Abstract
Exercise has been shown to modify the level/activity of the DNA damage repair enzyme 8-oxoguanine-DNA glycosylase (OGG1) in skeletal muscle. We have studied the impact of regular physical training (8 weeks of swimming) and detraining (8 weeks of rest after an 8-week training session) on the activity of OGG1 in the nucleus and mitochondria as well as its targeting to the mitochondrial matrix in skeletal muscle. Neither exercise training nor detraining altered the overall levels of reactive species; however, mitochondrial levels of carbonylated proteins were decreased in the trained group as assessed by electron spin resonance and biochemical approaches. Importantly, nuclear OGG1 activity was increased by daily exercise training, whereas detraining reversed the up-regulating effect of training. Interestingly, training decreased the outer-membrane-associated mitochondrial OGG1 levels, whereas detraining reversed this effect. These results suggest that exercise training improves OGG1 import into the mitochondrial matrix, thereby increasing OGG1-mediated repair of oxidized guanine bases. Taken together, our data suggest that physical inactivity could impair the mitochondrial targeting of OGG1; however, exercise training increases OGG1 levels/activity in the nucleus and specific activity of OGG1 in mitochondrial compartments, thereby augmenting the repair of oxidized nuclear and mitochondrial DNA bases.
Keywords: Exercise, Detraining, Base excision repair, Nuclear mitochondrial OGG1, Free radicals
Reactive oxygen species (ROS) generated both endogenously and exogenously induce oxidative DNA damage, along with oxidation of lipids and proteins. Whereas oxidized lipids and proteins are degraded by cellular processes, organisms repair DNA damage. Nearly all ROS-induced DNA lesions (except double-strand breaks) are repaired via the DNA base excision repair (BER) pathway [1,2]. BER processes are initiated by the excision of the damaged base by a specific DNA glycosylase, followed by cleavage of the DNA strand at the abasic site (s). Major mammalian DNA glycosylases for repair of oxidized bases include 8-oxoguanine (8-oxoG) DNA glycosylase (OGG1), NTH1 (the functional counterpart of Escherichia coli Nth, an endonuclease III homolog), and the E. coli MutY homolog MYH [3,4]. OGG1 primarily removes oxidized purines (8-oxoG and FapyG) from DNA. NTH1 preferentially removes oxidized pyrimidines, and MYH excises the misincorporated A opposite 8-oxoG during DNA replication [5].
8-Oxoguanine is one of the most frequently generated oxidative base lesions within DNA, owing to guanine’s lowest redox potential among the nucleic acid bases. When 8-oxoG lesions are not removed they have the potential to mispair with adenine, resulting in a G:C to T:A transversion [6]. OGG1 level/activity is thought to be differentially regulated in the nuclear and mitochondrial compartments, and nuclear OGG1 activity has been shown to stay the same or decrease, whereas mitochondria-associated OGG1 activity actually increases, during aging in liver [7]. The OGG1 gene encodes two major alternatively spliced mRNA forms (based on their last exon) that encode OGG1α (nuclear form) and OGG1β (mitochondrial form) [8].
Recent studies have revealed that aging causes impairment of OGG1β targeting into the mitochondrial matrix and that a transfer complex of outer-membrane-associated OGG1β is also isolated with purified mitochondria [9,10]. These findings suggest that overall levels of OGG1β activity could include outer mitochondrial membrane-associated OGG1β and OGG1β that internalized into the matrix for repair of mitochondrial DNA (mtDNA) [9,10]. Recently, we have shown that the activity of OGG1 is different in red and white skeletal muscle and that exercise training decreases the activity of OGG1β in the mitochondrial matrix, whereas it increases the activity of OGG1α [11]. We also showed decreased 8-oxoG content in the nuclear and mitochondrial DNA of hepatocytes, whereas the activity of OGG1 was different in these cell compartments [12].
Mitochondria are essential organelles in ATP synthesis and by default generate significant quantities of ROS owing to the escape of electrons during oxidative phosphorylation [13–16]. It has been shown that the oxidative damage to mtDNA is higher than that observed for nuclear (n) DNA, partly owing to its close proximity to the site of ROS generation and lack of protecting histones [17–19]. It is also possible that a less efficient DNA repair system in mitochondria contributes to a greater accumulation of oxidatively damaged bases of mtDNA compared to nDNA, as documented previously [20–22]. Exercise, especially acute/sudden physical activity, has long been known to increase the levels of tissue ROS and the abundance of oxidized proteins, lipids, and DNA bases, as well as inducing the expression of shock/stress proteins [23–28].
In this study, using a rat model we have tested the effects of regular daily exercise training on tissue ROS levels by electron spin resonance (ESR) spectroscopy and molecular approaches. We determined nuclear and mitochondrial OGG1 activities in rat skeletal muscle tissue as a function of exercise and mitochondrial targeting of OGG1β. Our data show that daily exercise results in adaptation to increased oxidative stress and a more efficient matrix targeting of OGG1 for repair of 8-oxoG in both the nuclear and the mitochondrial compartments.
Materials and methods
Animals
Twenty-one male Wistar rats (13 months of age) were used in the study and were cared for according to the Guiding Principles for the Care and Use of Animals based upon the Helsinki Declaration of 1964. The study was approved by the Animal Welfare Committee of Semmelweis University. Seven rats were randomly assigned to each of three groups: control (C), exercise trained (ET), and exercise trained/detrained (DT). ET and DT rats were subjected to swimming exercise for 8 weeks. Water temperature was maintained at 32°C and swimming duration was 60 min per day, 5 days a week for 4 weeks. For the remaining 4 weeks of exercise the duration was increased to 120 min a day for 5 days a week. After 8 weeks of exercise training the DT group was detrained for an additional 8 weeks. At the time of death, the ET group was 15, the C group 16, and the DT group 17 months of age. Importantly, we allowed 48 h to elapse between the last training session and sacrifice for the animals in the ET group to be able to exclude the effects of the final exercise session. Thus, we were able to study the impacts of regular daily exercise, rather than the acute effects of a single exercise. The animals were killed by decapitation and the red part of the quadriceps muscle was quickly removed and stored at −80°C.
