 ± 1.6 vs. HF: 44.2 ± 6.6 μmol/kg FFM • hr−1). The reduction in whole body protein breakdown was greater (P<0.05 group × infusion interaction effect) in HF patients compared to controls (change in whole body Ra: C: 6.5 ± 0.9 vs. HF: 18.4 ± 5.0 μmol/kg FFM • hr−1).
Figure 3.
Whole body rate of appearance (Ra) of phenylalanine in controls (filled bars) and HF patients (open bars) under fasting (fast) and hyperinsulinemic-hyperaminoacidemic (Ins+AA) conditions and the change between these two conditions. Values are mean ± SE. *, P<0.05.
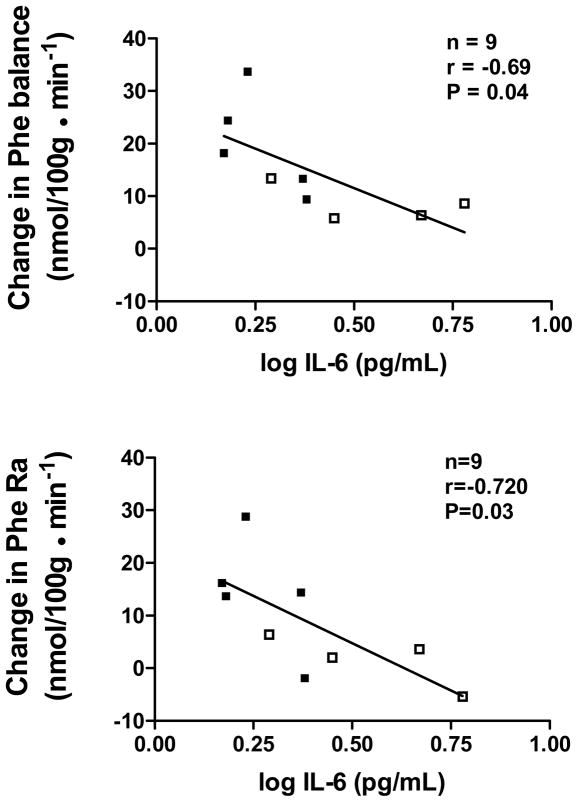
In an attempt to identify factors that might contribute to impaired anabolic response to insulin/amino acid infusion, we evaluated the relationship of circulating cytokines and insulin-stimulated glucose disposal to the change in leg phenylalanine balance and Ra. Group average data for plasma inflammatory markers and insulin-stimulated glucose uptake are shown in Table 3. No differences were found between groups in these potential correlates. Table 4 shows correlation coefficients for the relationship of these variables to phenylalanine balance and Ra. We found negative correlations between IL-6 and the change in phenylalanine balance and Ra. Scatterplots for these relationships are shown in Figure 4. Insulin-stimulated glucose disposal, an index of tissue insulin sensitivity, was not related to either variable (P=0.11 and 0.31, respectively).
Table 3.
Group differences in circulating hormones and insulin sensitivity variables used as potential correlates of changes in phenylalanine balance and kinetics.
| Controls | Heart failure | |
|---|---|---|
| C-reactive protein (μg/uL) | 2.23 ± 1.1 | 6.4 ± 2.2 |
| Tumor necrosis factor-α (pg/mL) | 1.69 ± 0.23 | 2.41 ± 0.71 |
| Tumor necrosis factor-α receptor II (pg/mL) | 2805 ± 192 | 3933 ± 588 |
| Interleukin 6 (pg/mL) | 1.89 ± 0.20 | 3.88± 0.93 |
| Interleukin 6 soluble receptor (ng/mL) | 31.4 ± 2.6 | 42.3 ± 0.8 |
| Insulin-stimulated glucose disposal (mg/kg FFM • min−1) | 4.52 ± 0.94 | 2.84 ± 0.68 |
Data are mean ± SE. No significant differences were found between groups.
Table 4.
Correlation coefficients for the relationship of circulating cytokine levels to the change in leg phenylalanine balance and phenylalanine appearance rate from the fasting to the hyperinsulinemic-hyperaminoacidemic conditions across the entire cohort.
| Variable | CRP | TNF-α | TNF-α RII | IL-6 | IL-6 sR | ISGU |
|---|---|---|---|---|---|---|
| Change in leg Phe balance | −0.574 | 0.022 | −0.440 | −0.690 * | −0.599 | 0.556 |
| Change in leg Phe Ra | −0.366 | −0.330 | −0.600 | −0.720 * | −0.468 | 0.381 |
Values represent Pearson correlation coefficients for n=9. CRP, c-reactive protein; TNF-α, tumor necrosis factor-α; TNF-α RII, TNF-α soluble receptor II; IL-6, interleukin-6; IL-6 sR, IL-6 soluble receptor; ISGU, insulin-stimulate glucose uptake.
