Abstract
Maternal consumption of fish during the gestational period exposes the fetus to both nutrients, especially the long-chain polyunsaturated fatty acids (LCPUFAs), believed to be beneficial for fetal brain development, as well as to the neurotoxicant methylmercury (MeHg). We recently reported that nutrients present in fish may modify MeHg neurotoxicity. Understanding the apparent interaction of MeHg exposure and nutrients present in fish is complicated by the limitations of modeling methods. In this study we fit varying coefficient function models to data from the Seychelles Child Development Nutrition Study (SCDNS) cohort to assess the association of dietary nutrients and children’s development. This cohort of mother-child pairs in the Republic of Seychelles had fish consumption averaging 9 meals per week. Maternal nutritional status was assessed for five different nutritional components known to be present in fish (n-3 LCPUFA, n-6 LCPUFA, iron status, iodine status, and choline) and associated with children’s neurological development. We also included prenatal MeHg exposure (measured in maternal hair). We examined two child neurodevelopmental outcomes (Bayley Scales Infant Development-II (BSID-II) Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI)), each administered at 9 and at 30 months. The varying coefficient models allow the possible interactions between each nutritional component and MeHg to be modeled as a smoothly varying function of MeHg as an effect modifier. Iron, iodine, choline, and n-6 LCPUFA had little or no observable modulation at different MeHg exposures. In contrast the n-3 LCPUFA docosahexaenoic acid (DHA) had beneficial effects on the BSID-II PDI that were reduced or absent at higher MeHg exposures. This study presents a useful modeling method that can be brought to bear on questions involving interactions between covariates, and illustrates the continuing importance of viewing fish consumption during pregnancy as a case of multiple exposures to nutrients and to MeHg. The results encourage more emphasis on a holistic view of the risks and benefits of fish consumption as it relates to infant development.
Keywords: Varying-coefficient function models, mercury exposure, neurodevelopment, interaction between nutritional status and toxic exposure
I. Introduction
Fish consumption during pregnancy exposes the fetus to the neurotoxicant methylmercury (MeHg). All fish naturally take up MeHg and it is biomagnified up the aquatic food chain. Maternal dietary fish consumption also provides an important source of nutrients, most notably the long chain polyunsaturated fatty acids (LCPUFA). These nutrients are important precursors to normal development of the central nervous system, and studies of cognitive and retinal development following maternal supplementation have shown benefits (Helland et al., 2003, Daniels et al., 2004, Dunstan et al., 2006, Judge et al., 2007). Recent reports from the Seychelles Child Development Nutrition Study (SCDNS) cohort suggested an adverse impact of MeHg exposure, accompanied by a simultaneous beneficial effect of n-3 LCPUFA on children’s development (Davidson et al., 2008; Strain et al., 2008). It is unclear whether this result was evidence for independent influences of MeHg and n-3 LCPUFA or effect modification. Bellinger (2008) has distinguished between these two types of confounding effects, suggesting that effect modification can be tested through including statistical interaction terms in a regression model, whereas independent effects can be tested with main effect terms. The primary analyses reported by Davidson and colleagues (2008) and Strain and colleagues (2008) employed ordinary multivariate regression to examine four primary endpoints and found an adverse association between prenatal MeHg exposure and the BSID-II PDI at 30 months which was statistically significant only when the model contained the LCPUFA (Strain et al., 2008). However, the nutrition variables did not have a significant association with that outcome (Davidson et al., 2008). For one outcome (BSID-II MDI at 9 months), overall model significance was not achieved, but secondary analyses indicated a beneficial association with n-3 LCPUFA. Davidson and colleagues (2008) utilized ordinary linear model interactions between MeHg and the n-3 LCPUFA docosahexaenoic acid (DHA), and the n-6 LCPUFA arachidonic acid (AA). The study did not find significant interactions between MeHg and AA and DHA (p = 0.53 and p = 0.30 for a 2 df test for MeHg*DHA and MeHg*AA, respectively). Those models utilized indicator variables to distinguish between tertiles of the sample distribution of the LCPUFAs, and examination of the interactions is limited by this specific, discretized form. Interactions present in other forms might not be visible in this discretized setting.