Assessment of lipid peroxides and glutathione level
Malondialdehyde (MDA) concentrations from cytosolic and mitochondrial fractions of the skeletal muscle samples were assessed by using a commercial kit (Bioxytech MDA 586; Oxis International, Foster City, CA, USA) according to the manufacturer’s recommendations. Data were normalized to the protein concentration. Total glutathione (GSH and GSSG) was measured in 100-µl samples gained from supernatants and mitochondrial extracts by recording the formation of 2-nitro-5-thiobenzoic acid at 412 nm (25°C) in a spectrophotometer (Genesys II) in the presence of 0.25 mM 5,5′-dithiobis-(2-nitrobenzoic acid), 0.4 mM NADPH, and 2 U type III glutathione reductase (Sigma, St. Louis, MO, USA). GSSG was determined by derivatizing 150-µl samples of supernatant with 3 µl of undiluted 2-vinylpyridine and assaying 100-µl aliquots of the derivatized sample as described for total GSH. Measured concentrations of GSH and GSSG were expressed per gram of wet tissue weight and as GSH:GSSG ratio.
Electron paramagnetic resonance
The electron paramagnetic resonance (EPR) measurements were carried out as previously described by [29]. In brief, the measurements were conducted with an X-Band computer-controlled EPR spectrometer constructed by Magnettech GmbH (Berlin, Germany). Approximately 100 mg of muscle was frozen into a rod-shaped form and spectra of the samples were recorded at 77 K using a quartz finger Dewar flask filled with liquid nitrogen. Instrument settings were 100 kHz modulation frequency, 0.7050 mT modulation amplitude, 18 mW microwave power, 1 min scan time, and 20.63 mT field sweep. For evaluation, a method of double integration of the EPR signals with Mn/MnO as an internal standard was used, and the data were expressed in arbitrary units.
Preparation of nuclear and mitochondrial fractions
The samples of the red portion of the quadriceps muscle were homogenized in buffer (HB) containing 20 mM Tris (pH 8.0), 1 mM EDTA, 1 mM dithiothreitol, 0.5 mM spermidine, 0.5 mM spermine, 50% glycerol, and protease inhibitors. The nuclear and mitochondrial fractions were separated by differential centrifugation. To prepare nuclear fractions, the hom ogenate was centrifuged at 1000 g for 10 min at 4°C, and the pellet was suspended in HB and recentrifuged. Then, the pellet was resuspended in HB with 0.5% NP-40 and centrifuged. Next, the pellet was washed twice in HB. After centrifugation, the final nuclear pellet was rocked for 30 min after the addition of a 1/10 (vol/vol) volume of 2.5 M KCl and centrifuged at 14,000 rpm for 30 min. The supernatant was divided into aliquots and stored at −80°C. The protein levels were measured using the BCA method. For the isolation of mitochondria, the supernatant from the first centrifugation was recentrifuged at 14,000 g for 30 min at 4°C. Then, the pellet was resuspended in HB (containing 200 mM sucrose and 50 mM mannitol) and recentrifuged three times. The pellet was suspended in 0.5 ml HB. The final mitochondrial pellet was suspended in HB containing 0.5% Triton X-100 and was kept on ice for 20 min. Protein concentrations were determined using the BCA method.
8-OxoG excision assay
The assay was carried out according to the protocol described by [30]. In brief, 20 pmol of synthetic substrate containing 8-oxodG (Trevigen, Gaithersburg, MD, USA) were labeled with 32P at the 5′end using polynucleotide T4 kinase (Boehringer Mannheim, Mannheim, Germany). For the nicking reaction, protein extract (2 µg) was mixed with 20 µl of reaction mixture containing 0.5 M N-(2-hydroxyethel) piperazine-N′-(ethanesulfonic acid), 0.1 M EDTA, 5 mM dithiothreitol, 400 mM KCl, purified BSA, and labeled probe (approximately 2000 cpm). The reaction was carried out at 30°C for 15 min and stopped by placing the mixture in ice. Next, 30 µl chloroform was added, samples were centrifuged, and 15 µl of the aqueous layer was mixed with loading buffer (90% formamide, 10 mM NaOH, and 10% blue-orange dye). After 3 min heating at 95°C, samples were chilled and loaded into polyacrylamide gel (20%) with 7 M urea and 1× TBE and run at 400 mV for 2 h. Radioactive signals of the cleavage product of the labeled substrate were quantified using a STORM bioimaging analyzer (Molecular Dynamics, Sunnyvale, CA, USA) loaded with ImageQuant software. The OGG1 8-oxodG-repair activity was determined and expressed as a percentage of the substrate cleaved [11,31].
Trypsin treatment of mitochondria and Western blots
Purified mitochondria (1 mg/ml) were resuspended in a buffer containing 10 mM Hepes–KOH (pH 7.4), 250 mM sucrose, 0.5 mM EGTA, 2 mM EDTA, and 1 mM DTT and treated with trypsin (10 µg/ml) for 20 min at room temperature, followed by the addition of an equivalent amount of bovine trypsin inhibitor to stop proteolysis [9]. Then the trypsin- and mock-treated samples were lysed and equal amounts were loaded into SDS–polyacrylamide gel, electrophoresed, and then transferred to PVDF membranes. Membranes were probed by anti-OGG1 antibody (Ab; Alpha Diagnostic, San Antonio, TX, USA). The binding of primary Abs was detected with horseradish peroxidase-conjugated secondary Ab (1:2000; anti-mouse or anti-rabbit IgG–horseradish peroxidase; Amersham Pharmacia, Arlington Heights, IL, USA). Subsequently, membranes were washed and incubated in ECL reagent (Amersham Pharmacia). Chemiluminograms were analyzed by using IMAGEQUANT software (Molecular Dynamics). The oxidative modification of amino acid residues of mitochondria was measured by the level of carbonyl derivatives using Western blot as described previously [31].
Statistical analyses
The statistical significance between groups of animals was expressed as mean±standard deviation (SD), calculated using one-way analysis of variance, followed by Scheffe’s post hoc test. Significance level was set at p<0.05.