P<0.05.
Figure 4.
Relationship of circulating interleukin-6 (IL-6) levels to the change in leg phenylalanine net balance and leg phenylalanine rate of appearance (Ra) from fasting to hyperinsulinemic-hyperaminoacidemic conditions. Controls are represented by closed squares and HF patients by open squares.
DISCUSSION
HF patients displayed a similar protein catabolic response to short-term (24 hr) dietary energy deficiency compared to controls. This result was somewhat unexpected given the elevated circulating levels of catabolic and reduced levels of anabolic hormones in these patients [26]. However, the absence of an exaggerated protein catabolic response to short-term energy deprivation is not unprecedented in clinical conditions characterized by muscle atrophy. In weight-losing cancer patients, for example, leg protein balance following an overnight fast (12 hr) is similar to weight-stable controls [27]. Moreover, prior studies from our laboratory showed that postabsorptive muscle protein synthesis rates and indices of protein breakdown do not differ between clinically-stable HF patients and controls [11, 28]. Our results differ from those of Morrison et al. [29], who found more negative leg tyrosine balance following an overnight fast in HF patients who had experienced weight loss (ie, cachectic) compared to controls, suggesting enhanced protein catabolism. Differing results between studies may relate to the patient population studied (ie, weight-stable vs. cachectic) and the timing of the measurements. Regarding the latter, patients studied by Morrison et al. [29] were evaluated directly following inpatient clinical management for their HF. Thus, their greater negative protein balance may be explained by the exacerbation of their HF, as acute illness is known to promote greater fasting protein catabolism [30, 31]. Taken together with our current and prior results [11, 28], these findings suggest that an enhanced catabolic response to short-term fasting is not an intrinsic feature of the HF syndrome, per se (ie, not present in clinically-stable patients), but may occur during or directly following periods of disease exacerbation and hospitalization [29].
Our results showed a strong trend toward a diminished protein anabolic response (ie, more negative net leg phenylalanine balance) to insulin/amino acid infusion in HF patients compared to controls (Figures 1). Practically speaking, net balance was positive in controls, indicating leg protein accretion; whereas, it remained slightly negative in HF patients, signifying continued protein loss. Although this difference did not reach statistical significance, it is similar in directionality and of greater magnitude when compared to protein imbalances found in other physiological/pathophysiological conditions that show a diminished protein anabolic response to meal-related stimuli, such as aging and cancer [27, 32]. Collectively, these data suggest that a diminished protein anabolic response to meal-related stimuli may be a common mechanism whereby muscle protein is lost in healthy and diseased elderly.
The blunted anabolic response in patients was explained by the trend towards a diminished suppression of leg phenylalanine Ra, an index of protein breakdown; whereas the stimulation of phenylalanine Rd, an index of protein synthesis, was similar in patients and controls (Figure 2). Group differences in phenylalanine Ra are not likely related to variation in hormone or substrate levels, as the degree of hyperinsulinemia and hyperaminoacidemia were comparable between groups. Because the suppression of muscle proteolysis is due primarily to insulin [33], it is reasonable to posit that tissue insulin resistance in HF patients [13, 24, 34] might explain our results. In particular, it could be argued that inclusion of two patients with NIDDM made our cohort excessively insulin resistant. However, the degree of insulin resistance observed in our cohort (−37%) is less than that reported in larger cohorts of non-diabetic HF patients (−58%; [13]) and insulin sensitivity was not related to the change in phenylalanine Ra from fasting to hyperinsulinemic-hyperaminoacidemic conditions. Although we clearly cannot discount a role for muscle insulin resistance in the diminished suppression of protein breakdown in HF patients, our data suggest other possible mediators.
IL-6 was negatively related to both the change in leg phenylalanine balance and Ra from fasting to hyperinsulinemic-hyperaminoacidemic conditions (Figure 4). Although the increased circulating IL-6 level in HF patients (+100%) did not reach significance, it was similar in magnitude to that observed in larger cohorts of HF patients [35]. Together with prior work from our laboratory in HF patients showing negative relationships of IL-6 and CRP levels to myofibrillar gene expression and protein synthesis rates, respectively [11, 12], we interpret these findings broadly as evidence for a role for immune activation in the regulation of skeletal muscle protein metabolism.