Effect modification has been defined as variation in the magnitude of an association between an exposure and an outcome measure across strata of a separate, effect modifying factor (Bellinger, 2000). Modeling statistical interaction between exposures and other measurable characteristics by including interaction terms in the regression models is the standard means to identify effect modification. Simple regression interaction terms, however, can fail to capture the variation in association between exposure and outcome when the change in that association differs in a complicated, perhaps nonlinear, fashion across levels of the effect modifying factor. Within the framework of linear models, methods for characterizing not just the presence but also the form and features of covariate interactions have been lacking. To address this need, the semiparametric varying coefficient (VC) model (Hastie and Tibshirani, 1993) has been proposed as a means of capturing features of covariate-by-covariate interactions between continuously varying covariates. Such models are uniquely suited to examining covariate interactions as they model the regression coefficient representing the relationship between a covariate and an outcome variable as a nonparametric smooth function of a separate, effect modifying covariate. Conditional on a fixed value of an effect modifier, the model for an outcome is linear in the modified predictors. In this study, we utilize the varying coefficient model to explore how the relationships between maternal nutritional covariates and a series of neurodevelopment outcomes are potentially modulated at different maternal MeHg exposures. We applied the VC models to data from the SCDNS cohort using the two child neurodevelopment outcomes measured at both 9 and 30 months that showed an association in prior analyses. The VC models examined here provide a more complete portrait of the interactions under consideration, allowing continuous-by-continuous covariate interactions to be represented via smooth functions. These models provide an important extension of previous analyses by helping to define the possible role of covariate interactions in this cohort, and illustrate a valuable methodology for examining complex biological exposures that have a wide applicability to a variety of data settings in which continuous covariate interactions might play a role in influencing outcome.
II. Methods
IIa. Data setting and developmental outcomes modeled
The Seychelles Child Development Study (SCDS) is an ongoing longitudinal study of neurodevelopmental effects arising from maternal MeHg exposure from a diet high in fish, conducted in the Republic of Seychelles (Myers et al., 2000). A cohort of 300 mother-infant pairs was recruited in 2001 to investigate the role that both MeHg exposure and maternal nutritional status have on developmental outcomes in the offspring. Mothers in this cohort were enrolled in the first trimester and completed a four day diet diary and a food use questionnaire at 28 weeks gestation. The primary developmental endpoints modeled were the Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) of the Bayley Scales of Infant Development-II (BSID-II), measured at 9 and 30 months. A total of n = 229 children completed developmental testing at both time points and had complete covariate information.
IIa1. Nutrition covariates
In the VC models for this cohort, we examined five primary nutrition indicators. These include two LCPUFA, (DHA, an n-3 fatty acid and AA, an n-6 fatty acid), maternal iodine status indicated by free thyroxine (T4), maternal iron status (Fe), and dietary choline (determined from the diet diary). These nutritional indicators were selected as they can be associated with fish consumption and child development (Strain et al., 2004). LCPUFA values were computed as geometric means of lipid values from nonfasting blood samples taken at 28 weeks gestation and 1 day post delivery. Data for some of the LCPUFA measurements missing at single time points were imputed prior to determination of the geometric means according to methods described in (Davidson et al., 2008). Maternal iodine status T4 was determined at 28 weeks gestation using competitive immunoassay. Iron status was determined as the total body iron store derived from the soluble transferrin receptor and ferritin measured at enrollment (prior to Fe supplementation). Dietary choline was estimated from the food use questionnaires (FUQs) and a 4-day diet diary as described in (Davidson et al., 2008).
IIa2. Exposure assessment and model covariates
The biomarker for prenatal MeHg exposure was the average maternal hair total Hg (THg) in ppm measured in hair growing during pregnancy. The Hg was measured using atomic absorption spectroscopy as described earlier (Cernichiari et al., 1995, Davidson et al., 2008). All models containing these exposure and nutritional metrics additionally include maternal- and child-related covariates chosen for their potential association with child development as described by Davidson and colleagues (2008). These include the continuous covariates socioeconomic status (SES) measured by the Hollingshead Four-Factor method modified for use in the Seychelles (Davidson et al., 1998), the Pediatric Review of Children’s Environmental Support and Stimulation (PROCESS), a measure of stimulation in the home environment, maternal intelligence measured using the Matrices subtest of the Kaufman Brief Intelligence Test (K-BIT) (Kaufman and Kaufman, 1990), maternal age, birth weight and weekly fish intake measured by the FUQ, as well as the categorical variables gender and family status (an indicator variable for presence of both parents in the home at age 9 months). Full details on the protocols for nutritional assessment and MeHg dosimetry are described in Davidson et al (2008).
IIb. Models
We are using varying coefficient models to characterize the interaction of mercury and nutritional covariates (Hastie and Tibshirani, 1993). The varying coefficient model is a regression model which is additive in the regressors, but the relationship between each regressor and the outcome is allowed to vary as a smooth function of additional, effect modifying regressors in the model. By allowing the regression coefficients to vary nonparametrically as functions of an effect modifying covariate, the relationship between a regressor and the outcome can change at different levels of the effect modifier. The VC model in which only one covariate operates as the effect modifier has the general form:
where X1, X2, …, Xp are explanatory covariates, R is the single effect modifying explanatory covariate, βi(R) is the smoothly varying coefficient function of R, for the i = 1, 2, …, p other explanatory variables, and ε is an error term. In this work, we are using MeHg as the effect modifier R, and the five nutritional status measurements as the modified explanatory covariates labeled as Xi in the above equation. Observe that if βi(R) = βi (a nonzero constant) for each i, the model reduces to an ordinary linear regression model. The conditional relationship between the explanatory variables Xi and R thus characterizes a type of interaction between those two covariates. Note that the intercept term β0(R) represents a main effect of covariate R, and is also modeled as a smoothly varying function of R. The coefficient functions βi(R) thus characterize the manner in which the relationship between covariate Xi and outcome changes as levels of the effect modifier change. For this model, methods of assessing constancy, linearity, or nonlinearity of the smoothed functions using testing have not been fully developed, and continued work to develop appropriate tests is ongoing.
