Abstract
The formation of CNS myelin is dependent on the differentiation of oligodendrocyte precursor cells (OPCs) and oligodendrocyte maturation. How the initiation of myelination is regulated is unclear but it is likely to depend on the development of competence by oligodendrocytes and receptivity by target axons. Here we identify an additional level of control of oligodendrocyte maturation mediated by interactions between the different cellular components of the oligodendrocyte lineage. During development oligodendrocyte precursors mature through a series of stages defined by labeling with monoclonal antibodies A2B5 and O4. Newly differentiated oligodendrocytes begin to express galactocerebroside recognized by O1 antibodies and subsequently mature to myelin basic protein (MBP) positive cells prior to formation of compact myelin. Using an in vitro brain slice culture system that supports robust myelination, the consequences of ablating cells at different stages of the oligodendrocyte lineage on myelination has been assayed. Elimination of all OPC lineage cells through A2B5+, O4+ and O1+ complement mediated cell lysis resulted in a delay in development of MBP cells and myelination. Selective elimination of early OPCs (A2B5+) also unexpectedly resulted in delayed MBP expression compared to controls suggesting early OPCs contribute to the timing of myelination onset. By contrast, elimination of differentiated (O1+) immature oligodendrocytes permanently inhibited the appearance of MBP+ cells suggesting that oligodendrocytes are critical to facilitate the maturation of OPCs. These data illuminate that the presence of intra-lineage feed-forward and feedback cues are important for timely myelination by oligodendrocytes.
Keywords: Oligodendrocyte precursors, oligodendrocytes, myelination, slice cultures
Introduction
The development of oligodendrocytes, the myelinating cells of the vertebrate central nervous system (CNS), has been studied extensively. In vivo oligodendrocyte precursors (OPCs) arise from neural stem cells in distinct regions of the embryonic neural tube as a result of localized signals that modulate the activity of a number of transcription factors such as the Olig genes. These OPCs migrate widely through the CNS in response to selected guidance cues and proliferate extensively in response to growth factors such as PDGF (Bogler et al., 1990) prior to differentiating and myelinating adjacent axons in a reproducible pattern. In vitro analyses of OPC development has been facilitated through cell culture and the utilization of antibodies or gene transcripts that distinguish particular stages in OPC development. Each stage is characterized by changes in proliferation, migratory abilities, and morphology (Bansal et al., 1989; Hardy and Reynolds, 1993; Lubetzki et al., 1991). Oligodendrocyte precursors (OPCs) express NG2 and mAb A2B5 (Raff, 1989; Raff et al., 1984a) (Raff et al., 1984b) and proliferate in response to platelet-derived growth factor (PDGF) (Noble et al., 1988; Richardson et al., 1988). Recent evidence suggests OPCs have stem cell like properties and can generate astrocytes and neurons in addition to oligodendrocytes (Kondo and Raff, 2004). Later in development, labeling with mAb O4 identifies OPCs and immature oligodendrocytes (Bansal et al., 1992). Newly differentiated oligodendrocytes begin to express galactocerebroside recognized by mAb O1 that, with further maturation, express myelin basic protein (MBP) followed by the full spectrum of myelin components and elaboration of compact myelin sheaths (Miller, 2002; Rosenberg et al., 2007).
Several mechanisms have been implicated in regulating the progression of OPCs to a myelinating cell. Clonal studies suggested the presence of a cell intrinsic timing mechanism controlling oligodendrocyte differentiation (Barres et al., 1994; Temple and Raff, 1986), a component of which may be the transcription factor GM98 (Emery et al., 2009), while the proliferative capacity of OPCs is mediated in part by p57Kip2 (Dugas et al., 2007). The cell intrinsic program of OPC differentiation is subject to significant external regulation. For example, in the presence of fibroblast growth Factor (FGF) and PDGF, OPCs continue to proliferate and fail to differentiate (McKinnon et al., 1993; Noble et al., 1988; Raff et al., 1988). Conversely, withdrawal of growth factors stimulates precocious OPC differentiation.
Several lines of evidence implicate interactions between cells of the oligodendrocyte lineage in regulating their behavior. Culture studies demonstrated that the final number of oligodendrocytes that develop is independent of the number of OPCs in the initial culture suggesting the lineage reaches equilibrium (Zhang and Miller, 1996). Similarly over- expression of PDGF in vivo generates more OPCs but no change in the final number of oligodendrocytes (Calver et al., 1998; Richardson et al., 1988). These normalizations of cell number may reflect a density-dependent inhibition of OPC proliferation mediated through control of P27KIP1 and Rb phosphorylation (Nakatsuji and Miller, 2001) as well as increases in oligodendrocyte apoptosis (Calver et al., 1998; Richardson et al., 1988). Further evidence of intra-OPC lineage interactions comes from the characterization of the fate of OPCs from distinct sources in the developing forebrain suggest that competition during normal development between early and late generated OPCs results in the elimination of cohorts of cells (Kessaris et al., 2006).
During development not all OPCs undergo differentiation into myelinating oligodendrocytes. Early studies identified “adult OPCs” that proliferate more slowly and in response to different mitogens than their embryonic counterparts (Bogler et al., 1990; Nishiyama et al., 1999; Wolswijk and Noble, 1992). Subsequently these adult OPCs have been shown to persist in significant numbers throughout life and their characterization in vivo reveals unique physiological properties implicating them in control of axonal excitability (De Biase et al.; Karadottir et al., 2008; Nishiyama et al., 1999; Wolswijk and Noble, 1992; Woodruff et al., 2004; Ziskin et al., 2007). Whether these cells represent a reservoir of OPCs for remyelination by replacing oligodendrocytes lost during adulthood is unclear. Remyelination can occur in the adult CNS, however in certain pathological conditions such as chronic demyelinated plaques seen in multiple sclerosis remyelination fails even though these regions contain cells with the characteristics of OPCs (Chang et al., 2000; Rudick and Trapp, 2009). Why these OPCs that are surrounded by naked axons fail to myelinate is currently unclear.
Slice cultures are emerging as an effective model with which to assess the regulation of myelination (Harrer et al., 2009). Using complement-mediated lysis to selectively eliminate distinct stages of the developing OPC population in slice and dissociated cell culture we show that the timely maturation of oligodendrocytes is dependent on all stages of the lineage. Oligodendrocyte maturation is delayed by selective removal of early OPCs but inhibited by selective removal of newly generated oligodendrocytes. These studies suggest interdependence among the cellular components of the oligodendrocyte lineage and reveal a novel regulation of oligodendrocyte maturation that may have important implications for development of effective myelin repair strategies.
Materials and Methods
Preparation of postnatal rat brain slice culture
All animals were purchased from Harlan Laboratories (Indianapolis, IN) and housed in the ARC facility of Case Western Reserve University School of Medicine. The Case ARC facility is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All animal procedures were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. Timed pregnant Sprague-Dawley (SD) rats were purchased and P2 rat brains dissected and kept in ice cold PBS buffer supplemented with 10% glucose (Sigma, USA) prior to embedding in 1% agar. Slices were taken from a region encompassing 1.1-0.2 relative to Bregma. All images and quantification were obtained from the region of the motor cortex, cingulum and underlying subcortical white matter. Slice cultures were prepared from 250um thick coronal slices using a Leica vibrating microtome (Leica, VT 1000S, Germany) and immediately placed into cell-culture inserts (0.4 μm, Millicell-CM, Millipore) and cultured in six-well culture dishes containing basal medium eagle (BME) medium supplement with 25% horse serum, 0.5% glucose, 2.5% Hank's solution and 1% L-glutamine. Slices were grown at 37°C/ 5% CO2 and the growth medium changed every second day.