Results
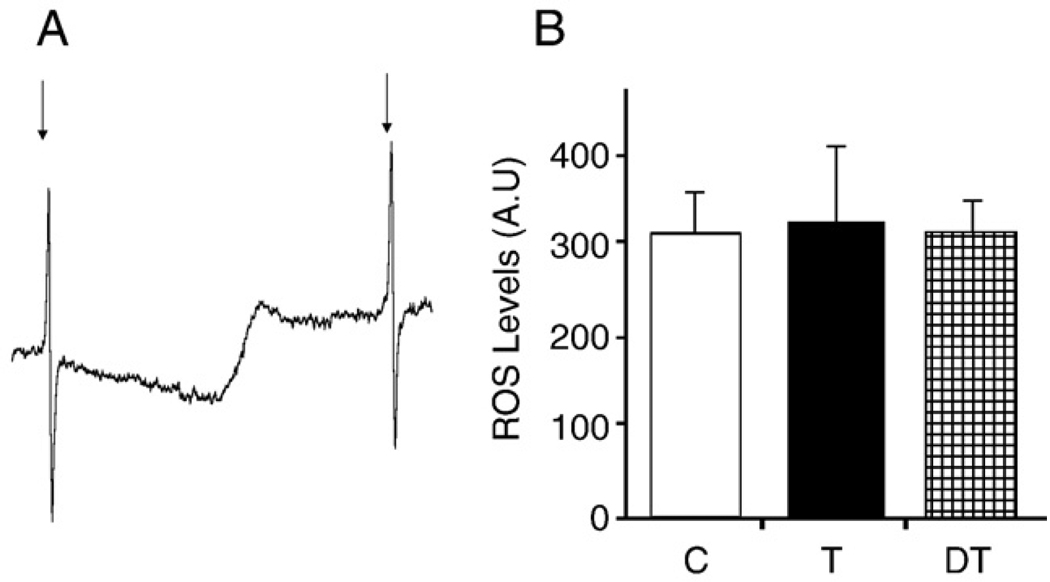
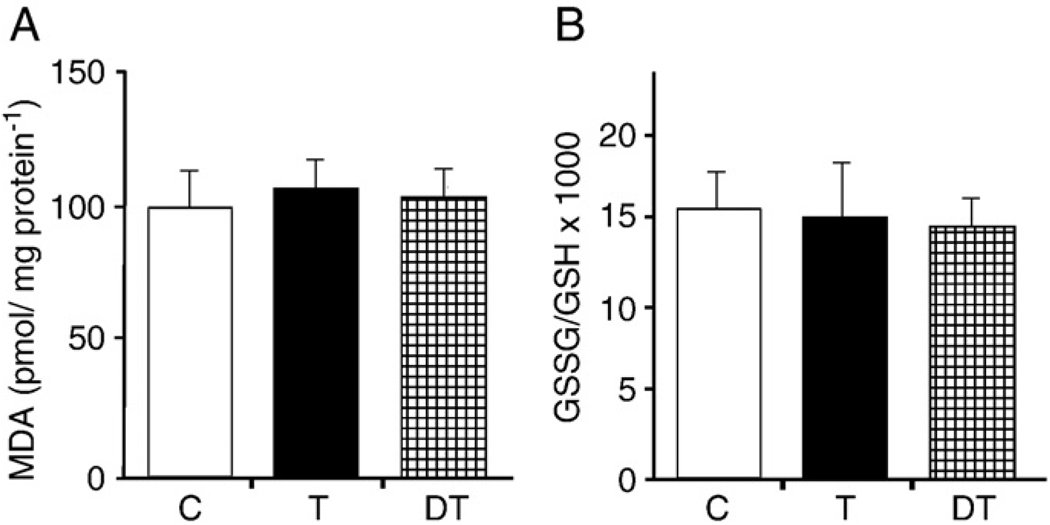
Physical activity without prior training enhances cellular oxidative stress levels and, consequently, levels of oxidatively damaged molecules including proteins, lipids, and DNA bases [17–21]. Here we tested whether regular daily exercise will result in tissue adaptation to oxidative stress at the level of activity and targeting of the repair protein OGG1. Our results show that 16 weeks of daily physical exercise did not significantly alter the overall levels of ROS in the skeletal muscle of animals, as assessed by ESR (Figs. 1A and B). Oxidative stress levels were similar to those of the control group and of the trained animals whose exercise was terminated for 8 weeks. To confirm the data generated by ESR, we determined the level of malondialdehyde as well as the GSH:GSSG ratio in lysates of muscle tissue. As shown in Figs. 2A and B, there were no significant differences in oxidative damage to lipids and the GSSG:GSH ratio did not change among controls and groups of animals subjected to daily physical exercise or detraining.
Fig. 1.
Free radical levels in skeletal muscle. (A) A typical ESR spectrum taken ex vivo at 77 K showing steady-state basal ROS levels in the red muscle tissue of rats. Spectrum contains two Mn/MnO signals as internal standards, indicated by arrows. The signal observed between the two signals of standards was double integrated for evaluation. (B) Graphical illustration of ESR data shows no change in overall ROS levels in the skeletal muscle of experimental animals after adaptation to physical exercise. Values are means±SD for six animals per group. *p<0.05 vs control.
Fig. 2.
(A) Overall levels of lipid peroxides and (B) GSSG:GSG ratios are unaltered in muscle. Malondialdehyde levels and GSSG:GSH ratios were assessed in muscle tissue lysates from trained (T), detrained (DT), and control (C) groups of animals as described under Materials and methods. Data are expressed as the means±SD for six animals per group.