The physiological interpretation of these correlations with IL-6 is unclear. They could represent an effect of IL-6 on skeletal muscle to increase protein breakdown [36] or to diminish insulin-induced suppression of protein breakdown [37]. Supporting the latter interpretation are data showing that both circulating and tissue IL-6 levels are inversely related to whole-body and adipocyte insulin sensitivity [38]. That both circulating and tissue IL-6 levels were related to tissue insulin sensitivity in this prior study [38] suggests that circulating IL-6 may serve as a biomarker of local IL-6 levels and/or action. In heart failure patients, studies have shown increased tissue expression of several cytokines, including IL-6 [39], which may spillover into the circulation [35]. In this context, plasma IL-6 could reflect the catabolic effects of local IL-6 production [40]. We should acknowledge, however, that the role of IL-6 in the regulation of muscle protein metabolism and/or effect on insulin action in humans is unclear. Recent studies have shown that, contrary to the relationships observed in the present study, acute elevation of IL-6 levels via infusion does not increase protein breakdown or diminish insulin action [10, 41]. Another tenable hypothesis to explain the relationship of IL-6 to protein metabolism is that it is functioning to antagonize another cytokine, such as TNF-α [42], which could antagonize the effects of insulin [43].
The reduction in whole body phenylalanine Ra with insulin/amino acid infusion, an index of whole body protein breakdown, was greater in HF patients (Figure 3), contrary to leg phenylalanine Ra. Considering that liver and skeletal muscle together constitute the majority of whole body protein turnover [44] and are the two main organ systems responsive to meal-related stimuli, group differences in the whole body response are most likely due to differential effects of insulin and/or amino acids on liver protein metabolism. Because protein breakdown in the splanchnic bed in humans is primarily affected by amino acids [45], not insulin, the differential effect of insulin/amino acid infusion on whole body phenylalanine appearance rate is likely due to the ability of amino acids to reduce hepatic proteolysis to a greater extent in HF patients. The reason for such an effect in HF patients is unclear, but may relate to altered hepatic protein metabolism secondary to circulatory deficiencies [46].
Several caveats to our study should be acknowledged. First, the phenylalanine balance and kinetic measurements across the leg cannot be attributed solely to muscle, since skin, bone and adipose tissue also contribute. However, because the large majority (85–90%) of metabolism of phenylalanine in the leg occurs in skeletal muscle [47] and the fact that there were no differences between groups in leg fat and fat-free mass (Table 1), our data are reasonable estimates of muscle protein balance and kinetics. Second, regarding protein kinetic measurements, the rates of disappearance and appearance of phenylalanine across the leg are not direct measures of protein synthesis and breakdown, respectively, because phenylalanine must transit the intramuscular amino acid pool prior to entering protein (via protein synthesis) or the circulation (from protein breakdown). Thus, the intracellular concentration and enrichment of phenylalanine must be constant for our kinetic estimates to reflect protein synthesis and breakdown. Of the amino acids typically used for limb balance measurements, phenylalanine is likely to give the most reliable estimates of these metabolic processes given that it is not synthesized or metabolized in muscle [22] and that it has a small intramuscular pool and rapid transmembrane transport rate [48].
In summary, our results suggest that heart failure patients are characterized by an impaired anabolic response to meal-related stimuli, particularly the effect of insulin to reduce protein breakdown. Moreover, the diminished anabolic response was related to increased circulating IL-6 levels. The fact that we studied clinically-stable patients with mild to moderate disease and without a history of cachexia suggests that our results are not merely a consequence of end stage disease or a metabolic adaptation to the cachectic state. Our results therefore define a potential mechanism whereby muscle protein could be lost in heart failure patients during bouts of negative energy imbalance and the possible involvement of inflammatory cytokines in this defect.
Acknowledgments
We thank all the volunteers who dedicated their valuable time to these studies.
FUNDING
This study was funded by grants from the National Institutes of Health AM-02125, AG-17949 and RR-00109.