Preparation of dissociated cell cultures
The brain region containing the corpus callosum was isolated from P2 SD rats, chopped and digested in trypsin with 0.25% EDTA in MEM for 20-25 minutes at 37°C. Cell suspensions were filtered through a 30-μm nylon mesh to remove large tissue clumps and cells plated on 12-mm glass coverslips coated with poly-L-lysine (Sigma) at a density of 2 × 105 cells/coverslip. Cells were grown in medium (DMEM medium supplemented with 1% FBS-c, PDGF and N2) overnight before antibody-complement treatment using a protocol described below.
Preparation of slices for electron microscopy
To assess the extent of myelination 1um Epon embedded sections were labeled with Toluidine blue and ultrathin sections were examined on a Joel 100CX electron microscope at 80KV. Slices were fixed in glutaraldehyde (2%) and paraformaldehyde (4%) followed by 1% OsO4, block stained with saturated uranyl acetate, dehydrated through graded alcohols and embedded in Epon 812. Sections were cut in a plane parallel to the surface of the slice.
Antibodies and complement mediated cell lysis
The primary antibodies used in the study include the following: mouse monoclonals A2B5, O4 and O1 IgM hybridoma supernatant as previously described (Gao et al., 2006; Tsai et al., 2006). NG2+ cells were identified by labeling with the anti-NG2 chondroitin sulfate proteoglycan rabbit polyclonal antibody (Millipore, AB5320). A mouse monoclonal antibody against MBP (Covance, Princeton, NJ) was used as a marker of mature oligodendrocytes. Polyclonal rabbit Neurofilament 200 antibody was used to label axons (Sigma, N4142). Anti-iba-1 polyclonal antibody (Wako Chemicals USA, Inc., VA), purified from rabbit antisera with specific reactivity to microglia and macrophages was used to assess microglial response. Guinea Pig complement was purchased from Sigma (St. Louis, MO) and cells were eliminated using the following protocol: P2 coronal slices were sectioned and incubated at 37°C incubation overnight. The following day slices were incubated in A2B5, O4 or O1 antibodies diluted 1:1 in 10% horse serum in BME at 37°C for 60 minutes. Guinea pig complement was then added at a dilution of 1:10 and incubated for 2 hours at 37°C. After washing with BME, the slices were maintained in growth medium overnight. To insure the majority of the selected cell population was depleted, the procedure was repeated the following day. Following antibody mediated complement cell lysis, slices were maintained at various time points (P2+3DIV, P2+7DIV and P2+14DIV) prior to immunostaining. Controls included incubation in complement or antibody alone and neither significantly altered the number of oligodendrocyte lineage cells in the slice. To assess the effectiveness of the cell depletion, slices were labeled with the same antibodies 24 hrs after complement treatment. Primary antibody labeling was visualized by conjugated secondary Alexa antibodies (Invitrogen, USA) and slices mounted in anti-fading fluorescence medium with or without DAPI from Vector Laboratories (Burlingame, CA). The extent of myelination was confirmed by Black Gold. Black Gold (Chemicon, Product# AG390) is a novel haloaurophosphate complex that localizes both normal and pathologic myelin within the CNS.
Immunocytochemistry and myelin labeling
MBP immunocytochemistry in brain slice culture
Cultured slices were fixed in 4% paraformaldehyde for 30 minutes at room temperature. After rinsing in PBS, slices were de-lipidated with 5% acetic acid in 95% ethanol for 20 minutes at 4°C, blocked with 10% normal goat serum in PBS for 1 hour at room temperature and incubated with primary mouse monoclonal anti-MBP antibody (1:500) overnight at 4°C. After rinsing, slices were incubated in fluorescence-conjugated anti-mouse secondary antibody Alexa 596 (1:500) for 2 hours. Cultured slices were mounted and analyzed using Leica DFC 500 fluorescence or confocal microscope.
Double or single labeling of OPCs in slice cultures
Slice cultures were fixed in 4% paraformaldehyde for 30 minutes at room temperature. After washing in PBS, slices were blocked in 5% goat serum in PBS with 0.03% triton for 20 minutes at room temperature. Double immunostaining of polyclonal rabbit Neurofilament 200 (1:250) and MBP (1:500) or single primary antibodies (A2B5, NG2, O1) were incubated overnight at 4 °C. After rinsing, slices were incubated in fluorescence-conjugated anti-mouse or anti-rabbit secondary antibody Alexa 488 (1:500) or 596 (1:1000) for 2 hours at room temperature and labeled slices were mounted on slides.
Immunostaining in dissociated brain cell culture
A2B5, O4 and O1 staining was performed on live cells in dissociated cell culture as previously described (Nishiyama et al., 1999; Zhang and Miller, 1996). For labeling with mouse anti-MBP antibody, cultured cells were fixed with 4% paraformaldehyde and incubated in mouse monoclonal MBP antibody followed by anti-mouse secondary antibody Alexa 596 for 2 hours at room temperature.
Black-Gold
Slices were fixed with 4% paraformaldehyde for 1 hour at room temperature, transferred into pre-warmed Black Gold solution at 60°C (0.2% Black-Gold solution: 100mg of Black-Gold, 50ml of 0.9% NaCl) for 30-40 minutes. After rinsing, slices were fixed for 3 minutes in sodium thiosulfate solution and rinsed, air-dried, and mounted in Citifluor (Ted Pella, INC. CA, USA).
Quantification of MBP+ expression in brain slice cultures
To quantify the expression of MBP protein in slice cultures, the histogram statistic feature of Adobe Photoshop CS3 was used. For each antibody-complement treated group, six randomly selected regions were measured and the intensity of MBP expression assessed. Each observation was repeated at least 3 times. The average intensity value of MBP expression was calculated and compared between conditions. Each study was repeated at least 3 times and the data presented as mean+/-SD. Statistical significance was determined using paired student t test or ANOVA test and set at p>0.05.
In slice and dissociated cell cultures, double-blinded cell counts were performed using a Leica Fluorescence microscope with a 20× or 40× objective lens and a reticule (10×10 grid). For each experiment, 5-9 fields were randomly selected on each slice or coverslip and the total number of A2B5+, O1+ or MBP+ immunopositive cells and the total number of cell nuclei stained with DAPI counted. The number of immunopositive cells in the total cell number was calculated and compared between treated cultures and non-treated controls. The Person Chi-square or ANOVA test was used for statistical analysis between treated and non-treated groups. The studies were repeated a minimum of 3 times and significance determined as p>0.05
Results
Development of myelination in slice culture
To define the timing of oligodendrocyte maturation and myelination coronal brain slice cultures including corpus callosum established from postnatal day 2 (P2) forebrain were labeled with anti-MBP antibodies after different culture intervals. After 3 days in vitro (P2+3DIV) individual MBP+ cells were widely distributed throughout the slice. Within the developing motor cortex and underlying presumptive white matter these cells had few short processes (Figure 1a) that emerged in a radial manner from the cell body and resembled premyelinating oligodendrocytes seen in vivo. After two additional days in vitro (P2+5 DIV) the number of MBP+ cells increased from approximately 3% to 6% of total cells and their morphology became more complex (Figure 1b). Compared to cells in younger slices, after 5 days in vitro many MBP-positive cells had an increased number of processes that were significantly longer. Although individual MBP cells occupied a larger area, the processes from an individual cell rarely overlapped with adjacent cells. With a further 2 days in vitro (P2+7 DIV) there was no significant increase in the density of MBP+ cells that remained at approximately 7% of total cells, however the processes of many MBP+ cells were oriented parallel to adjacent axons but still did not significantly overlap with each other (Figure 1c). By P2+14 DIV a dramatic up-regulation of MBP+ expression to about 20% of total cells was seen and both cell bodies and processes had increased MBP expression (Figure 1d). Adjacent oligodendrocytes appeared to have formed continuous coverage along neighboring axons and individual oligodendrocyte domains were no longer discernable. These observations demonstrate that oligodendrocyte maturation follows a similar temporal sequence in slice cultures as in the intact CNS where myelination begins in the first postnatal week and peaks in the first postnatal month (Benjamins and Morell, 1978).
