Abstract
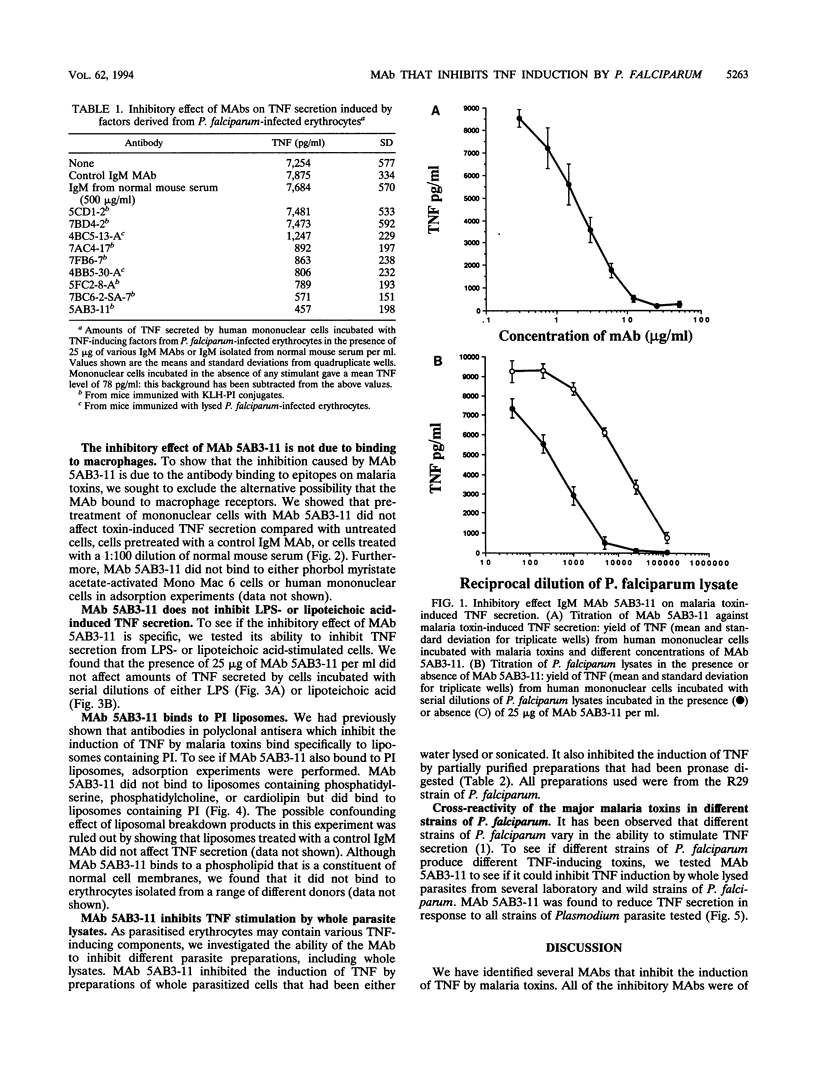
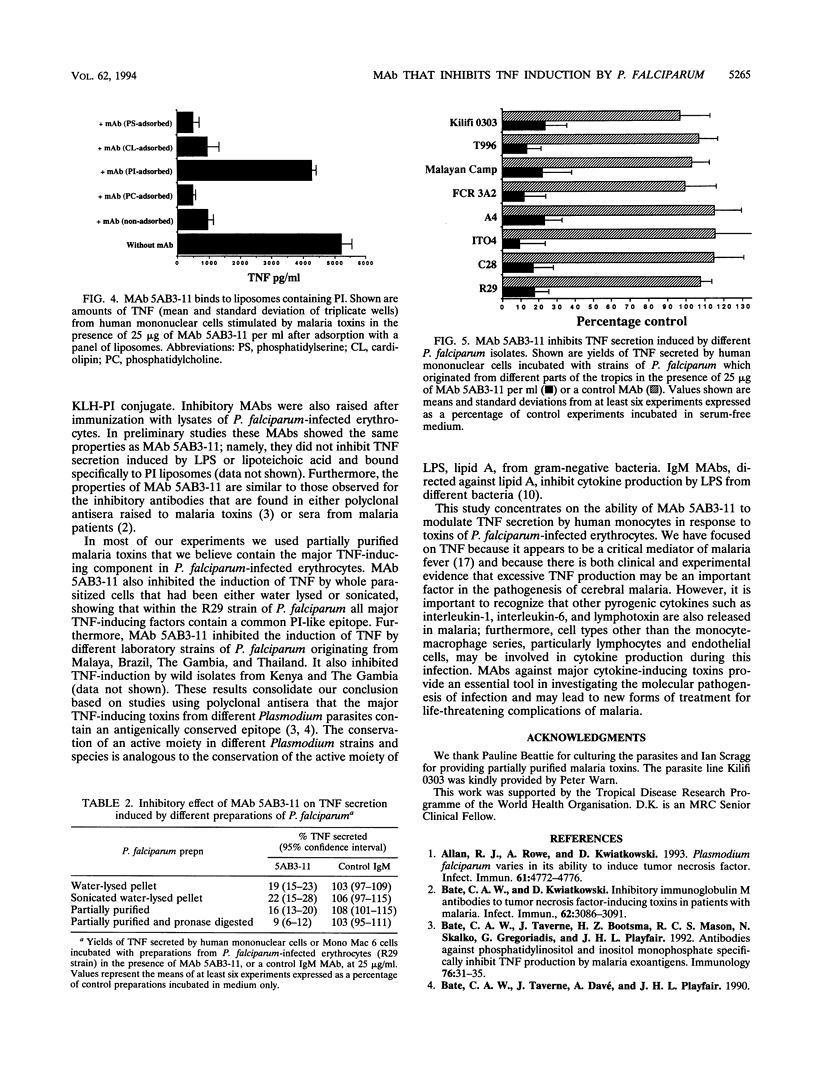
The clinical symptoms of human malaria are mediated, at least in part, by the release of tumor necrosis factor alpha (TNF) by monocytes and macrophages. We have found that lysates of Plasmodium falciparum-infected erythrocytes stimulate the secretion of TNF from human mononuclear cells, and we have generated several immunoglobulin M monoclonal antibodies (MAbs) that inhibit the induction of TNF by such lysates. Here we describe the properties of MAb 5AB3-11, which causes dose-dependent inhibition of the TNF-inducing factors derived from P. falciparum-infected erythrocytes, with a 50% reduction in TNF secretion at nanomolar concentrations (1 to 2 micrograms/ml). The inhibitory effect appears to be malaria specific in that the induction of TNF by either lipopolysaccharide or lipoteichoic acid is not affected. MAb 5AB3-11 binds to liposomes containing phosphatidylinositol but not to other phospholipid liposomes, showing that it recognizes a phosphatidylinositol-like epitope. MAb 5AB3-11 inhibits the induction of TNF by whole lysates from several strains of P. falciparum which originated from different parts of the tropics, indicating that all of the major TNF-inducing factors derived from Plasmodium-infected erythrocytes contain a common epitope. A phosphatidylinositol-like epitope expressed by Plasmodium-infected erythrocytes may be a suitable immunological target for the prevention or treatment of severe malaria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan R. J., Rowe A., Kwiatkowski D. Plasmodium falciparum varies in its ability to induce tumor necrosis factor. Infect Immun. 1993 Nov;61(11):4772–4776. doi: 10.1128/iai.61.11.4772-4776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Bootsma H. J., Mason R. C., Skalko N., Gregoriadis G., Playfair J. H. Antibodies against phosphatidylinositol and inositol monophosphate specifically inhibit tumour necrosis factor induction by malaria exoantigens. Immunology. 1992 May;76(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Davé A., Playfair J. H. Malaria exoantigens induce T-independent antibody that blocks their ability to induce TNF. Immunology. 1990 Jul;70(3):315–320. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Kwiatkowski D., Playfair J. H. Phospholipids coupled to a carrier induce IgG antibody that blocks tumour necrosis factor induction by toxic malaria antigens. Immunology. 