References
- 1.Harrington D, Anker S, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, Coats AJS. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 2.Toth MJ, Gottlieb SS, Fisher ML, Poehlman ET. Skeletal muscle atrophy and peak oxygen consumption in heart failure. Am J Cardiol. 1997;79:1267–1269. doi: 10.1016/s0002-9149(97)00098-2. [DOI] [PubMed] [Google Scholar]
- 3.Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077–1083. doi: 10.1016/S0140-6736(03)12892-9. [DOI] [PubMed] [Google Scholar]
- 4.McNurlan MA, Garlick PJ. Influence of nutrient intake on protein turnover. Diabetes Metab Rev. 1989;5:165–189. doi: 10.1002/dmr.5610050206. [DOI] [PubMed] [Google Scholar]
- 5.Mann DL. Inflammatory mediators and the failing heart: past, present and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 6.Anker SD, Clark AL, Kemp M, Salsbury C, Teixeira MM, Hellewell PG, Coats AJS. Tumor necrosis factor and steroid metabolism in chronic heart failure: possible relation to muscle wasting. J Am Coll Cardiol. 1997;30:997–1001. doi: 10.1016/s0735-1097(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 7.Anker SD, Ponikowski PP, Clark AL, Leyva F, Rauchhaus M, Kemp M, Teixeira MM, Hellewell PG, Hooper J, Poole-Wilson PA, Coats AJS. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J. 1999;20:683–693. doi: 10.1053/euhj.1998.1446. [DOI] [PubMed] [Google Scholar]
- 8.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol. 2004;287:C834–843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 9.Petersen AM, Plomgaard P, Fischer CP, Ibfelt T, Pedersen BK, van Hall G. Acute moderate elevation of TNF-{alpha} does not affect systemic and skeletal muscle protein turnover in healthy humans. J Clin Endocrinol Metab. 2009;94:294–299. doi: 10.1210/jc.2008-1110. [DOI] [PubMed] [Google Scholar]
- 10.van Hall G, Steensberg A, Fischer C, Keller C, Moller K, Moseley P, Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab. 2008;93:2851–2858. doi: 10.1210/jc.2007-2223. [DOI] [PubMed] [Google Scholar]
- 11.Toth MJ, Matthews DE, Ades PA, Tischler MD, Van Buren P, Previs M, LeWinter MM. Skeletal muscle myofibrillar protein metabolism in heart failure: relationship to immune activation and functional capacity. Am J Physiol. 2005;288:E685–E692. doi: 10.1152/ajpendo.00444.2004. [DOI] [PubMed] [Google Scholar]
- 12.Toth MJ, Ades PA, LeWinter MM, Tracy RP, Tchernof A. Skeletal muscle myofibrillar mRNA expression in heart failure: relationship to local and circulating hormones. J Appl Physiol. 2006;100:35–41. doi: 10.1152/japplphysiol.00570.2005. [DOI] [PubMed] [Google Scholar]
- 13.Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJS. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30:527–532. doi: 10.1016/s0735-1097(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 14.Cooper BC, Sites CK, Fairhurst PA, Toth MJ. Evidence against a role for ovarian hormones in the regulation of blood flow. Fertil Steril. 2006;86:440–447. doi: 10.1016/j.fertnstert.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Toth MJ, Sites CK, Cefalu WT, Matthews DE, Poehlman ET. Determinants of insulin-stimulated glucose disposal in middle-aged, premenopausal women. Am J Physiol. 2001;281:E113–E121. doi: 10.1152/ajpendo.2001.281.1.E113. [DOI] [PubMed] [Google Scholar]
- 16.Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson RN. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–218. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 17.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 18.Matthews DE, Pesola G, Campbell RG. Effect of epinephrine on amino acid and energy metabolism in humans. Am J Physiol. 1990;258:E948–E956. doi: 10.1152/ajpendo.1990.258.6.E948. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol. 1993;264:E109–E118. doi: 10.1152/ajpendo.1993.264.1.E109. [DOI] [PubMed] [Google Scholar]
- 20.Gilker CD, Pesola GR, Matthews DE. A mass spectrometric method for measuring glycerol levels and enrichments in plasma using 13C and 2H isotopic tracers. Anal Biochem. 1992;205:172–178. doi: 10.1016/0003-2697(92)90595-x. [DOI] [PubMed] [Google Scholar]
- 21.Daurman D, Froguel P, Rongier M, Robert JJ. Amino acid exchange between plasma and erythrocytes in vivo in humans. J Appl Physiol. 1989;67:2383–2388. doi: 10.1152/jappl.1989.67.6.2383. [DOI] [PubMed] [Google Scholar]
- 22.Williams IH, Sugden PH, Morgan HE. Use of aromatic amino acids as monitors of protein turnover. Am J Physiol. 1981;249:E677–E681. doi: 10.1152/ajpendo.1981.240.6.E677. [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 24.Paolisso G, De Riu S, Marrazzo G, Verza M, Varricchio M, D’Onofrio F. Insulin resistance and hyperinsulinemia in patients with chronic congestive heart failure. Metab Clin Exp. 1991;40:972–977. doi: 10.1016/0026-0495(91)90075-8. [DOI] [PubMed] [Google Scholar]