Figure 1.

Development of myelination in rat brain slice cultures. P2 brain coronal slices taken from the region 1.1-02 relative to Bregma were grown in vitro for 3, 5, 7 or 14 days. Immature MBP+ cells with few processes were seen in the developing motor cortex and underlying white matter at P2+3 DIV (a). In the cultures at P2+5 DIV (b) and P2+7 DIV (c), more MBP+ cells with extended processes had developed by P2+14 DIV (d), MBP is expressed both in oligodendrocyte cell bodies and in processes surrounding axons. Black-Gold® staining (e) confirms the presence of myelination in P2+14 DIV slice cultures. The close association of axons and oligodendrocyte process is demonstrated by the high level of coincidence of MBP (red) co-localized with neuronal axons, which is stained by neurofilament (green) in (f). Confocal imaging (g) confirms that axons are wrapped by MBP+ process. Electron microscopy (EM) shows an axon in longitudinal section surrounded by a compacted myelin sheath (arrows) at P2+14 DIV (h). cx = cortex, cc = corpus callosum. Scale bar = 25μm.
Regional differences in the pattern of oligodendrocyte development and myelination were preserved in slice cultures. In the corpus callosum of older slices (14 DIV), densely packed MPB+ cell processes were oriented largely parallel to callosal axons and multiple MBP+ cell bodies were present (Figure 1d, e). By contrast in cortical regions the majority of the MBP+ processes were oriented radially and were far sparser than in presumptive white matter. A similar pattern was observed using independent indicators of myelination. For example labeling with Black-Gold, that stains normal as well as degenerating myelin demonstrated that the majority of the myelin was oriented parallel in the corpus callosum (cc) but radially in the cortex (cx) (Fig. 1e). The close association of the axons and oligodendrocyte processes was evident in slices double labeled with antibodies to neurofilament (NF200) and MBP where the labeling appeared coincident at the level of the light microscope (Fig. 1f, arrows). Confocal imaging (Figure 1g) confirmed the organization of the MBP processes. To unambiguously demonstrate the presence of compact myelin, slices were prepared for electron microscopy and sections cut parallel to the surface of the slice. Compared to the robust expression of MBP and Black Gold labeling, relatively few compact myelinated axons were seen (Figure 1h, arrows). Rather the majority of axons appeared to be surrounded by oligodendrocyte processes that had yet to form compact myelin. By 21DIV extensive myelination was seen through the slice (data not shown). These data suggest that while the maturation of oligodendrocytes is efficiently supported in slice cultures, the generation of compact myelin is delayed relative to in vivo.
Selective ablation of distinct stages of the oligodendrocyte lineage
A powerful approach to understanding the interrelationships between distinct cell types is to assess the consequences of selective cell ablation. To determine whether specific cell subpopulations within the oligodendrocyte lineage could be eliminated, coronal slice cultures from P2 rat brains were exposed after 1 day in vitro to 2 rounds of complement mediated cell lysis using either mAb A2B5 to eliminate cells early in the lineage, or mAb O1 to eliminate newly formed oligodendrocytes. Twenty-four hours after the second complement treatment slices were labeled with A2B5 or O1 antibodies and the number of labeled cells was determined. In slices treated with A2B5 antibody and complement, the number of A2B5+ cells was reduced dramatically 24hrs later (Figure 2A, b) with the residual labeling mainly on cell debris. By contrast the number of O1+ cells was essentially unaffected compared to controls (Figure 2A, e). Likewise in slices treated with O1 antibody and complement the number of O1+ cells was virtually zero after 24hrs (Figure 2A, f) while the number of A2B5+ cells (Figure 2A, c) was essentially unaffected compared to controls (Figure 2A, a, d). At this stage neither treated nor control slices contained significant numbers of MBP+ cells. Quantitative data is shown in Figure 2B and 2C.
Figure 2.
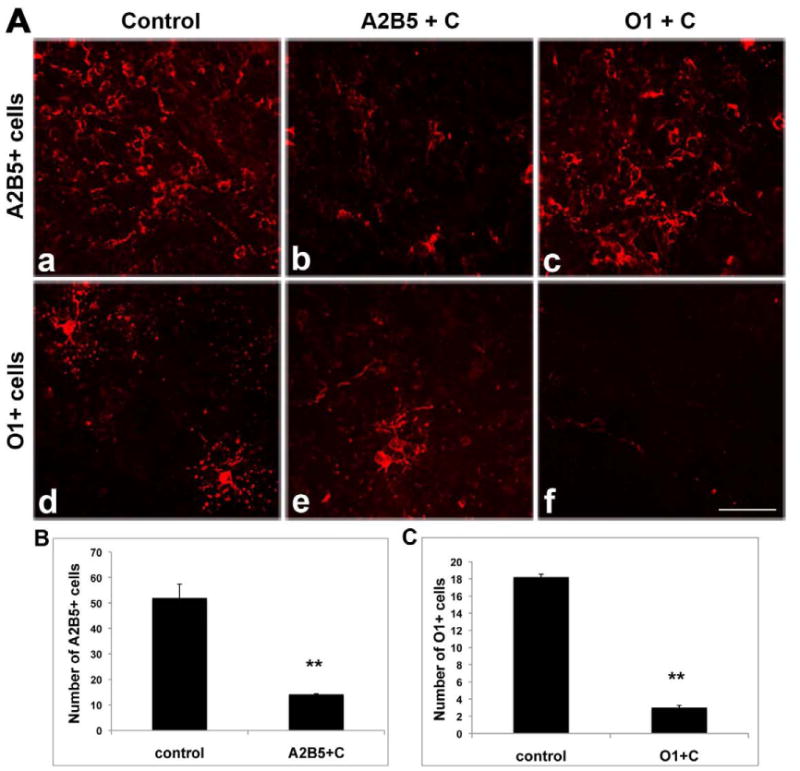
Reduction of A2B5+ or O1+ cells in slice cultures after complement-mediated lysis to selectively ablate A2B5+ or O1+ cells. A dramatic reduction in the number of A2B5+ cells (A,b) was seen following treatment with A2B5 plus complement (A2B5+C), compared to the untreated control (A,a) whereas the density of O1+ cells remains at a similar level to control (A,e). Likewise, treatment with O1 antibody plus complement resulted in substantial loss of O1+ cells (A,f) while sparing A2B5+ cells (A,c). Quantitative data are shown in B and C. **p<0.01, treated groups versus controls. Scale bar =25μm
In parallel studies, the efficacy of selective cell ablation was assessed in dissociated cell cultures. In control cultures, A2B5-immunopositive cells account for 85%, O4-immunopositive cells for 8% and O1-immunopositive cells for 6% of the total cell number. After complement-mediated lysis of the A2B5+ cells, as expected the number of A2B5+ cells was reduced nearly to zero. The total number of O1-immunopositive cells, however, was not statistically different in A2B5-depleted cultures (Figure 3, B) when compared with non-treated cell culture (Figure 3, A). Quantification of these results (Figure 3, C) confirms first that O1+ immature oligodendrocytes have been generated at the time of complement lysis of the mixed cell cultures and second, that the A2B5 progenitors can be selectively depleted with little to no change in the number of O1 oligodendrocytes retained in the culture. Together, these data demonstrate the capability of eliminating distinct populations of cells of the oligodendrocyte lineage through complement mediated cell lysis. Ablation of A2B5+ cells eliminates a subset but not all NG2+ cells while ablation of O1+ immature oligodendrocytes does not affect the population of OPCs.
Figure 3.