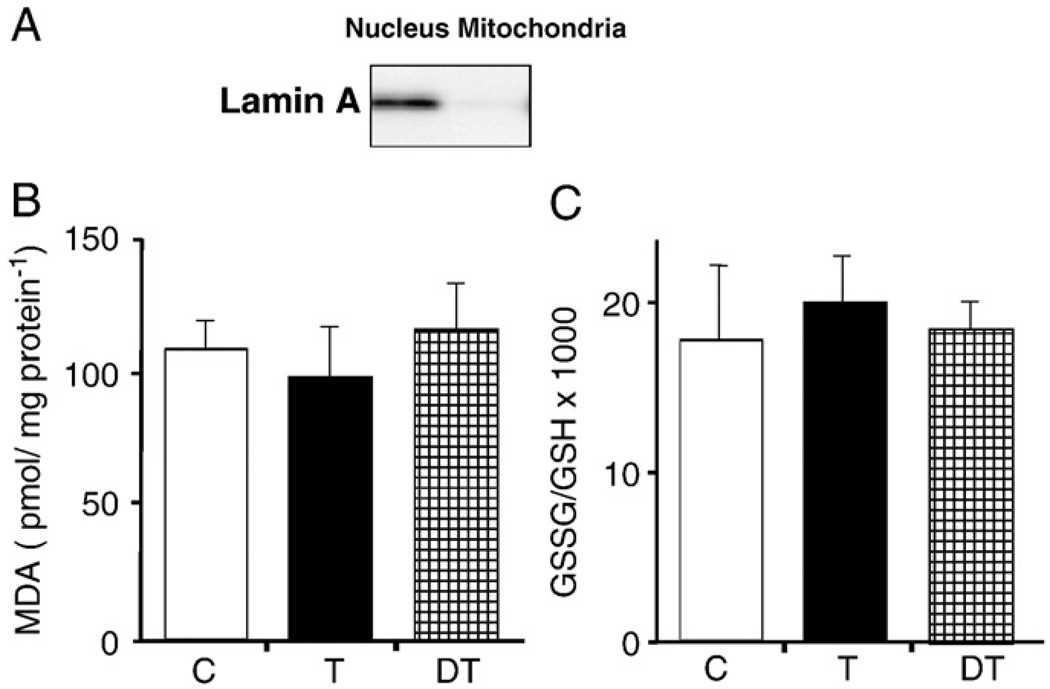
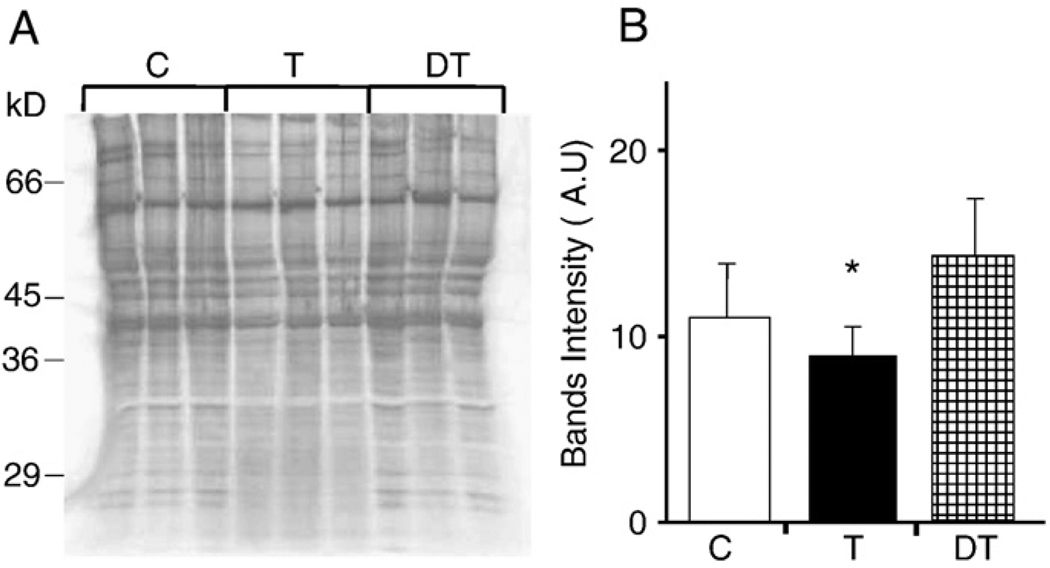
Because overall levels of ROS in a tissue do not ultimately mean that the free radical level is unaltered in the cellular compartment, we determined changes in the GSSG:GSH ratio, MDA, and protein carbonyl levels of mitochondrial lysates. Significant differences were not found in the level of MDA or GSSG:GSH ratio (Figs. 3A and B); in contrast, data from Western blot analysis showed that mitochondrial levels of carbonylated proteins were higher in control and detrained animals (8 weeks after physical exercise was terminated) compared to those adapted by daily exercise (trained). Especially, proteins in the low-molecular-weight range accumulated less oxidative damage in mitochondria from animals trained daily (Figs. 4A and B). These data are in line with previous publications documenting that acute exercise increased oxidative stress levels and induction of shock/stress proteins [23–25]. Whether decreased levels of protein carbonyl in the adapted exercise group mean efficient removal of damaged molecules or less generation of ROS is the focus of future investigations.
Fig. 3.
(B) The levels of lipid peroxidation and (C) GSSG:GSH ratios are not changed in the mitochondrial lysates of skeletal muscle. (A) The purity of mitochondrial and nuclear extracts was evaluated by the concentration of the nuclear envelope marker lamin A (Abcam 133A2, Cambridge, UK). Malondialdehyde levels and GSSG:GSH ratios were assessed from mitochondrial lysates from trained (T), detrained (DT), and control (C) groups of animals as described under Materials and methods. Data are expressed as the means±SD for six animals per group.
Fig. 4.
Decreases in mitochondrial protein carbonyl levels in animals adapted to exercise. (A) A representative image showing the abundance of protein carbonyls in individual control (C), trained (T), and detrained (DT) animals. (B) Graphical illustration of changes in protein carbonyl levels from densitometry of autoradiograms (N=6). The levels of protein carbonylation were similar in control and detrained animals. The trained group showed significantly less damage, especially in low-molecular-weight proteins. *p<0.05.