For this study, interest centered on the manner in which the nutritional impact of a high-fish diet and the maternal MeHg burden combine to influence child developmental outcomes. The VC model was employed using MeHg as the effect modifying covariate, with the five nutrition covariates modeled as having coefficient functions that vary as smooth functions of MeHg. The interpretation of this model is that the slope of the linear relationship between a given nutrition covariate and a developmental endpoint will change at different levels of maternal MeHg exposure, according to the varying coefficient slope function βi(R). We also modeled the MeHg main effect by β0(R), also varying in R, and included the additional, non-nutrition covariates in the model as associated with ordinary linear regression fixed coefficients. The model for this dataset has the form:
for nutritional covariates denoted as Nutri, i = 1, 2, …, 5, and additional covariates associated with nonvarying coefficients denoted Xj, j = 6, …, 13. Thus the model included six smooth functions: one varying coefficient intercept function (main effect of MeHg), and the five varying coefficient slope functions associated with each nutritional covariate entering the model.
IIc. Statistical Model Fitting
The smooth nonparametric coefficient functions were fit using a penalized spline representation (see Ruppert et al., 2003). For the splines, linear truncated power basis functions were used, and models were fit using two sets of knot values (12 and 20), with knots chosen based on quantiles of the data. No substantial alterations in model behavior were observed between results obtained for each knot set, so results will be presented only for the models using 20 knots. Penalized spline model representations have been demonstrated as having a mathematical equivalence to a mixed effect (ME) model representation, with the estimate of the nonparametric coefficient functions written as the best linear unbiased predictor (BLUP) of an appropriately parameterized mixed effect model (Brumbeck et al., 1999). This equivalence allowed fitting to proceed using existing mixed effect model procedures in the statistical software packages SAS and S+ (SAS Institute Inc., 2002, Insightful, 2007, Ngo and Wand, 2003). Selection of the penalty parameter in the penalized spline representation can be accomplished automatically in the equivalent ME model setting, by utilizing ratios of the restricted maximum likelihood (REML) estimates of variance parameters in the ME model (Ruppert et al., 2003). It is important to note, though, that this method of smoothing parameter selection resulted in substantial oversmoothing for some of the coefficient functions, as well as different degrees of freedom to be expended for each smoothed function. To facilitate comparison of the VC functions and reduce the oversmoothing, hand-tuning of the smoothing parameters was utilized to fix the amount of smoothing for each fitted VC function at approximately 3 degrees of freedom per curve, with degrees of freedom of the fit for the selected value of the smoothing parameter computed as the trace of the appropriate smoother matrix.
III. Results
Mothers in this cohort consumed an average of 9 fish meals per week, for an average fish intake of 537 g/week based on food use diaries (Davidson et al., 2008). For these data, MeHg showed a significant correlation with the n-3 fatty acid DHA (Pearson r = 0.32 (p < 0.0001)). The correlations between MeHg and the other nutrition covariates were very low, (less than 0.1 in absolute value), and were not significant (Table 1).
Table 1.
Pearson’s product moment correlations between mercury and nutritional measures (p-values are for the test of the null hypothesis Ho: correlation = 0).
