Abstract
We tested the hypothesis that intramuscular immunization with a multisubunit chlamydial vaccine candidate will induce long lasting immune responses in mice. Accordingly, groups of female C57BL/6 mice were immunized intramuscularly with Vibrio cholerae ghosts (VCG) expressing the Poring B and polymorphic membrane protein-D proteins of C. trachomatis or a control antigen. Humoral and cell-mediated immune responses were evaluated following immunization and after live chlamydial infection. Immunization induced an anamnestic response characterized by chlamydial-specific IgG2a and IgA antibodies in sera and vaginal lavage as well as specific genital and splenic T cell responses. The results also revealed that the local mucosal and systemic cellular and humoral immune effectors induced in mice following immunization with the vaccine candidate are long lasting. Vaccinated mice cleared intravaginal challenge with 105 chlamydial inclusion forming units within 12 days compared to control mice, which shed up to 2×103 IFUs at this time point. Moreover, rechallenge of mice 98 days after resolution of the primary infection resulted in the recall and retention of a relatively high frequency of chlamydial-specific Th1 cells and IgG2a in the genital mucosa. These results provide the first evidence that a VCG-based multisubunit chlamydial vaccine is capable of effectively stimulating anamnestic systemic and mucosal immune responses in mice. The data support further vaccine evaluation and testing for induction of long-term protective immunity.
Keywords: Chlamydia, vaccine, delivery, immunity
1. Introduction
Chlamydia trachomatis genital infections constitute a major public health challenge due to the significant morbidity that includes pelvic inflammatory disease, ectopic pregnancy and infertility {Schechter, 1998 #3068;Brunham, 1999 #468}. The frequent asymptomatic infection, especially in women, usually precludes early diagnosis and treatment, making clinical presentation of sequelae often the first indication of infection. In the USA alone more than $2 billion is spent annually in the management of chlamydial genital infections {Igietseme, 2003 #4101}. Consequently, a vaccine capable of protecting against infection or even ameliorating severe disease would be the most promising and effective strategy to control Chlamydia {Stagg, 1998 #3271;Igietseme, 2002 #3982;Igietseme, 2003 #4101} and prevent sequelae of infection.
The current immunologic paradigms for designing and evaluating chlamydial vaccines include the requirement for a T-helper Type 1 (Th1) immune response {Morrison, 2002 #4100;Igietseme, 2003 #4101}. However, recent findings indicate that antibodies of the IgG2a and IgA isotype enhance Th1 activation against chlamydiae {Morrison, 2000 #2282;Moore, 2002 #4226;Igietseme, 2004 #4225}. Furthermore, the selection of a suitable vaccine candidate capable of inducing the required immune effectors and the development of an effective delivery system to boost such immune responses, are additional requirements for an efficacious chlamydial vaccine. The use of whole chlamydial agents as vaccines is unattractive due to the potential existence of immunopathogenic components {Brunham, 1994 #467} and the inability to genetically modify chlamydiae to produce safe, attenuated vaccine strains. Thus, the current focus is to develop vaccines based on chlamydial subunit components. The focus on a multisubunit approach in chlamydial vaccine design imposes a major challenge: to determine the appropriate combination of immunogenic components that can be delivered to the immune system to elicit an optimal immune response. Advances in chlamydial genomics have predicted several immunogenic proteins in addition to the chlamydial outer membrane protein, MOMP {Read, 2000 #2799;Stephens, 1998 #3309;Stephens, 2000 #3310} that may serve as potential vaccine candidates. Among these are the polymorphic outer membrane proteins (POMPs or Pmps) {Longbottom, 1998 #4222;Grimwood, 2001 #4152;Niessner, 2003 #4153} and the conserved PorB family of membrane proteins {Kubo, 2000 #4150;Kubo, 2001 #4151;Kawa, 2002 #4148}. PmpD and PorB are major protective antigens on the surface of chlamydial elementary bodies (EBs) {Brunham, 1994 #467;Kawa, 2002 #4148;Crane, 2006 #4404;Kubo, 2000 #4150} that could generate neutralizing antibodies. Both proteins are evolutionarily conserved and involved in chlamydial attachment to host cells {Wehrl, 2004 #4405;Kubo, 2000 #4150}. Also, PmpD and PorB are highly immunogenic and induce protective immunity in mice {Kawa, 2004 #4149} {Ifere, 2007 #4500}. A delivery platform that would simultaneously present multiple antigens may represent a viable immunization and vaccine regimen to induce protective immunity against Chlamydia. In this respect, the recombinant Vibrio cholerae ghost (rVCG) platform is an effective carrier and delivery system for cloned C. trachomatis proteins, eliciting chlamydial-specific immune responses and protection following immunization and challenge {Eko, 2003 #4099;Eko, 2004 #4135}. We have previously shown that intramuscular delivery of rVCG-based chlamydial vaccines is highly effective at inducing antigen-specific mucosal immune responses in the genital tract and provided significant protection against vaginal infection in mice {Eko, 2004 #4135;Eko, 2003 #4099;Ifere, 2007 #4500;Ekong, 2009 #4755}. In addition, the rVCG system is capable of simultaneously delivering multiple antigens to the immune system {Eko, 2004 #4135; Ifere, 2007 #4500}.