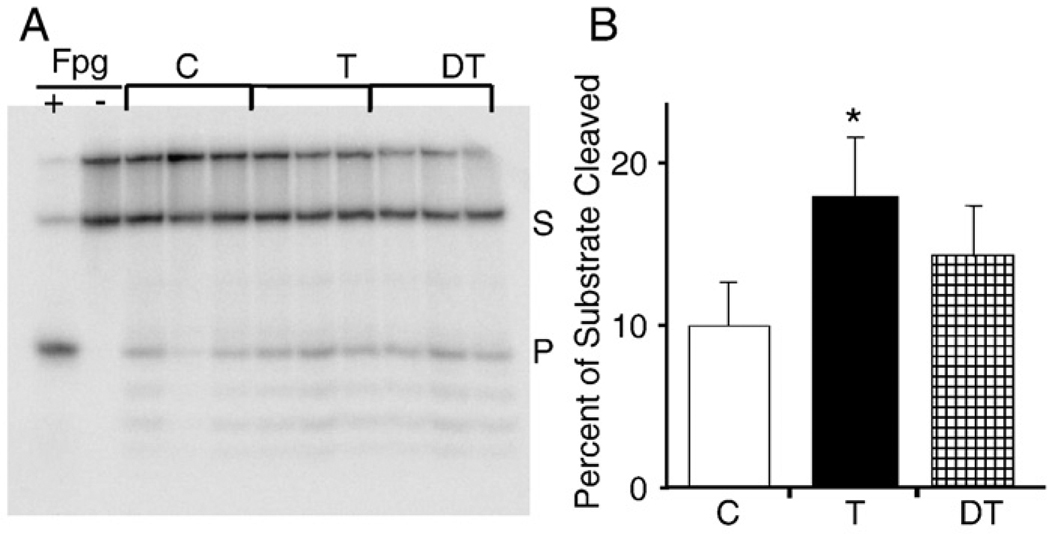
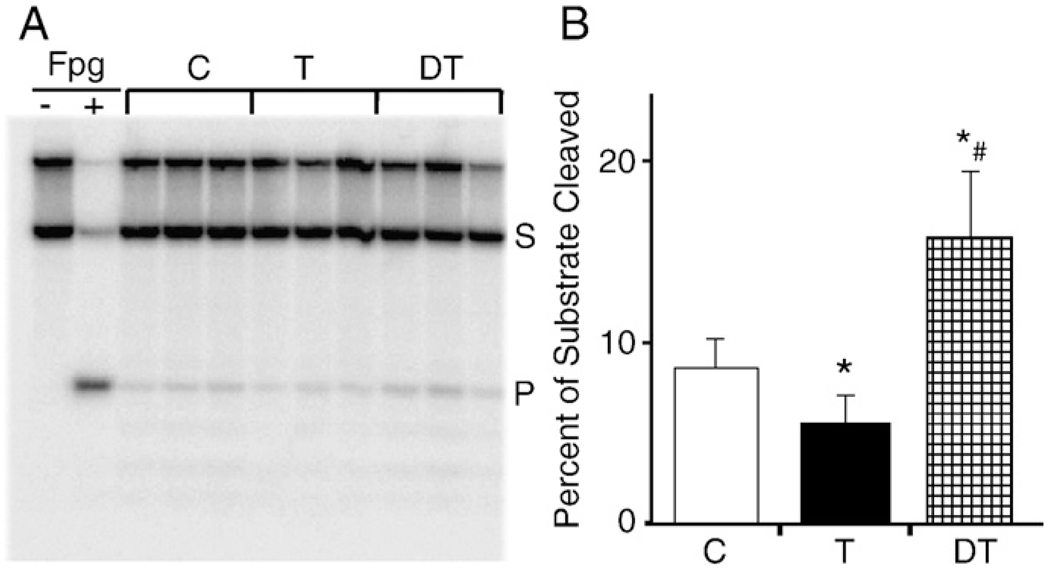
8-OxoG is one of the most frequently generated oxidatively damaged DNA base lesions; therefore it is a sensitive measure of oxidative overload of cells/tissues. Here we determined whether OGG1 activity (8-oxoG repair assay) was different in nuclei and mitochondria from the muscle of trained, detrained, and control animals. Our data in Figs. 5A and B) showthat daily training of animals resulted in a statistically significant increase in OGG1 activity in the nuclear lysate (p<0.05). Eight weeks after the training was terminated (detrained group) there was no significant difference in OGG1 activity in the nucleus compared to control (no physical exercise). Surprisingly, total OGG1 activity was decreased in mitochondrial lysates from animals subjected to daily physical exercise/training (p<0.05). In contrast, a higher total OGG1 activity was observed in the mitochondrial lysates isolated from muscle of animals subjected to rest for 8 weeks (detrained) and in controls (Figs. 6A and B).
Fig. 5.
Exercise increases the activity of OGG1 in the nucleus. Two micrograms of nuclear extract was mixed with 20 pmol of 32P-labeled synthetic substrate containing 8-oxoG in 20 µl of reaction buffer and incubated at 30°C for 15 min. Incision products were separated by polyacrylamide (20%) with 7 M urea gel and the gels were analyzed using a STORM bioimaging analyzer (Materials and methods). The OGG1 8-oxoG-repair activity was determined and expressed as a percentage of the substrate cleaved [24,15]. (A) Image showing increased nOGG1 activity as a result of exercise training, whereas detraining did not alter the activity. Fpg, formamidopyrimidine DNA glycosylase. (B) Graphical illustration of densitometric data obtained from six animals for each group as described under Materials and methods. Assays were run three times and representative data for three animals from each group are shown in (A). S, substrate; P, product. *p<0.05 vs control.
Fig. 6.
Exercise decreases the activity of mitochondria-associated OGG1. The activities of OGG1β in mitochondrial lysates were determined as described for Fig. 4. (A) Image illustrating the changes in OGG1β activity in mitochondrial lysates of individual animals, trained (T), detrained (DT), and controls (C). Fpg, formamidopyrimidine DNA glycosylase. Assays were run three times and a representative gel is shown. (B) Graphical illustration of densitometric data obtained from six animals for each group as described under Materials and methods. S, substrate; P, product. *p<0.05 vs control, #p<0.05 vs trained.