| Nutritional component | correlation | p-value |
|---|---|---|
| AA | 0.073 | 0.272 |
| DHA | 0.318 | < 0.001 |
| Iodine | 0.042 | 0.527 |
| Iron | −0.006 | 0.927 |
| Choline | 0.094 | 0.155 |
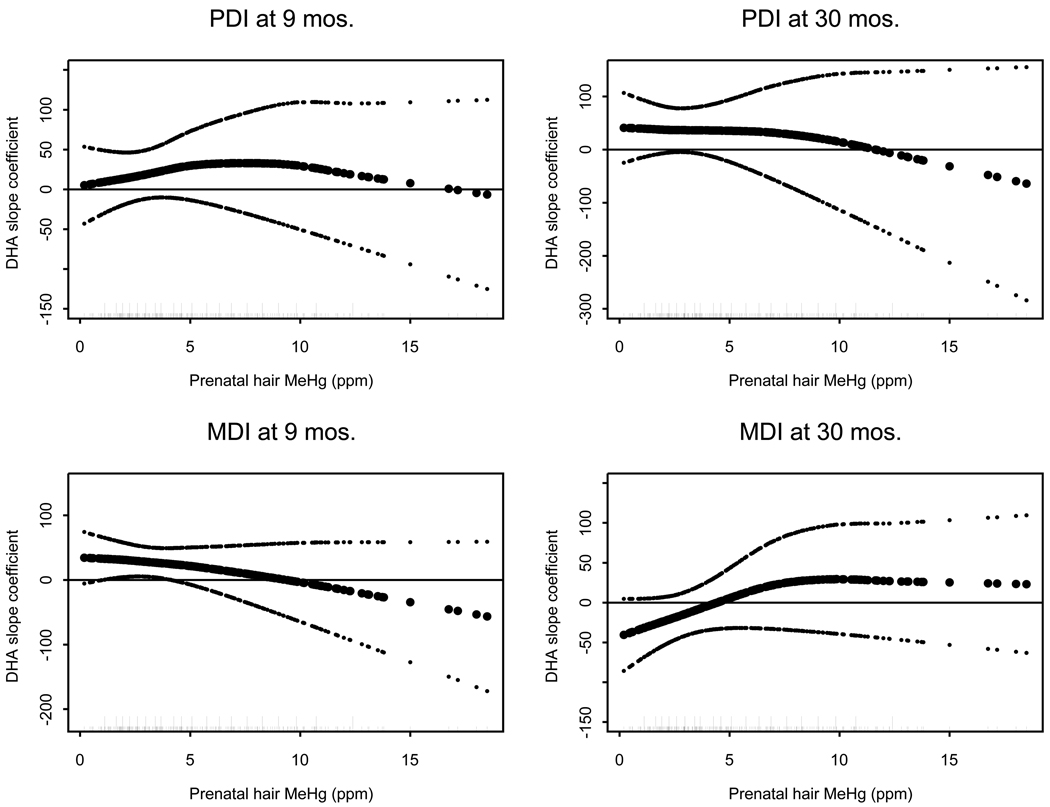
Graphical results from the VC models did not indicate modification by MeHg of the effects of the nutrition covariates iron, iodine, AA, or choline. For the nutrition covariate DHA, however, results from the VC models suggested possible interaction with MeHg. Figure 1 presents the varying coefficient slope functions for DHA for all four outcomes (BSID-II MDI and PDI at 9 and 30 months). The plots display estimates of the slope curve β associated with the nutritional covariate DHA, as a function of increasing MeHg exposure. These curves provide a means of representing how the relationship of DHA with the outcomes shifts as MeHg exposure increases. Where the slope values are positive, this would indicate a positive association of DHA with the developmental outcome. Where the slope values fall below zero, this would indicate that the positive association of DHA with the developmental outcome is lost. Decreasing slope values, indicated by downwardly trending curves, would suggest increasing levels of MeHg exposure are associated with loss of benefit from the nutritional covariate DHA. This finding is observed for all four outcomes at the higher levels of MeHg exposure. A representation of pointwise variability in the estimated slope coefficient functions is shown, computed as function estimate +/− one standard error. While precise error margins for VC models with penalized splines have not been established in the statistical literature, we calculated the pointwise SE based on variability from the mixed model representation of the splines. These assist in demonstrating the increase in variability in function estimates in areas where the data are sparse. For the PDI outcome at 30 months, the slope values were positive and decreasing as MeHg exposure levels increase, suggesting that the beneficial effect of DHA is diminished as MeHg exposure increased. At approximately 11 ppm exposure, the slope function became negative, and DHA was no longer positively associated with outcome. In the similar analyses on this cohort utilizing ordinary linear regression models from Davidson and colleagues (2008), the point estimate of the slope coefficient of DHA was β = 24.97, with a non-significant p-value 0.34. This earlier point estimate was not capable of capturing the possible decline in DHA effect with increasing MeHg exposure that was demonstrated in the current VC models, which are geared towards representing the dynamic relationship between MeHg exposure and DHA. Similar to the 30 month PDI outcome, the slope function for the BSID-II 9 month MDI outcome decreased across the entire range of MeHg exposure, and changed from a positive to a negative coefficient value at approximately a MeHg exposure level of 9 ppm. For the other two outcomes (MDI at 30 months and PDI at 9 months), there was an increasing relationship between MeHg and slope at the lower levels of MeHg exposure, but that increase stopped and either decreased (for 9 month PDI) or leveled off and slightly decreased (for MDI at 30 months) at approximately 8 ppm in both of these outcomes.
Figure 1.