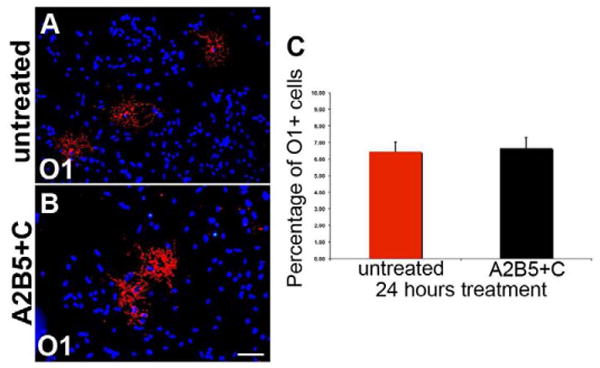
The number of O1+ immature oligodendrocytes remains unchanged before and after complement mediated A2B5+ cell ablation in dissociated cell cultures. A, O1-positive cells were found in untreated primary brain mixed culture after 24 hours. In cultures exposed to A2B5 plus complement mediated lysis (A2B5+C), O1-positive cells are still present (B) and quantification (C) shows that the number of O1-positive cells is not significantly difference in treated and untreated cell cultures (p>0.05). Scale bar = 25μm.
In control slice cultures there is a significant overlap in the expression of A2B5 (red) and NG2 (Green) on OPCs (Figure 4a-c), however a discrete population of NG2+/A2B5- cells is also present and are retained following ablation of A2B5+ cells (Figure 4 d-f). Parallel studies with O1 targeted complement lysis do not substantially alter the composition of the pool of OPCs (Figure 4 g-i). These data suggest that there is a residual population of potential OPCs after A2B5 mediated complement cell lysis that could replace eliminated cells.
Figure 4.
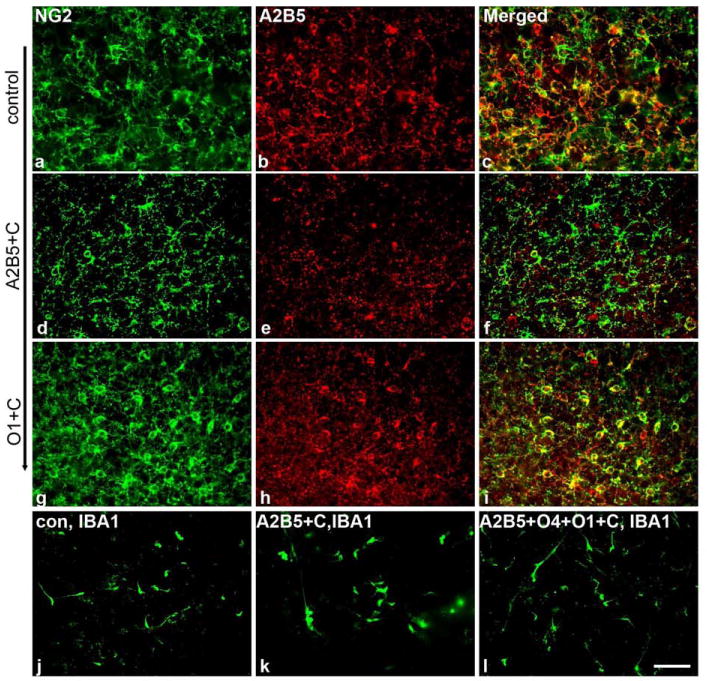
Ablation of A2B5+ cells reduces the density of NG2+ cells while ablation of O1+ cells does not dramatically affect NG2+ cell numbers. Complement mediated cell lysis in slice cultures does not stimulate a widespread microglial response. Control cultures show significant overlap in expression of A2B5 (red) and NG2 (green) on OPCs (a-c). A discrete population of NG2+ cells is also present and is retained following ablation of A2B5+ cells (d-f). Parallel studies with O1 targeted complement lysis do not substantially alter the composition of the pool of OPCs (g-i). To determine whether complement mediated cell lysis stimulated a microglial response, slice cultures were labeled with IBA1 antibodies in controls and after A2B5 or triple antibody mediated cell lysis (j-l). No increase in IBA1 staining obviously accompanied cell lysis. Scale bar = 25μm.
To determine whether complement mediated cell lysis stimulated a microglial response, slice cultures were labeled with IBA1 antibodies in control slices and in slices after A2B5 or triple antibody mediated cell lysis (Figure 4 j-l). No increase in IBA1 labeling was seen in slices in which cell lysis was induced, suggesting either that in this setting the microglia are not stimulated by complement mediated cell lysis or that the microglia are already highly stimulated by the slice preparation.
Oligodendrocyte maturation and myelin formation are delayed after ablation of both OPCs and immature oligodendrocytes
To determine the effects of ablation of both OPCs and early oligodendrocytes on the development of MBP+ cells and myelination, slices were treated with a combination of A2B5, O4, and O1 antibodies and complement and the number of MBP+ cells compared to controls. After 2 rounds treatments, treated slices were cultured in vitro for 3 days (P2+3 DIV). While normal numbers of MBP+ cells developed in slice cultures exposed to antibodies or complement alone, very few MBP+ cells were present in experimental slice cultures suggesting that removal of OPCs and early oligodendrocytes delayed the development of MBP+ cells (Figure 5A, d) (the residual MBP labeling was associated with cell debris). This delay was transient. At P2+7 DIV increasing numbers of MBP+ cells developed (Figure 5A, e) although these cells were relatively immature in their morphology with short radial processes. By P2+14DIV (Figure 5A, f) significantly more MBP+ cells were present in both treated and untreated slices and many of these cells possessed long processes. Compared to control slices (Fig 5A, a-c) however, the number of MBP+ oligodendrocytes was significantly reduced and the orientation of the oligodendrocyte processes far more random. This likely reflects alterations in the cytoarchitecture of the slice following cell ablation. Quantification of MBP expression confirmed the developmental delay. In both control and experimental slices the level of MBP expression increased over the 14 day culture interval (Fig 5B) although the levels in slices treated with antibodies and complement never achieved the same level of expression as seen in controls. These studies demonstrate the potential for recovery of myelinating oligodendrocytes following the elimination of the majority of oligodendrocyte lineage cells in slice cultures. To determine whether functional recovery was restricted to slice preparations, similar studies were conducted on dissociated cell cultures. Ablation of A2B5+, O4+ and O1+ cells resulted in significantly reduced numbers of MBP+ cells after three days (Figure 5A, j & 5C). The number of MBP+ cells increased over time and developed increasingly complex morphologies (Figure 5A, k & l) such that by P2+14 DIV they extended numerous MBP+ processes. Although the proportion of MBP+ cells increased in experimental cultures they failed to recover to control levels within 14DIV. Together these data suggest that the residual oligodendrocytes lineage cells in complement treated cultures are capable of generating MBP+ cells in reduced numbers and after a temporal delay. The delayed appearance of MBP+ cells likely reflects the recruitment and maturation of residual and pre-A2B5+ cells in experimental cultures.
Figure 5.