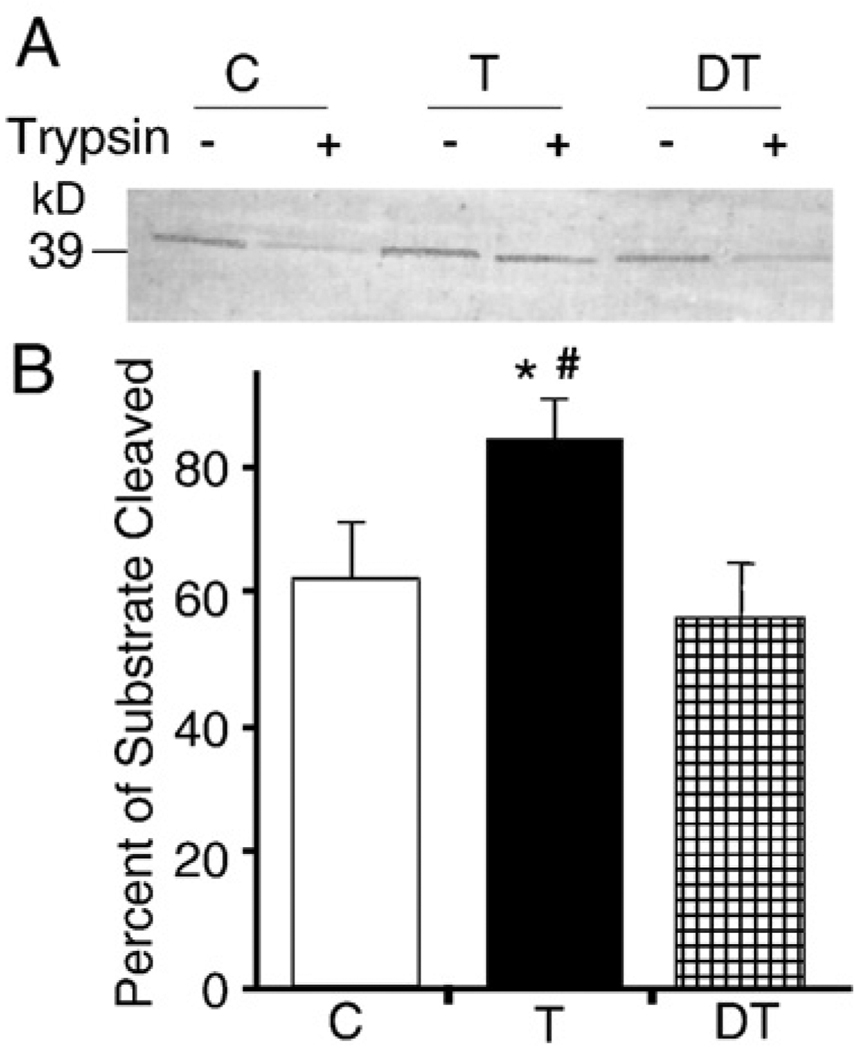
It has been shown that elevated levels of ROS (such as occur during the aging process) result in impaired transport of OGG1β into the mitochondrial matrix [13,14]. Thus, we asked whether the higher overall mitochondria-associated OGG1 activity was due to increased levels of outer-membrane-attached OGG1β. As we expected, trypsin treatment of mitochondrial suspensions lowered levels of OGG1 in the untrained (control) and in detrained animals (lacking training for 8 weeks) compared to those animals subjected to 16 weeks of daily training (Fig. 7A). These observations indicate that significant amounts of OGG1 were attached to the outer membranes of the mitochondria from physically inactive animals. These findings suggest that daily physical exercise training improved the import of OGG1 into the mitochondrial matrix. To test this hypothesis, we assayed lysates of mitochondria +/− trypsin treatment for their 8-oxoG incision activity. We found that OGG1 activity in the mitochondrial lysate frommuscles of animals trained dailywas significantly higher than that of control or detrained animals (Fig. 7B). How daily physical exercise lowers levels of OGG1 in parallel with increased mitochondrial OGG1 activity is the focus of our present investigation.
Fig. 7.
OGG1β level and activity in mitochondrial lysates before and after trypsin digestion. (A) Purified mitochondrial suspensions from control (C), trained (T), and detrained (DT) groups of animals were subjected to mild trypsin digestion or mock treatment and OGG1β levels were assessed as described under Materials and methods. Trypsin treatment resulted in significantly decreased OGG1β levels in control and detrained groups, suggesting that OGG1β was attached to the outer membrane. (B) Mitochondria-associated OGG1β activity is decreased by trypsin digestion in C and DT groups compared to T animals. OGG1 activity was determined as for Fig. 4. *p<0.05 vs control, #p<0.05 vs detrained.
Discussion
Physical exercise augments the generation of reactive species in skeletal muscle, resulting in oxidative stress. ROS modulate gene expression via redox-sensitive transcription pathways, which has been suggested to be involved in the processes of training adaptation [26]. Adaptation of musclesT antioxidant defense against oxidative stress conditions represents a potential mechanism responsible for skeletal muscle tolerance of exercise-induced oxidative damage and altered signaling. Here, we show data on adaptive responses at the level of activity of the DNA repair system and cellular processes that target OGG1 to the site of DNA damage. The data from this study show that regular daily physical exercise increases the incision activity of OGG1 in the nucleus and mitochondria. Remarkably, daily exercise led to a decrease in OGG1 level in the mitochondria without sacrificing efficient 8-oxoG repair. Although these data are seemingly contradictory, they are in accordance with our previous studies [11,12]. Thus our data suggest that training adaptation exists at the level of base excision DNA repair that ensures the sequence fidelity of DNA during exercise-induced stress conditions in muscle.
We have evaluated the impact of regular daily exercise on the oxidative status of muscle tissue. To our surprise, at the tissue level there were no significantly increased ROS levels detectable by ESR. Furthermore, we found that exercise training actually decreased the accumulation of protein carbonyls in mitochondria. These results are only partly in line with the accumulation of mitochondrial protein carbonyls, demonstrated earlier [32]. The extent of oxidative damage of proteins is higher than that of lipids and DNA [33], suggesting that mitochondrial proteins may serve as a buffer against ROS and protect DNA.