Varying coefficient functions for the DHA slope values, for the four outcomes analyzed, as functions of prenatal MeHg exposure (ppm). Fitted points for coefficient functions are shown in black (▪), with a reference line at zero provided (---). Pointwise error bands are included, calculated as described in the text. All plots are from models fit using 20 knots with smoothing parameter controlled to give 3 degrees of freedom fit per varying coefficient function. Short tick marks along the abscissa indicate the location of MeHg values, and longer tick marks indicate the location of knot positions used in model fitting.
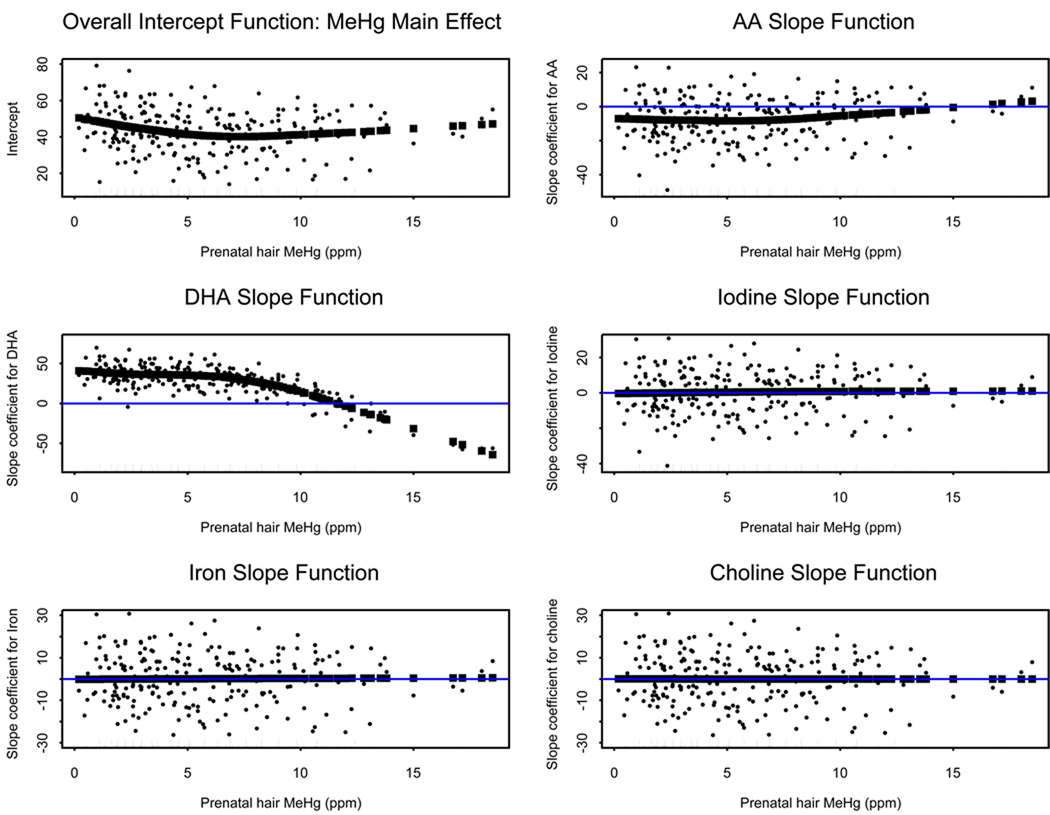
Figure 2 shows the full set of the six varying coefficient function graphs for the outcome PDI at 30 months, each fit using approximately 3 degrees of freedom per curve. This outcome was the only one in which a significant adverse association with prenatal MeHg exposure was observed in our earlier analyses of these data (Davidson et al., 2008). These graphs present the overall main effect of prenatal MeHg exposure on the outcome, as well as the five VC slope functions for each of the nutrition covariates. All were functions of the effect modifying covariate MeHg, and demonstrate how the slope values of each nutrition covariate change with increasing MeHg exposure. The overall MeHg intercept function is seen to decrease over much of the exposure range except at the very highest levels of exposure. This result corresponds to a decrease in 30 month PDI score with increasing MeHg exposure over much of the exposure range. It is important to note that sparsity of data at the very high exposures result in few knot values utilized for fitting the model in that region. Curvature of the function suggests a possibly nonlinear association between MeHg and the BSID-II 30 month PDI, such as has been previously reported (Huang et al., 2005). For the BSID-II PDI at 9 months, and MDI at 9 and 30 months, the overall trend of the MeHg intercept function was increasing. This result does not correspond with results obtained in similar ordinary linear regression models on these data, where non-significant small but negative values were observed for the point estimate of the MeHg coefficient (Davidson et al., 2008). This apparent discrepancy between the two model results may reflect possible absorption of any MeHg negative effects in the overall negative DHA effects at high MeHg concentrations, as the locations of alterations in the behavior of the overall intercepts functions correspond to the locations of change of behavior in the DHA slope functions.
Figure 2.