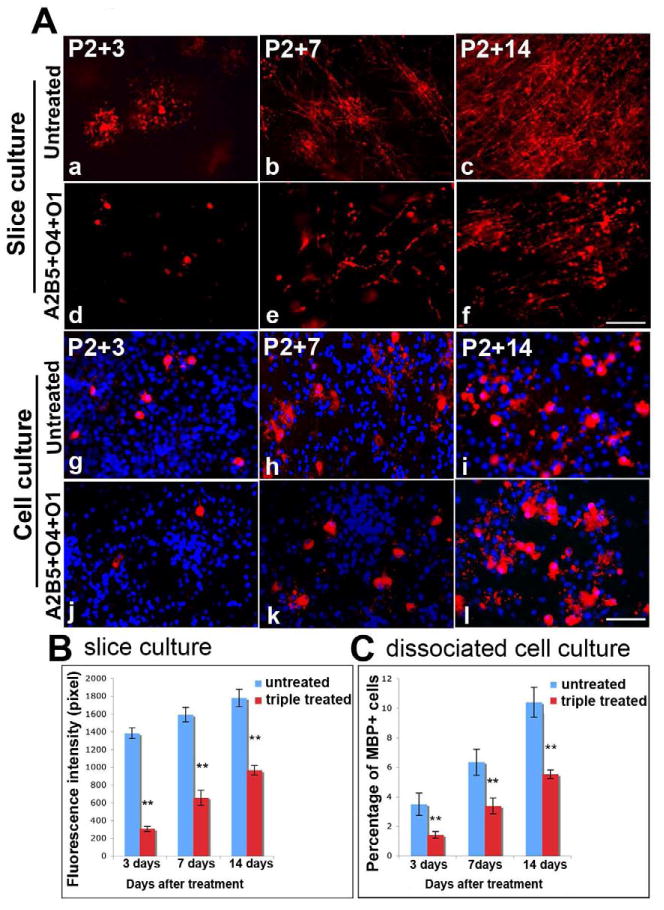
Myelin formation is delayed in the absence of all stages of the committed OPC lineage. Cultured slices were treated with A2B5, O4 and O1 antibodies simultaneously, followed by complement-mediated lysis. While some occasional MBP+ process were detectable, few MBP+ cells (red) were seen P2+3 DIV (A,d) and appeared unhealthy compared to untreated P2+3DIV slices (A,a). In the treated groups, MBP+ cells with few myelinated fibrils appear by P2+7 DIV (A,e) and increase by P2+14 DIV(A,f), but myelination was significantly reduced compared to the non-treated slices at the same ages (A,b-c). Similar results were found in primary dissociated brain mixed cell cultures (A,g-l). Quantitation of MBP fluorescence intensity (pixel) shows significantly decreased MBP intensity in triple-treated slice cultures (P<0.01) compared to untreated slice cultures (B). Quantitation of the percent of MBP+ cells in treated dissociated cell cultures demonstrated a significant decrease (p<0.01) compared to untreated cultures (C). Scale bar =25μm.
Myelination is delayed after ablation of early stage progenitors
Since ablation of OPCs and early oligodendrocytes reduces the number and delays development of MBP+ cells we hypothesized that selective ablation of early OPCs would reduce the initial number of OPC lineage cells but not alter the timing of MBP+ cell appearance. To test this hypothesis slice cultures were treated with A2B5+ and complement to eliminate OPCs but retain O1+ immature oligodendrocytes and the appearance of MBP+ cells was assayed. In contrast to the anticipated results the appearance of MBP+ cells was significantly delayed after treatment with A2B5 and complement (Figure 6). For example, treated slices contained only occasional MBP+ cells, presumably derived from A2B5+ progenitors until P2+14 DIV (Figure 6A, d-f) while control slices contained substantial numbers of MBP+ cells by P2+3 DIV (Figure 6A, a). Since A2B5 plus complement mediated lysis did not alter O1+ immature oligodendrocyte numbers after 24hrs (Figure 3) these data suggest that in the absence of A2B5+ OPCs the maturation of oligodendrocytes is delayed. A similar delay in the appearance of MBP+ cells was seen using a combination of A2B5 and O4 to target cell ablation (Figure 6A, g-i). The delay in MBP+ cell appearance was supported by quantification of MBP expression where the average intensity (pixel) of labeling was significantly lower in experimental cultures than in untreated controls at P2+DIV3 and P2+DIV7 (Figure 6B, a, c). The reduced expression of MBP persisted until P2+14 DIV, the longest time point examined. Together these data imply that OPCs influence the timing of onset of myelination by oligodendrocytes.
Figure 6.
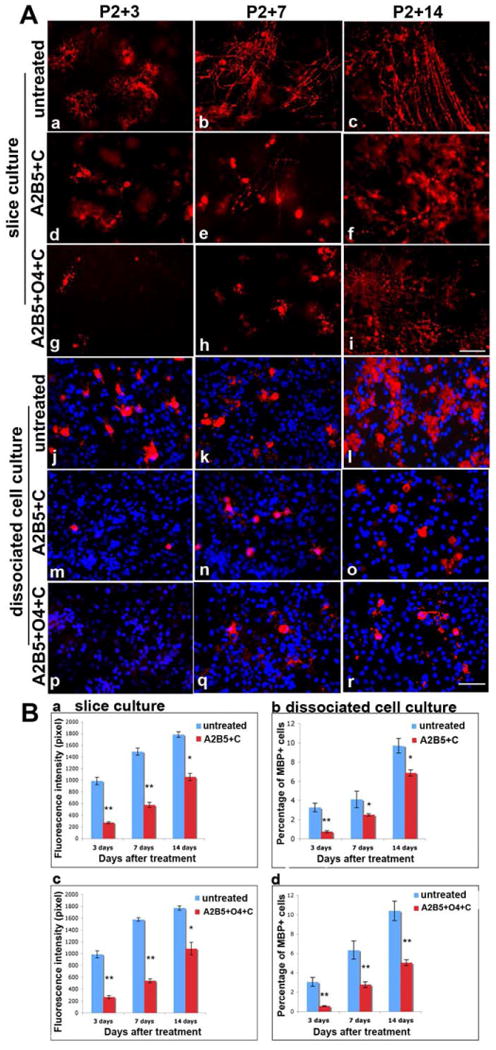
Myelination is delayed after depleting early stage OPC progenitors. After depleting early progenitors (A2B5+ cells or A2B5+ and O4+ cells), only a few immature MBP+ cells are seen at P2+ 3 DIV (A, d, g) and at P2+7 DIV(A, e, h). In untreated cultures, MBP+ cells were morphologically healthy and more mature at P2+3 DIV (A, a), and process extension started by P2+7 DIV (A, b). By P2+14 DIV, MBP+ processes appeared in the treated culture (A, f, i), but were delayed and disorganized compared to the untreated slice cultures that contain robust, parallel myelinated fibrils at P2+14 DIV (A, c). Similar results were found in primary brain dissociated mixed cell cultures after ablation of A2B5+ cell alone (A, m-o) or A2B5+ and O4+ cells together (A, p-r). Untreated cell cultures are shown in A, j-l. Quantitative analysis of MBP fluorescence intensity (pixel) and the percentage of MBP positive cells in cultures are shown in B, a-d. After depletion of A2B5+ cells alone or A2B5+ plus O4+ progenitors in slice cultures, the intensity of MBP fluorescence is significantly decreased (**p<0.01) compared to untreated slice cultures (B, a, c). Even at P2+14 DIV, when MBP intensity continues to increase, it is still significantly lower than in untreated slice cultures (*p<0.05). The percentage of MBP-positive cells is significant decreased (**p<0.01) in brain dissociated mix cell cultures (B, b, d). Scale bar = 25μm.
Ablation of A2B5+ OPCs also inhibited oligodendrocyte maturation in dissociated cell culture. While control cultures contained significant numbers of MBP+ cells at P2+3 DIV (Figure 6A, j) and these increased by P2+7 DIV (Figure 6A, k), cultures treated with A2B5 antibody and complement contained only an occasional MBP+ cell at P2+3 DIV (Figure 6A, m, p) and very few at P2+7 DIV (Figure 6A, n, q). By P2+14 DIV, the number of MBP+ cells in A2B5+C or A2B5+O4+C treated cultures had increased (Figure 6A, o, r) but failed to achieve levels seen in controls (Figure 6A, l). Furthermore in A2B5+C treated cultures, the expression of MBP on individual cells was relatively low and restricted to the cell body with a relatively immature morphology (Figure 6A, o, r).
Failure of MBP+ cells to develop after ablation of immature oligodendrocytes
Ablation of O1+ immature oligodendrocytes, the direct precursor of MBP+ cells, would be predicted to delay the appearance of MBP+ cells in slice cultures. To test this hypothesis O1+ cells were ablated by complement mediated cell lysis at P2 slice cultures and the development of MBP expression assessed.