Since the severe sequelae associated with chlamydial infection are thought to be the consequence of repeated infections caused by poor immunological memory to previous infection, a vaccine capable of protecting against infection and inducing long lasting immunity would be the most effective strategy to control Chlamydia. The present study was undertaken to investigate whether maintenance of immunological memory against a genital chlamydial infection can be established by intramuscular immunization with an rVCG-based multisubunit chlamydial vaccine. The results revealed that the local mucosal and systemic cellular and humoral immune effectors induced in mice following immunization with the vaccine candidate are long lasting.
2. Materials and Method
2.1. Chlamydia stocks and antigens
Stock preparations of C. trachomatis serovar D strain were generated by propagating elementary bodies (EBs) in HeLa cells as previously described {Ramsey, 1988 #2758}. All stocks were titrated on HeLa cell monolayer’s followed by purification of EBs over engrain gradients {Ramsey, 1988 #2758} and stored at −70°C. Chlamydial antigens were prepared by UV-inactivation of EBs for 3 h and stored at −70°C.
Production of rVCG expressing the vaccine antigens was essentially as previously described {Eko, 2000 #3976}. Lyophilized ghost preparations were stored at room temperature until used.
2.2. Mice
All mice used in these studies were of the C57BL/6 strain (female, aged 6 to 8 weeks) from The Jackson Laboratory (Bar Harbor, ME). They were housed in the animal facility of Morehouse School of Medicine and animal study protocols were performed in compliance with institutional IACUC and federal guidelines.
2.3. Immunization, challenge and analysis of protective immunity
Mice (10 mice/group) were immunized intramuscularly (IM) with 50 µl PBS containing 2 mg of lyophilized rVCG-PmpD/PorB on days 0, 14 and 28 as previously described {Eko, 2004 #4135;Igietseme, 1998 #1512}. Also, two groups of 6 mice each were mock-immunized with PBS or PBS containing 1mg of rVCG-gD2 (rVCG expressing glycoprotein D from HSV-2, a chlamydial irrelevant antigen). A dose of 1 mg of lyophilized rVCG corresponds to approximately 2×109 cuff. Animals were anesthetized by intraperitoneal injection of 200 µl of a 5% sodium pentobarbital solution (Sigma-Aldrich, Milwaukee, WI) before vaccine administration. Seven days after the last immunization, the animals were administered Depo Provera (2.5 mg/mouse; UpJohn Co., Kalamazoo, MI) to synchronize the estrous cycle and facilitate a productive infection and challenged intravaginally one week later with 105 inclusion forming units (IFUs) of live C. trachomatis serovar D. After challenge, mice were observed twice daily to monitor health status, such as clinical signs of adverse reaction to infection. To assess the level of infection, cervicovaginal swabs were collected from each animal every 3 days following the challenge and chlamydiae were isolated from swabs in tissue culture by standard methods {Ramsey, 1988 #2758}. Ninety-eight days after initial primary challenge, a time when mice vaginally infected with live chlamydiae are usually susceptible to reinfection, mice were rechallenged with 2.5×104 IFU of serovar D per mouse. Serum and vaginal lavage samples were collected at different time points and stored as previously described {Macmillan, 2007 #4488}.