1993 May;79(1):138–145. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Malarial parasites induce TNF production by macrophages. Immunology. 1988 Jun;64(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Román E., Moreno C., Playfair J. H. Tumour necrosis factor induction by malaria exoantigens depends upon phospholipid. Immunology. 1992 Jan;75(1):129–135. [PMC free article] [PubMed] [Google Scholar]
- Berendt A. R., Simmons D. L., Tansey J., Newbold C. I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989 Sep 7;341(6237):57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- Brewster D. R., Kwiatkowski D., White N. J. Neurological sequelae of cerebral malaria in children. Lancet. 1990 Oct 27;336(8722):1039–1043. doi: 10.1016/0140-6736(90)92498-7. [DOI] [PubMed] [Google Scholar]
- Fang I. S., Wisniewski M. A., Huntenburg C. C., Knight L. S., Bubbers J. E., Schneidkraut M. J. Inhibition of lipopolysaccharide-associated endotoxin activities in vitro and in vivo by the human anti-lipid A monoclonal antibody SdJ5-1.17.15. Infect Immun. 1993 Sep;61(9):3873–3878. doi: 10.1128/iai.61.9.3873-3878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Karunaweera N. D., Grau G. E., Gamage P., Carter R., Mendis K. N. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3200–3203. doi: 10.1073/pnas.89.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P., Hemmer C. J., Van Damme J., Gruss H. J., Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989 Aug;87(2):139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990 May 2;82(9):772–776. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Molyneux M. E., Stephens S., Curtis N., Klein N., Pointaire P., Smit M., Allan R., Brewster D. R., Grau G. E. Anti-TNF therapy inhibits fever in cerebral malaria. Q J Med. 1993 Feb;86(2):91–98. [PubMed] [Google Scholar]
- Pradines-Figueres A., Raetz C. R. Processing and secretion of tumor necrosis factor alpha in endotoxin-treated Mono Mac 6 cells are dependent on phorbol myristate acetate. J Biol Chem. 1992 Nov 15;267(32):23261–23268. [PubMed] [Google Scholar]
- Roberts D. J., Craig A. G., Berendt A. R., Pinches R., Nash G., Marsh K., Newbold C. I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992 Jun 25;357(6380):689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993 Jan 1;177(1):145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]