Selective ablation of O1+ cells inhibited the appearance of MBP+ cells. In contrast to the delay seen after ablation of OPCs and early oligodendrocytes, in O1+ complement treated slices the development of MBP+ cells was dramatically inhibited up to 14 days in culture, the longest time examined. In contrast to controls, while an occasional MBP+ cell was present at P2+3 and P2 +7, the numbers failed to increase (Figure 7A, d, e). Not only were MBP+ cell bodies absent but also no myelinated axons were detectable in O1+ complement treated slice cultures even when these were maintained for P2+14 (Figure 7A, f). In contrast, control cultures developed a significant number of myelinated axons (Figure 7A, a-c). The failure of MBP+ cells to develop after O1 and complement mediated cell lysis was not restricted to slice cultures. Ablation of O1+ immature oligodendrocytes in mixed dissociated cell culture resulted in a permanent loss of MBP+ cells, at least up to P2+14DIV. In control cultures, the total number of process bearing MBP+ cells increased throughout the culture period (Figure 7A, g-i). By contrast, only occasional morphologically immature MBP+ cells were detected in treated cultures at all stages examined (Figure 7A, j-l). Statistical quantification of significant difference of the number of MBP+ cells (Figure 7B) and the florescence intensity (pixel) of MBP expression (Figure 7C) confirmed the permanent reduction seen in cultures following elimination of O1+ cells. These data suggest that in the absence of an appropriate cohort of O1+ immature oligodendrocytes, residual progenitors are unable to mature to MBP+ cells.
Figure 7.
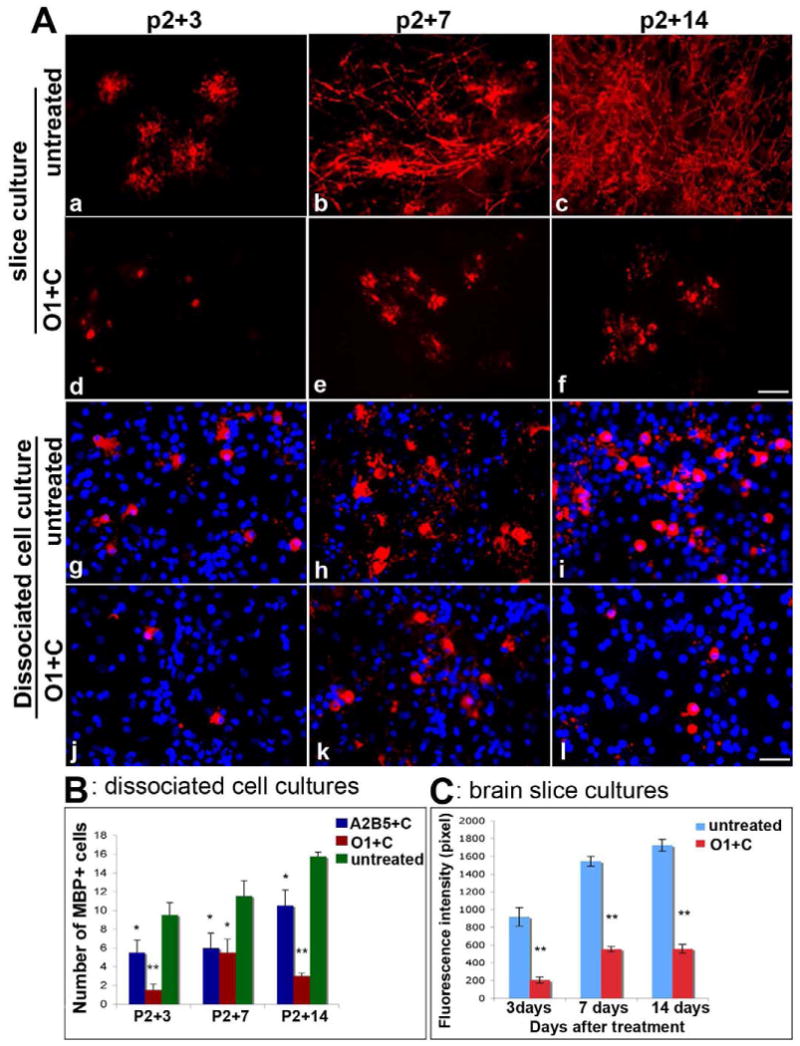
Lack of myelin formation and oligodendrocyte maturation in the absence of O1+ immature oligodendrocytes. In slice cultures (a-f) and dissociated cell cultures (g-i) the maturation of oligodendrocytes is dependent on the presence of O1+ cells. Few MBP+ cell bodies were found at P2+3 DIV (A, d) and P2+7 DIV (A, e) following elimination of O1+ cells. MBP+ processes failed to develop in treated cultures even at P2+14 DIV (A,f). Untreated slice cultures are shown in A, a-c. In mixed cell cultures (g-i) treated with O1 plus complement (O1+C), MBP+ cells were reduced and showed no extended MBP+ processes even at P2+14 DIV (A, j-l). In untreated cell cultures, advanced MBP+ cells were seen in increased numbers and had complex and extended processes (A, g-i). Quantitative analysis is shown in B for dissociated cell cultures and in C for brain slice cultures. *p<0.05 versus control; **p<0.01 versus control. Scale bar = 25μm.
Growth factor stimulation partially overcomes the O1+ cells ablation induced block of oligodendrocyte maturation
Previous studies have demonstrated that platelet-derived growth factor (PDGF) can modulate proliferation and survival of OPCs (Hart et al., 1989; Noble et al., 1988; Woodruff et al., 2004). Addition of exogenous PDGF (10ng/ml) to O1+ cell depleted slice cultures resulted in a partial recovery of the number of MBP+ cells after P2+ 7 and P2+14 (Figure 8, c-d) compared to cultures without PDGF (Figure 8, a-b) suggesting that the inhibition of oligodendrocyte maturation induced by ablation of O1+ cells can be partially overcome by extrinsic signals.
Figure 8.
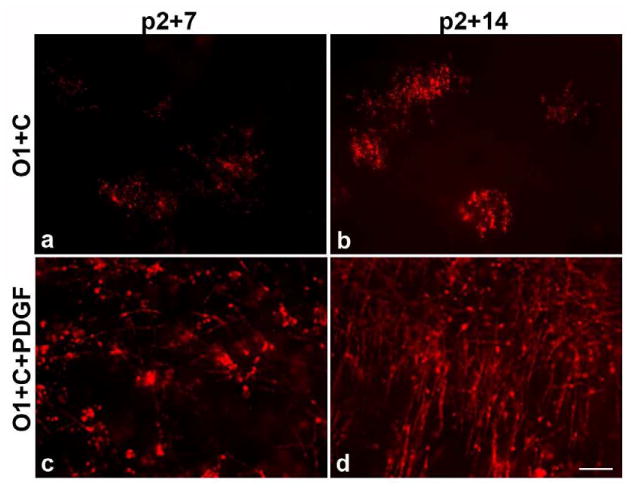
PDGF promotes myelination even in the absence of O1+ immature oligodendrocytes in slice cultures. After addition of 0.1% PDGF to slice cultures lacking O1+ progenitors, MBP+ myelinated fibrils appear by P2+7 in slice culture (c) and robust MBP+ myelination was seen in P2+14 slices (d). In contrast, no myelinated axons were observed in P2+14 slices after ablation of O1+ immature oligodendrocytes without the addition of PDGF (a-b). Scale bar = 25μm.
Discussion
The development of oligodendrocytes and myelin formation is critical for the normal functioning of the vertebrate central nervous system. Studies in dissociated cell culture have provided considerable insights into OPC biology (Barres and Raff, 1994; Miller, 2002), however less is known about the onset of myelination by oligodendrocytes in part due to the lack of robust easily manipulated models. Here we utilize a developing forebrain slice culture that allows for the analysis of all stages of the oligodendrocyte lineage including the onset of myelination. We show that in control slices the timing of OPC development, oligodendrocyte differentiation and maturation as well as the onset of myelination closely reflects that seen in vivo, consistent with previous studies (Notterpek et al., 1993).