Our previous studies provide evidence that exercise decreases the level of 8-oxoG in nDNA and this is associated with increased OGG1 activity [12,31]. In contrast, our present data show that acute physical exercise decreases the level of OGG1 in the mitochondrial lysates. These observations were unexpected, because 8-oxoG levels in nDNA and mtDNA were decreased in liver tissue as a result of exercise training [12]. One may speculate that nuclear and mitochondrial OGG1 activities are similarly altered in liver and skeletal muscle by physical exercise training, thus the levels of 8-oxoG in mtDNA and nDNA change similarly. Despite the similar responses in liver and skeletal muscle to exercise-induced adaptation to oxidative challenge, an equivalence between these findings cannot be drawn, owing to a possible tissue-specific adaptive profile. It is straightforward that lower nuclear 8-oxoG content is associated with increased OGG1 activity, whereas it is difficult to envision how low levels of mtDNA damage (in liver) are accompanied by decreased activity of OGG1 in the mitochondria.
Intriguing observations showing delayed transport of OGG1 into the mitochondrial matrix in aged cells [9] encouraged us to use a similar approach to study the possible adaptation by exercise training at the level of OGG1Ts import into mitochondria. Therefore, we applied mild trypsin digestion to cleave those proteins that were attached to the outer membrane of mitochondria. Our data showed that, indeed, exercise training improved the import of OGG1 into the mitochondrial matrix and suggest that physical inactivity (control and detrained animals) delays the mitochondrial import of OGG1. The relative activity (OGG1 level before vs after trypsin digestion) of OGG1 demonstrates that exercise training increases, whereas physical inactivity decreases, the matrix level of mitochondrial OGG1. The fact that exercise improves protein transfer into mitochondria has been published previously [34].
Mitochondria contain several hundred proteins, of which only 13 are encoded by the mtDNA. All other proteins are encoded by the nuclear DNA and targeted to various mitochondrial compartments [34]. Protein import may depend on molecular mass and structure, among other things, and the intensity of cell signaling required for posttranslational modifications of proteins. An unsolved question is how daily exercise increases OGG1 import. It is possible that lower levels of damage to proteins may facilitate efficient targeting of OGG1 to the mitochondrial matrix. Aerobic exercise training, as was used in the present study, is known to enhance mitochondrial biogenesis so it is also possible that the membranes of newly formed mitochondria are more accessible for transport than those of “aged” mitochondria. Alternatively, exercise training may induce specific changes in mitochondrial transport systems or in OGG1 polypeptide by posttranslational modifications for efficient targeting to matrix.
In summary, data suggest that the levels of free radicals and adaptive capabilities of the subsarcolemmal and intermyofibrillar subpopulations of mitochondria in skeletal muscle are differently regulated [35–37]. Hence, it cannot be excluded that OGG1 import and activity could differ in the mitochondrial subpopulations, but the clarification of this issue needs an independent investigation. Taken together, our data show that regular exercise training induces adaptation to oxidative overload at the level of the DNA repair enzyme OGG1, although with different characteristics in nuclear and mitochondrial extracts. It is intriguing that this adaptation response took place at the level of protein targeting into the mitochondrial matrix and it is reversed by detraining (8 weeks after exercise was terminated).
Acknowledgments
The present work was supported by Hungarian Grants ETT 38388 and TT JAP13/02, awarded to Z. Radk, and by AG 021830 (I.B.) from the U.S. NIH/NIA.
References
- 1.Krokan HE, Nilsen H, Skorpen F, Otterlei M, Slupphaug G. Base excision repair of DNA in mammalian cells. FEBS Lett. 2000;476:73–77. doi: 10.1016/s0014-5793(00)01674-4. [DOI] [PubMed] [Google Scholar]
- 2.Mitra S, Izumi T, Boldogh I, Bhakat KK, Hill JW, Hazra TK. Choreography of oxidative damage repair in mammalian genomes. Free Radic. Biol. Med. 2002;33:15–28. doi: 10.1016/s0891-5849(02)00819-5. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda S, Biswas T, Roy R, Izumi T, Boldogh I, Kurosky A, Sarker AH, Seki S, Mitra S. Purification and characterization of human hNTH1, a homolog of Escherichia coli endonuclease III: direct identification of Lys-212 as the active nucleophilic residue. J. Biol. Chem. 1998;273:21585–21593. doi: 10.1074/jbc.273.34.21585. [DOI] [PubMed] [Google Scholar]
- 4.Aspinwall R, Rothwell DG, Roldan-Arjona T, Anselmino C, Ward CJ, Cheadle JP, Sampson JR, Lindahl T, Harris PC, Hickson ID. Cloning and characterization of a functional human homolog of Escherichia coli endonuclease III. Proc. Natl. Acad. Sci. USA. 1997;94:109–114. doi: 10.1073/pnas.94.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slupska MM, Baiialov C, Luther WM, Chiang J-H, Wei YH, Miller JH. Cloning and sequencing a human homolog (hMYH) of the Escherichia coli mutY gene whose function is required for the repair of oxidative DNA damage. J. Bacteriol. 1996;178:3885–3892. doi: 10.1128/jb.178.13.3885-3892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993;9:246–249. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- 7.de Souza-Pinto NC, Hogue BA, Bohr VA. DNA repair and aging in mouse liver:8-oxodG glycosylase activity increase in mitochondrial but not in nuclear extracts. Free Radic. Biol. Med. 2001;30:916–923. doi: 10.1016/s0891-5849(01)00483-x. [DOI] [PubMed] [Google Scholar]