Varying coefficient functions for the BSID-II PDI at 30 months. The VC intercept function (top left) and five slope coefficient functions for each nutrition covariate are shown. Fitted points for coefficient functions are shown in black (▪), with a reference line at zero provided ( ) where appropriate. Model residuals around the fitted values are shown. All plots are from models fit using 20 knots with smoothing parameter controlled to give 3 degrees of freedom fit per varying coefficient function. Short tick marks along the abscissa indicate the location of MeHg values, and longer tick marks indicate the location of knot positions used in model fitting.
) where appropriate. Model residuals around the fitted values are shown. All plots are from models fit using 20 knots with smoothing parameter controlled to give 3 degrees of freedom fit per varying coefficient function. Short tick marks along the abscissa indicate the location of MeHg values, and longer tick marks indicate the location of knot positions used in model fitting.
Figure 2 also shows that the slope functions for iron and choline indicated no interaction with the BSID-II 30 month PDI at any prenatal MeHg exposure level, a pattern that was observed for all four of the examined outcomes. The essentially constant zero-slope line for the varying coefficient slope function of all four nutritional covariates demonstrates how the effect of these covariates on the PDI 30 month outcome remains essentially unchanged at all observed levels of MeHg exposure. The iodine biomarker T4 showed a very slight upward trend in the slope function, although the slope values were close to zero. For the BSID-II PDI at 9 months, and MDI at 9 and 30 months, the slope function for maternal iodine status showed an approximately linear and slightly decreasing trend, again over a very narrow range of small values which changed from positive to negative slope values in the 6 – 8 ppm range.
Figure 2 also shows that the slope function for AA was generally negative over the range of prenatal MeHg exposure, a pattern that was observed for all outcomes except the BSID-II 9 month PDI, where the slope function was positive and increased over the prenatal MeHg exposure range. All of the nutrition covariates except DHA had very low correlations with MeHg, consistent with results from the VC model suggesting only slight alteration of the slope coefficients for these nutrition covariates as functions of the effect modifier prenatal MeHg exposure.
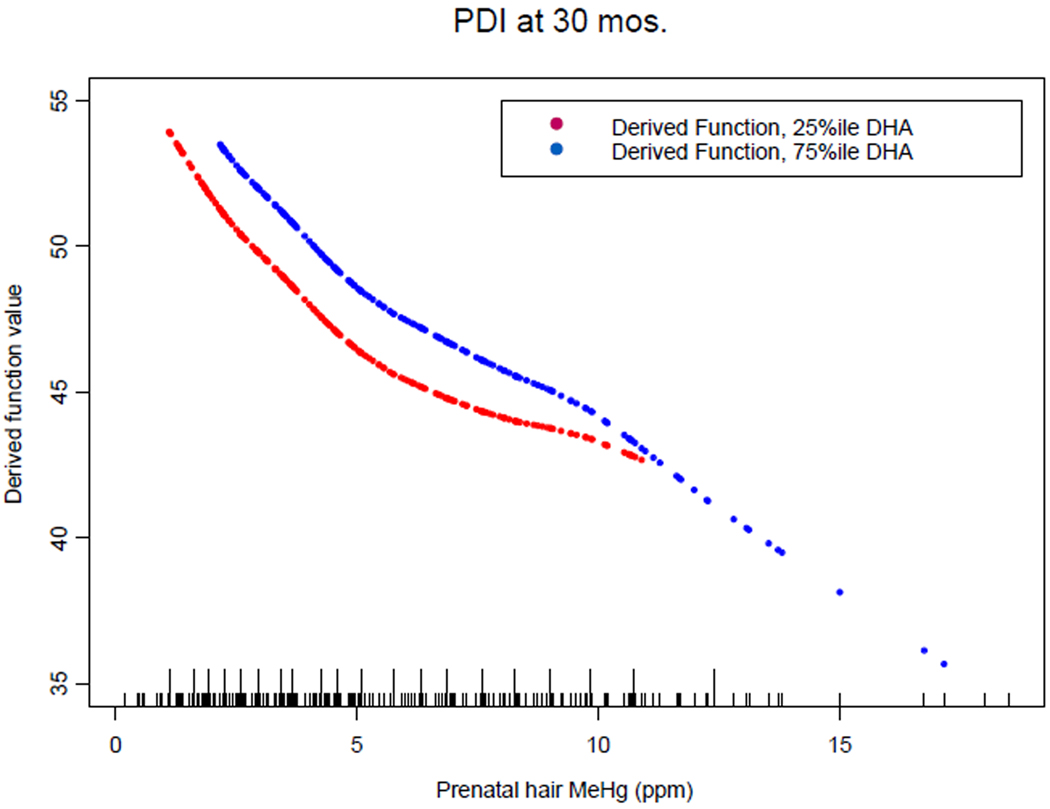
Figure 3 shows an overall function derived from the fitted varying coefficient functions, presented for the outcome BSID-II PDI at 30 months, which yields an additional means of examining the combined effects of MeHg and DHA. The derived function comprises the MeHg varying coefficient intercept function added to the DHA slope function*quantile of DHA, plotted versus an appropriate range of mercury values. The quantiles used were 25% and 75%iles, because these provide a reasonable bracketing of a wide section of the actual observed DHA levels. Appropriate prenatal MeHg ranges for the two quantiles utilized were determined by taking the range endpoints of values of MeHg associated with DHA in a window of ± 0.01 units of DHA (Supplementary Information Figure 1). Owing to the positive correlation between DHA and MeHg, DHA values at the lower quantile were only associated with about half of the observed range of MeHg exposures, whereas DHA values near the upper quantile were associated with MeHg over nearly its full observed range. Figure 3 suggests that where the MeHg exposures are coincident, a higher exposure to DHA is associated with a higher derived function value relative to the lower exposure to DHA, observable in the position of the 75%ile curve above the 25%ile curve. However, Figure 3 also provides evidence for increased MeHg effects at higher DHA consumption, seen where the 75%ile DHA curve overtakes the lower DHA curve at around 10 ppm MeHg, the location at which the DHA slope function becomes negative for this outcome (see Fig. 2). The plots in Figure 3 suggest that over the range of prenatal MeHg exposure that was coincident with either the upper or lower quantile of DHA, higher DHA concentration was beneficial to BSID-II performance, and presents an additional demonstration of the manner in which the interaction of MeHg and DHA generally leads to loss of beneficial DHA effects on outcomes.