Through the selective ablation of distinct populations of cells we reveal an unexpected regulatory mechanism of oligodendrocyte maturation dependent upon interactions between stages of the oligodendrocyte lineage that modulates the timing of cell maturation and onset of myelination. Targeting complement mediated cell ablation with mAb A2B5 eliminates essentially all A2B5+ cells but has no immediate appreciable effect on the number of O1+ cells. Likewise, targeting complement mediated cell lysis with mAb O1 eliminates essentially all O1+ cells but has no immediate appreciable effect on the number of A2B5+ cells demonstrating the capability to selectively eliminate cells at distinct stages of oligodendrocyte maturation. Using a combination of A2B5, O4 and O1 antibodies together to target complement mediated cell lysis, the majority of cells of the oligodendrocyte lineage were selectively eliminated in slice cultures. This cell loss resulted in a significant delay in the appearance of mature (MBP+) oligodendrocytes as well as a reduction in their overall numbers. Selective ablation of A2B5+ oligodendrocyte precursors unexpectedly resulted in a similar delay in the appearance of MBP+ cells. This delay in oligodendrocyte maturation was not restricted to slice cultures. Parallel studies with dissociated cell cultures resulted in similarly delayed MBP appearance. In contrast, selective ablation of O1+ immature oligodendrocytes in either slice or dissociated cell cultures resulted in a blockade of MBP cell appearance even though the numbers of A2B5+ progenitor cells were unaffected. These data suggest that maturation of cells of the oligodendrocyte lineage is regulated in part through feed-forward and feedback mechanisms between the stages of the oligodendrocyte lineage. Such regulatory mechanisms may have important implications for both the development of myelination and remyelination.
Two mechanisms could account for the capacity of slice cultures to recover MBP+ cells after ablation of multiple stages of the oligodendrocyte lineage. It may be that a residual pool of A2B5+ OPCs remains in the slice following complement mediated lysis and these cells subsequently proliferate and repopulate the entire lineage. Alternatively, a pre-OPC population (Armstrong, 2007; Baracskay et al., 2007; Nait-Oumesmar et al., 1999) could regenerate the entire lineage. Several sources of pre-OPCs have been described (Nait-Oumesmar et al., 1999; Wu et al., 2009). For example, the slices contain regions of the ventricular zones that are known to harbor neural stem cells (Wu et al., 2009) while in the developing cortex NG2+ cells represent OPCs and may be the ancestors to A2B5+ cells (Baracskay et al., 2007; Nishiyama et al., 2009; Yoo and Wrathall, 2007; Zhu et al., 2008). These cells are retained after ablation of A2B5+ cells may subsequently repopulate the lineage. Regardless of the specific cellular source, the ability of the oligodendrocyte lineage to generate MBP+ cells after removal of the majority of OPCs and oligodendrocytes demonstrates the remarkable regenerative capacity of this lineage. At the time of cell ablation no MBP+ cells had developed in the slice cultures so their appearance 3 days after treatment and the increase in cell numbers that approximately parallels that of controls over the subsequent 11 days suggests MBP+ cells can develop from pre-OPCs within 3 days.
While the delay in MBP+ cell appearance after ablation of the majority of cells of oligodendrocyte lineage is easily understood, it is less obvious why elimination of A2B5+ OPCs results in delayed appearance of MBP+ cells. Given that the number of O1+ cells was not immediately affected by A2B5 targeted complement mediated cell lysis it might have been anticipated that the residual, more mature oligodendrocyte lineage cells would have differentiated according to their intrinsic schedule (Temple and Raff, 1986) resulting in no changes in the timing of initial appearance but a reduction in subsequent number of MBP+ cells. By contrast, the delay in MBP+ cell expression strongly suggests that the timing of oligodendrocyte maturation is influenced by the number or density of OPCs (Nakatsuji and Miller, 2001; Zhang and Miller, 1996). These findings implicating a community effect are consistent with a model in which a critical number of OPCs are generated prior to the initiation of myelination (Calver et al., 1998; Zhang and Miller, 1996) and would explain how specific regions of the CNS can myelinate extremely rapidly during development. In addition, morphological studies have suggested that some chronic demyelinated plaques in patients with multiples sclerosis contain significant numbers of premyelinating oligodendrocytes in close association with viable axons (Chang et al., 2000; Zawadzka and Franklin, 2007). It may be that the paucity of OPCs in these regions contributes to the blockade of the further maturation and myelination of these cells. Other mechanisms such as the presence of elevated levels of inhibitors of OPC differentiation such as LINGO-1 or the lack of appropriate stimuli are also likely to contribute to the blockade of remyelination (Mi et al., 2005; Mi et al., 2009; Mi et al., 2008; Miller and Mi, 2007).
The inability of the oligodendrocyte lineage to recover from ablation of O1+ cells although the number of A2B5+ cells is unaffected is unexpected. Since in the setting of slice cultures MBP+ cells can develop from pre-OPCs within 3 days, their appearance on a similar time frame was anticipated. By contrast, even after 14 days MBP+ cells failed to emerge in significant numbers after O1 targeted ablation. These findings may reflect a mechanism by which developing OPCs have a specific window in which to initiate myelination and mAb O1 complement mediated ablation disrupts that window (Dugas et al., 2007; Emery et al., 2009). More likely, the residual A2B5+ cells in the slice are dependent on the presence of O1+ cells for their continued maturation. One possible mechanism for such an effect is that O1+ cells facilitate the production of OPC mitogens such as PDGF from astrocytes and other cells in the surrounding tissue possibly by enhancing axonal electrical activity (Fields, 2005; Fields and Stevens-Graham, 2002; Ishibashi et al., 2006) and that this mitogenic activity drives OPC differentiation and maturation. Several lines of evidence are consistent with this hypothesis. First, there is a close relationship between OPC proliferation and differentiation (Raff et al., 1984b) that may be mediated by cell intrinsic regulatory mechanisms (Cayouette et al., 2003; Dugas et al., 2007; Gao et al., 2006; Miller, 2002; Temple and Raff, 1986). Second, in spinal cord cultures oligodendrocytes tend to differentiate in spatially defined clusters that are not always clonally related (Zhang and Miller, 1996) suggesting that the presence of differentiated OPCs enhances the differentiation potential of adjacent cells. Third, addition of exogenous PDGF to O1+ cell depleted slices resulted in a partial recovery of MBP+ cells. Alternatively, the presence of sufficient numbers of O1+ cells may provide protection for the survival of premyelinating OPCs that is known to be dependent on PDGF (Murtie et al., 2005).
Together, these data argue that the different stages of OPC development and oligodendrocyte maturation are intimately interrelated and interact with each other during the development process. There is an unexpected feedback relationship from the later to the earlier stage cells that has a strong influence on the ability of the earlier stages to complete their differentiation into myelin-forming oligodendrocytes. Local environmental cues as well as balance among the different stages of the oligodendrocyte lineage are critical in regulating the differentiation of oligodendrocytes to mature myelin-forming cells. Identifying the molecular mediators of this complex phenomenon will have important implications for understanding critical aspects of neural development and generating novel effective repair strategies for demyelinating diseases.