- 25.Paolisso G, Gambarella A, Marrazzo G, Verza M, Teasuro P, Varricchio M, D’Onofrio F. Metabolic and cardiovascular benefits deriving from β-adrenergic blockade in chronic congestive heart failure. Am Heart J. 1992;123:103–110. doi: 10.1016/0002-8703(92)90753-i. [DOI] [PubMed] [Google Scholar]
- 26.Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJS. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–534. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 27.Lundholm K, Bennegard K, Eden E, Svaninger G, Emery PW, Rennie MJ. Efflux of 3-methylhistidine from the leg in cancer patients who experience weight loss. Cancer Res. 1982;42:4807–4811. [PubMed] [Google Scholar]
- 28.Miller MS, Van Buren P, LeWinter MM, Lecker SH, Selby DE, Palmer BM, Maughan DW, Ades PA, Toth MJ. Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function. Circ: Heart Fail. 2009;2:700–706. doi: 10.1161/CIRCHEARTFAILURE.109.876433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison WL, Gibson JNA, Rennie MJ. Skeletal muscle and whole body protein turnover in cardiac cachexia: influence of branched-chain amino acid administration. Eur J Clin Invest. 1988;18:648–654. doi: 10.1111/j.1365-2362.1988.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 30.Bennegard K, Lindmark L, Eden E, Svaninger G, Lundholm K. Flux of amino acids across the leg in weight-losing cancer patients. Cancer Res. 1984;44:386–393. [PubMed] [Google Scholar]
- 31.Clowes GHA, Randall HT, Cha CJ. Amino acid and energy metabolism in septic and traumatized patients. J Paren Enter Nutr. 1980;4:195–200. doi: 10.1177/014860718000400225. [DOI] [PubMed] [Google Scholar]
- 32.Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K, Rennie MJ. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr. 2009;90:1343–1350. doi: 10.3945/ajcn.2009.27543. [DOI] [PubMed] [Google Scholar]
- 33.Hillier TA, Fryburg DA, Jahn LA, Barrett EJ. Extreme hyperinsulinemia unmasks insulin’s effect to stimulate protein synthesis in the human forearm. Am J Physiol. 1998;274:E1067–E1074. doi: 10.1152/ajpendo.1998.274.6.E1067. [DOI] [PubMed] [Google Scholar]
- 34.Kemppainen J, Tsuchida H, Stolen K, Karlsson H, Bjonholm M, Heinonen OJ, Nuutila P, Krook A, Knuuti J, Zierath JR. Insulin signalling and resistance in patients with chronic heart failure. J Physiol. 2003;550:305–315. doi: 10.1113/jphysiol.2003.042648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congetive heart failure. J Am Coll Cardiol. 1998;31:391–398. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 36.Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med. 1994;205:182–185. doi: 10.3181/00379727-205-43695. [DOI] [PubMed] [Google Scholar]
- 37.Vary TC, Dardevet D, Grizard J, Voisin L, Buffiere C, Denis P, Breuille D, Obled C. Differential regulation of skeletal muscle protein turnover by insulin and IGF-I after bacteremia. Am J Physiol. 1998;275:E584–593. doi: 10.1152/ajpendo.1998.275.4.E584. [DOI] [PubMed] [Google Scholar]
- 38.Bastard JP, Maachi M, van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084–2089. doi: 10.1210/jcem.87.5.8450. [DOI] [PubMed] [Google Scholar]
- 39.Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42:861–868. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 40.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 41.Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen BK, Steensberg A, Keller P, Keller C, Fischer C, Hiscock N, van Hall G, Plomgaard P, Febbraio MA. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pfluggers Arch. 2003;446:9–16. doi: 10.1007/s00424-002-0981-z. [DOI] [PubMed] [Google Scholar]
- 43.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- 44.Matthews DE. Proteins and amino acids. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. Lippincott; Philadelphia: 2006. pp. 23–60. [Google Scholar]
- 45.Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes. 2003;52:1377–1385. doi: 10.2337/diabetes.52.6.1377. [DOI] [PubMed] [Google Scholar]
- 46.Kubo SH, Walter BA, John DHA, Clark M, Cody RJ. Liver function abnormalities in chronic heart failure: influence of systemic hemodynamics. Arch Intern Med. 1987;147:1227–1230. [PubMed] [Google Scholar]
- 47.Biolo G, Gastaldelli A, Zhang XJ, Wolfe RR. Protein synthesis and breakdown in skin and muscle: a leg model of amino acid kinetics. Am J Physiol. 1994;267:E467–474. doi: 10.1152/ajpendo.1994.267.3.E467. [DOI] [PubMed] [Google Scholar]
- 48.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol. 1995;268:E74–E85. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]