Figure 3.
Plot of derived function of overall MeHg and DHA combined effect on BSID-II PDI at 30 months, consisting of fitted VC intercept function added to DHA (Ω-3 LCPUFA) slope function*quantile of DHA. The quantiles shown are 25%ile ( ) and 75%ile (
) and 75%ile ( ). Models were fit using 20 knots with smoothing parameter controlled to give 3 degrees of freedom fit per VC function. Short tick marks along the abscissa indicate the location of MeHg values, and longer tick marks indicate the location of knot positions used in model fitting.
). Models were fit using 20 knots with smoothing parameter controlled to give 3 degrees of freedom fit per VC function. Short tick marks along the abscissa indicate the location of MeHg values, and longer tick marks indicate the location of knot positions used in model fitting.
IV: Discussion
These VC models represent an additional statistical methodology that can help begin to clarify the complex relationships between maternal nutrition and prenatal MeHg exposure from a high fish diet. For DHA, the VC model agrees generally with the point estimates of slope values in our earlier paper, where positive slope point estimates indicating beneficial effects were obtained for all outcomes (Davidson et al., 2008). In addition, the VC model suggests that the beneficial impact of DHA on developmental outcomes may be increasingly attenuated as prenatal MeHg exposure increases. The fixed coefficients in our earlier analyses did not capture the modulation of DHA impact on development by prenatal MeHg exposure, as observed in the VC model setting. Our results from the varying coefficient model analysis for the influence of iron, choline, and to a lesser extent iodine, demonstrate nearly constant slope coefficient functions suggesting a lack of interaction with MeHg for these nutrition covariates. These results are consistent with our original report for these three nutritional indicators using multiple linear regression (Davidson et al., 2008). The VC model results demonstrate trends consistent with those from our earlier models (Davidson et al., 2008). However, they also permit a more detailed view of alterations in the relationship between nutrient status and prenatal MeHg.
Oxidative damage to membrane lipids in the brain has been suggested as a major pathway of MeHg neurotoxicity (Kaur et al., 2006). The n-3 LCPUFA have anti-inflammatory effects that might limit such oxidative damage (Simopoulos et al., 2006), even though paradoxically these highly unsaturated LCPUFA should be very susceptible to an inflammatory insult, such as oxidative damage. In addition, DHA affects development independently of any ameliorating effects on MeHg toxicity as DHA is a major structural component of brain lipids and plays important functional roles in visual and neural processes through mechanisms involving neurotransmitter metabolism, ion channel activity and gene expression (Innes, 2003; 2005). As has been suggested by Hill (1965), the presence of an association does not imply causal relationships, but the biological plausibility of the associations should be taken into account in observational studies when evaluating these types of associations.
While methods for hypothesis testing which would yield p-values to assist in fully evaluating the presence and functional nature of possible interactions are under development at this time, the VC models can still provide insight into the interactions that cannot be accessed via ordinary multiple regression methods. The interesting representation which emerges from the VC models of the interactions between mercury toxicity and maternal nutritional status warrant a more complete analysis with a larger cohort, and the development of model selection and testing procedures suitable for this model setting which will facilitate further refinement of our understanding of the complex interplay of the competing factors that influence child neurodevelopment.