- 8.Nishioka K, Ohtsubo T, Oda H, Fujiwara T, Kang D, Sugimachi K, Nakabeppu Y. Expression and differential intracellular localization of two major forms of human 8-oxoguanine DNA glycosylase encoded by alternatively spliced OGG1 mRNAs. Mol. Biol. Cell. 1999;10:1637–1652. doi: 10.1091/mbc.10.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szczesny B, Hazra TK, Papaconstantinou J, Mitra S, Boldogh I. Age-dependent deficiency in import of mitochondrial DNA glycosylases required for repair of oxidatively damaged bases. Proc. Natl. Acad. Sci. USA. 2003;100:10670–10675. doi: 10.1073/pnas.1932854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szczesny B, Bhakat KK, Mitra S, Boldogh I. Age-dependent modulation of DNA repair enzymes by covalent modification and subcellular distribution. Mech. Ageing Dev. 2004;125:755–765. doi: 10.1016/j.mad.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Radak Z, Kumagai S, Nakamoto H, Goto S. 8-Oxoguanosine and uracil repair of nuclear and mitochondrial DNA in red and white skeletal muscle of exercise, trained old rats. J. Appl. Physiol. 2007;102:1696–1701. doi: 10.1152/japplphysiol.01051.2006. [DOI] [PubMed] [Google Scholar]
- 12.Nakamoto H, Kaneko T, Tahara S, Hayashi E, Naito H, Radak Z, Goto S. Regular exercise reduces 8-oxodG in the nuclear and mitochondrial DNA and modulates the DNA repair activity in the liver of old rats. Exp. Gerontol. 2007;42:287–295. doi: 10.1016/j.exger.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- 15.Cadenas E, Davies KJA. Mitochondrial free radical production, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–2230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 16.Barja G. Free radicals and aging. Trends Neurosci. 2004;10:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Henle ES, Linn S. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. J. Biol. Chem. 1997;272:19095–19098. doi: 10.1074/jbc.272.31.19095. [DOI] [PubMed] [Google Scholar]
- 18.Hudson EK, Houge BA, Souza-Pinto NC, Croteau DL, Anson RM, Bohr VA, Hansford RG. Age-associated change in mitochondrialDNA damage. Free Radic. Res. 1998;29:573–579. doi: 10.1080/10715769800300611. [DOI] [PubMed] [Google Scholar]
- 19.Richter C. Do mitochondrial DNA fragments promote cancer and aging? FEBS Lett. 1988;241:1–5. doi: 10.1016/0014-5793(88)81018-4. [DOI] [PubMed] [Google Scholar]
- 20.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 21.Karahalil B, Hogue BA, de Souza-Pinto NC, Bohr VA. Base excision repair capacity inmitochondria and nuclei: tissue-specific variations. FASEB J. 2002;16:1895–1902. doi: 10.1096/fj.02-0463com. [DOI] [PubMed] [Google Scholar]
- 22.Bohr VA, Stevnsner T, de Souza-Pinto NC. Mitochondrial DNA repair of oxidative damage in mammalian cells. Gene. 2002;286:127–134. doi: 10.1016/s0378-1119(01)00813-7. [DOI] [PubMed] [Google Scholar]
- 23.Davies KJA, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 24.Davies KJA, Hochstein P. Ubisemiquinone radicals in liver: implications for a mitochondrial Q cycle in vivo. Biochem. Biophys. Res. Commun. 1982;107:1292–1299. doi: 10.1016/s0006-291x(82)80138-1. [DOI] [PubMed] [Google Scholar]
- 25.Salo DC, Donovan CM, Davies KJA. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic. Biol. Med. 1991;11:239–246. doi: 10.1016/0891-5849(91)90119-n. [DOI] [PubMed] [Google Scholar]
- 26.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic. Biol. Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 27.Radak Z, Pucsok J, Mecseki S, Csont T, Ferdinandy P. Muscle soreness-induced reduction in force generation is accompanied by increased nitric oxide content and DNA damage in human skeletal muscle. Free Radic. Biol. Med. 1999;26:1059–1063. doi: 10.1016/s0891-5849(98)00309-8. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y, Nanri H, Ohta M, Kasai H, Ikeda M. Increase of human MTH1 and decrease of 8-hydroxydeoxyguanosine in leukocyte DNA by acute and chronic exercise in healthy male subjects. Biochem. Biophys. Res. Commun. 2003;305:333–338. doi: 10.1016/s0006-291x(03)00774-5. [DOI] [PubMed] [Google Scholar]
- 29.Stadler K, Jenei V, von Bolcshazy G, Somogyi A, Jakus J. Increased nitric oxide levels as an early sign of premature aging in diabetes. Free Radic. Biol. Med. 2003;35:1240–1251. doi: 10.1016/s0891-5849(03)00499-4. [DOI] [PubMed] [Google Scholar]
- 30.Cardozo-Pelaez F, Stedeford TJ, Brooks PJ, Song S, Sanchez-Ramos JR. Effects of diethylmaleate on DNA damage and repair in the mouse brain. Free Radic. Biol. Med. 2002;33:292–298. doi: 10.1016/s0891-5849(02)00881-x. [DOI] [PubMed] [Google Scholar]
- 31.Radak Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002;445:273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- 32.Radak Z, Kaneko T, Tahara S, Nakamoto H, Ohno H, Sasvari M, Nakamoto H, Goto S. The effect of exercise training on oxidative damage of lipids, proteins, and DNA in rat skeletal muscle: evidence for beneficial outcomes. Free Radic. Biol. Med. 1999;27:69–74. doi: 10.1016/s0891-5849(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 33.Radak Z, Taylor AW, Ohno H, Goto S. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc. Immunol. Rev. 2001;7:90–107. [PubMed] [Google Scholar]
- 34.Hood DA, Adhihetty PJ, Colavecchia M, Gordon JW, Irrcher I, Joseph AM. Mitochondrial biogenesis and the role of the protein import pathway. Med. Sci. Sports Exerc. 2003;35:86–94. doi: 10.1097/00005768-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Truscott KN, Brandner K, Pfanner N. Mechanisms of protein import into mitochondria. Curr. Biol. 2003;13 doi: 10.1016/s0960-9822(03)00239-2. R326–237. [DOI] [PubMed] [Google Scholar]
- 36.Adhihetty PJ, Ljubicic V, Hood DA. Effect of chronic contractile activity on SS and IMF mitochondrial apoptotic susceptibility in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2007;292:E748–E755. doi: 10.1152/ajpendo.00311.2006. [DOI] [PubMed] [Google Scholar]
- 37.Servais S, Courturier K, Koubi H, Rouanet JL, Desplanches D, Sornay-Mayet MH, Sempore B, Lavoie JM, Facier R. Effect of voluntary exercise on H2O2 release by subsarcolemmal and intermyofibrillar mitochondria. Free Radic. Biol. Med. 2003;35:24–32. doi: 10.1016/s0891-5849(03)00177-1. [DOI] [PubMed] [Google Scholar]