Acknowledgments
This work is supported by NIH RO1 (NS30800) and the Myelin Repair Foundation. The authors would like to thanks for Anne DeChant for manuscript edits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong RC. Growth factor regulation of remyelination: behind the growing interest in endogenous cell repair of the CNS. Future Neurol. 2007;2:689–697. doi: 10.2217/14796708.2.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, Stefansson K, Pfeiffer SE. Proligodendroblast antigen (POA), a developmental antigen expressed by A007/O4-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. J Neurochem. 1992;58:2221–9. doi: 10.1111/j.1471-4159.1992.tb10967.x. [DOI] [PubMed] [Google Scholar]
- Bansal R, Warrington AE, Gard AL, Ranscht B, Pfeiffer SE. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res. 1989;24:548–57. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- Baracskay KL, Kidd GJ, Miller RH, Trapp BD. NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia. 2007;55:1001–10. doi: 10.1002/glia.20519. [DOI] [PubMed] [Google Scholar]
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–42. doi: 10.1016/0896-6273(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Benjamins JA, Morell P. Proteins of myelin and their metabolism. Neurochem Res. 1978;3:137–74. doi: 10.1007/BF00964057. [DOI] [PubMed] [Google Scholar]
- Bogler O, Wren D, Barnett SC, Land H, Noble M. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci U S A. 1990;87:6368–72. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–82. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Cayouette M, Barres BA, Raff M. Importance of intrinsic mechanisms in cell fate decisions in the developing rat retina. Neuron. 2003;40:897–904. doi: 10.1016/s0896-6273(03)00756-6. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–12. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase LM, Nishiyama A, Bergles DE. Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci. 30:3600–11. doi: 10.1523/JNEUROSCI.6000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas JC, Ibrahim A, Barres BA. A crucial role for p57(Kip2) in the intracellular timer that controls oligodendrocyte differentiation. J Neurosci. 2007;27:6185–96. doi: 10.1523/JNEUROSCI.0628-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, Barres BA. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–85. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528–31. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–62. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Macklin W, Gerson J, Miller RH. Intrinsic and extrinsic inhibition of oligodendrocyte development by rat retina. Dev Biol. 2006;290:277–86. doi: 10.1016/j.ydbio.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Hardy R, Reynolds R. Neuron-oligodendroglial interactions during central nervous system development. J Neurosci Res. 1993;36:121–6. doi: 10.1002/jnr.490360202. [DOI] [PubMed] [Google Scholar]
- Harrer MD, von Budingen HC, Stoppini L, Alliod C, Pouly S, Fischer K, Goebels N. Live imaging of remyelination after antibody-mediated demyelination in and ex-vivo model for immune mediated CNS damage. Exp Neurol. 2009;216:431–8. doi: 10.1016/j.expneurol.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Hart IK, Richardson WD, Bolsover SR, Raff MC. PDGF and intracellular signaling in the timing of oligodendrocyte differentiation. J Cell Biol. 1989;109:3411–7. doi: 10.1083/jcb.109.6.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–32. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–6. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–9. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff MC. A role for Noggin in the development of oligodendrocyte precursor cells. Dev Biol. 2004;267:242–51. doi: 10.1016/j.ydbio.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Lubetzki C, Goujet-Zalc C, Gansmuller A, Monge M, Brillat A, Zalc B. Morphological, biochemical, and functional characterization of bulk isolated glial progenitor cells. J Neurochem. 1991;56:671–80. doi: 10.1111/j.1471-4159.1991.tb08202.x. [DOI] [PubMed] [Google Scholar]
- McKinnon RD, Smith C, Behar T, Smith T, Dubois-Dalcq M. Distinct effects of bFGF and PDGF on oligodendrocyte progenitor cells. Glia. 1993;7:245–54. doi: 10.1002/glia.440070308. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, Hession C, Sah D, Trapp B, He Z, Jung V, McCoy JM, Pepinsky RB. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–51. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, Zhang Y, Shields CB, Miklasz S, Shea D, Mason J, Franklin RJ, Ji B, Shao Z, Chedotal A, Bernard F, Roulois A, Xu J, Jung V, Pepinsky B. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009;65:304–15. doi: 10.1002/ana.21581. [DOI] [PubMed] [Google Scholar]
- Mi S, Sandrock A, Miller RH. LINGO-1 and its role in CNS repair. Int J Biochem Cell Biol. 2008;40:1971–8. doi: 10.1016/j.biocel.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate. CNS Prog Neurobiol. 2002;67:451–67. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Miller RH, Mi S. Dissecting demyelination. Nat Neurosci. 2007;10:1351–4. doi: 10.1038/nn1995. [DOI] [PubMed] [Google Scholar]
- Murtie JC, Zhou YX, Le TQ, Vana AC, Armstrong RC. PDGF and FGF2 pathways regulate distinct oligodendrocyte lineage responses in experimental demyelination with spontaneous remyelination. Neurobiol Dis. 2005;19:171–82. doi: 10.1016/j.nbd.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–66. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Nakatsuji Y, Miller RH. Density dependent modulation of cell cycle protein expression in astrocytes. J Neurosci Res. 2001;66:487–96. doi: 10.1002/jnr.1240. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113–24. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560–2. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Notterpek LM, Bullock PN, Malek-Hedayat S, Fisher R, Rome LH. Myelination in cerebellar slice cultures: development of a system amenable to biochemical analysis. J Neurosci Res. 1993;36:621–34. doi: 10.1002/jnr.490360603. [DOI] [PubMed] [Google Scholar]
- Raff MC. Glial cell diversification in the rat optic nerve. Science. 1989;243:1450–5. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- Raff MC, Abney ER, Miller RH. Two glial cell lineages diverge prenatally in rat optic nerve. Dev Biol. 1984a;106:53–60. doi: 10.1016/0012-1606(84)90060-5. [DOI] [PubMed] [Google Scholar]
- Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988;333:562–5. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Raff MC, Williams BP, Miller RH. The in vitro differentiation of a bipotential glial progenitor cell. EMBO J. 1984b;3:1857–64. doi: 10.1002/j.1460-2075.1984.tb02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WD, Pringle N, Mosley MJ, Westermark B, Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53:309–19. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg SS, Powell BL, Chan JR. Receiving mixed signals: uncoupling oligodendrocyte differentiation and myelination. Cell Mol Life Sci. 2007;64:3059–68. doi: 10.1007/s00018-007-7265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick RA, Trapp BD. Gray-matter injury in multiple sclerosis. N Engl J Med. 2009;361:1505–6. doi: 10.1056/NEJMcibr0905482. [DOI] [PubMed] [Google Scholar]
- Temple S, Raff MC. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell. 1986;44:773–9. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Macklin WB, Miller RH. Netrin-1 is required for the normal development of spinal cord oligodendrocytes. J Neurosci. 2006;26:1913–22. doi: 10.1523/JNEUROSCI.3571-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2Aadult progenitor cells to rapidly dividing cells with characteristics of O-2Aperinatal progenitor cells. J Cell Biol. 1992;118:889–900. doi: 10.1083/jcb.118.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci. 2004;25:252–62. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Wu C, Chang A, Smith MC, Won R, Yin X, Staugaitis SM, Agamanolis D, Kidd GJ, Miller RH, Trapp BD. Beta4 tubulin identifies a primitive cell source for oligodendrocytes in the mammalian brain. J Neurosci. 2009;29:7649–57. doi: 10.1523/JNEUROSCI.1027-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S, Wrathall JR. Mixed primary culture and clonal analysis provide evidence that NG2 proteoglycan-expressing cells after spinal cord injury are glial progenitors. Dev Neurobiol. 2007;67:860–74. doi: 10.1002/dneu.20369. [DOI] [PubMed] [Google Scholar]
- Zawadzka M, Franklin RJ. Myelin regeneration in demyelinating disorders: new developments in biology and clinical pathology. Curr Opin Neurol. 2007;20:294–8. doi: 10.1097/WCO.0b013e32813aee7f. [DOI] [PubMed] [Google Scholar]
- Zhang H, Miller RH. Density-dependent feedback inhibition of oligodendrocyte precursor expansion. J Neurosci. 1996;16:6886–95. doi: 10.1523/JNEUROSCI.16-21-06886.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–57. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–30. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]