Supplementary Material
Supplementary Information Figure 1. Scatterplot of DHA versus MeHg values demonstrating typical ranges of MeHg exposures associated with a ±0.01 unit window (shaded regions) around 25% and 75% quantiles (solid lines) of DHA. Regression line (dot-dash line) of DHA regressed on MeHg included to show the positive correlation between MeHg and DHA.
Acknowledgment
This research was supported by grants 5-RO1-ES010219 and T32-ES007271 from the US National Institute of Environmental Health Sciences, National Institutes of Health and by the Government of the Republic of Seychelles. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No authors have any conflict of interests.
Protection of Human Subjects
This research was reviewed and approved by the Institutional Review Boards of both the University of Rochester and the Republic of Seychelles in accordance with national and institutional guidelines for the protection of human subjects.
References
- Bellinger DC. Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicology and Teratology. 2000;22:133–140. doi: 10.1016/s0892-0362(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Lead neurotoxicity and socioeconomic status: Conceptual and analytical issues. NeuroToxicology. 2008;29:828–832. doi: 10.1016/j.neuro.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbeck BA, Ruppert D, Wand MP. Comment on Shively, Kohn, and Wood. Journal of the American Statistical Association. 1999;93:961–964. [Google Scholar]
- Cernichiari E, Toribara TY, Liang L, et al. The biological monitoring of mercury in the Seychelles Study. Neurotoxicology. 1995;16:613–628. [PubMed] [Google Scholar]
- Daniels JL, Longnecker MP, Rowland AS, et al. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology. 2004;15:394–402. doi: 10.1097/01.ede.0000129514.46451.ce. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Strain JJ, Myers GJ, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. NeuroToxicology. 2008;29:767–775. doi: 10.1016/j.neuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan JA, Simmer K, Dixon G, et al. Cognitive assessment of children at age 2.5 years after maternal fish oil supplementation in pregnancy: a randomized controlled trial. In: Fetal Neonatal, editor. Archives of Diseases in Childhood. 2006. December 21, 2006 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani R. Varying coefficient models. Journal of the Royal Statistical Society: Series B. 1993;58:379–396. [Google Scholar]
- Helland IB, Smith L, Saarem K, et al. Maternal supplementation with very long chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics. 2003;111:E39–E44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- Hill AB. The Environment and Disease: Association or Causation? Proceedings of the Royal Society of Medicine. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- Huang L-S, Cox C, Myers GJ, et al. Exploring nonlinear association between prenatal methylmercury exposure from fish consumption and child development: evaluation of the Seychelles Child Development Study nine-year data using semiparametric additive models. Environmental Research. 2005;97:100–108. doi: 10.1016/j.envres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Innes SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J. Pediatr. 2003;143:S1–S8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- Innes SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26:S69–S75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Insightful Corp. S-Plus 8.0 for Windows Enterprise Developer. 2007. [Google Scholar]
- Judge MP, Harel O, Lammi-Keefe CJ, et al. Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 months. Am J Clin Nutr. 2007;85:1572–1577. doi: 10.1093/ajcn/85.6.1572. [DOI] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kaur P, Aschner M, Syversen T. Glutathione modulation influences methyl mercury induced neurotoxicity in primary cell cultures of neurons and astrocytes. Neurotoxicology. 2006;27:492–500. doi: 10.1016/j.neuro.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, et al. Twenty-seven years studying the human neurotoxicity of methylmercury exposure. Environmental Research. 2000;83(3):275–285. doi: 10.1006/enrs.2000.4065. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles Child Development Study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Ngo L, Wand MP. Smoothing with mixed model software. J Stat Software. 2004;9(1):1–54. [Google Scholar]
- Ruppert D, Wand MP, Carroll RJ. Semiparametric Regression. NY, NY: Cambridge U Press; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS 9.1.3 Service Pack 4 for Windows. Cary, NC: SAS Institute Inc.; 2002 – 2003. [Google Scholar]
- Simopoulos AP. Evolutionary aspects of diet; the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic disease. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- Strain JJ, Davidson PW, Bonham MP, et al. Associations of maternal long-chain polyunsaturated fatty acids, methylmercury, and infant development in the Seychelles Child Development Nutrition Study. NeuroToxicology. 2008;29:776–782. doi: 10.1016/j.neuro.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JJ, Bonham MP, Duffy EM, et al. Nutrition and neurodevelopment : the search for candidate nutrients in the Seychelles Child Development Nutrition Study. Seychelles Med Dent J. 2004;7:77–83. doi: 10.1016/j.neuro.2020.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Figure 1. Scatterplot of DHA versus MeHg values demonstrating typical ranges of MeHg exposures associated with a ±0.01 unit window (shaded regions) around 25% and 75% quantiles (solid lines) of DHA. Regression line (dot-dash line) of DHA regressed on MeHg included to show the positive correlation between MeHg and DHA.