2.4. Purification of CD4+ T cells
At indicated time points after immunization or challenge, animals designated for immunogenicity studies were sacrificed and the genital tract (GT), iliac lymph nodes (ILN) draining the genital tract and spleens (SPL) were harvested. Immune T cell-enriched cells were prepared from tissues of immunized and control mice by the nylon wool enrichment procedure described previously {Igietseme, 1991 #1502}. T cells were then purified by the Midi magnetic bead-activated cell sorting (MidiMACS) purification system, by positive selection of CD4+ T cells, using CD4-specific MACS microbeads (Miltenyi Biotech, Auburn, CA). The purity of the CD4+ T cell population was determined to be at least >95% by flow cytometry using an APC-conjugated anti-CD4 monoclonal antibody (Pharmingen, San Diego, CA). A separate pool of splenocytes prepared from naive animals and treated with mitomycin C (25 µg/107 cells) for 20 min was used as a source of antigen-presenting cells (APCs).
2.5. Detection of cytokine production by ELISA
The level of Chlamydia-specific Th1 and Th2 response was assayed by measuring the antigen-specific Th1 (IFN-γ) and Th2 (IL-4, IL-5) cytokine production by each cell population, respectively. Briefly, purified CD4+ T cells were plated in quadruplicate wells of 96-well tissue culture plates at 2 × 105 cells/well and cultured with APCs (2 × 105) and 10 µg/ml UV-irradiated C. trachomatis EBs (chlamydial antigen) for 5 days. Control cultures contained APCs and T cells without antigen. At the end of the incubation period, supernatants were harvested and assayed for cytokines using the Bio-Plex cytokine assay kit in combination with the Bio-Plex Manager software (Bio-Rad, Hercules, CA). The mean and SD of all replicate cultures were calculated. The experiment was repeated three times.
2.6. Measurement of the frequency of Chlamydia-specific Th1 cells
A modified procedure for the limiting dilution technique reported previously {Kees, 1984 #1706;Igietseme, 2000 #1516} was used to measure the frequency of Chlamydia-specific Th1 cells (assayed as antigen-specific IFN-γ production) by each cell population. In brief, purified T cells were seeded in a serial doubling dilution into 96-well round-bottom tissue culture plates at 24 wells/dilution. The T cells were stimulated with APCs from wild-type (wt) mice (2 × 105 cells/well) and chlamydial antigen (10 µg/ml). Background cultures contained 24 wells with APCs and chlamydial antigen. After 5 days of incubation, the supernatants were assayed for IFN-γ using the Bio-Plex cytokine assay kit in combination with the Bio-Plex Manager software (Bio-Rad, Hercules, CA). The mean and SD of all 24 replicates of background cultures were calculated. Three times the value of the SD was added to the mean value, and the sum was the baseline for positive experimental wells. After determination of the number of positive and negative wells per dilution of each T cell preparation, the data were analyzed by a limiting dilution computer program (LIDIA) {Kees, 1984 #1706}, which provided both the Th1 frequency and the conformity of the input data with a single-hit Poisson model. The data are expressed as the Th1 frequency per 106 responding T cells in each cell preparation. T cells from naive wild type mice have a Th1 frequency of 15 (range, 9–21) per 106 cells.
2.7. Measurement of T cell proliferation
Purified immune T cells were assessed for their ability to proliferate in response to in vitro restimulation in culture with chlamydial antigen using the 5-Bromo-2’-deoxy-uridine (BrdU) cell proliferation assay kit according to the manufacturer’s instructions (Roche Molecular Biochemicals, Indianapolis, IN). Purified CD4+ T cells (106 cells/ml) were cultured with γ-irradiated (3,000 rads) splenic feeder cells (106/ml) in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% heat-inactivated FBS in the presence or absence of chlamydial antigen (10 µg/ml UV-irradiated EBs) at 37°C in 5% CO2. After five days of ex vivo antigen-restimulation, 106 cells/ml were transferred to round-bottom 96-well plates (Corning Glass Work, Acton, MA) and 10 µl of BrdU labeling solution (10 µM/ml final concentration per well) was added and the plates incubated for 18 h at 37°C in 5 % CO2. The cells were then fixed and incubated with 100 µl of nuclease per well for 30 min at 37°C (to remove DNA). After washing with complete medium the cells were incubated with peroxidase-coupled anti-BrdU antibody for 1h at 37°C and developed by adding 100 µl of 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) substrate for 30 min. BrdU incorporation was detected using a scanning multi-well spectrophotometer (Spectra-Max 250 ELISA reader, Molecular Devices, Sunnyvale, CA) and the stimulation index (SI), the ratio between stimulated and non-stimulated cells, was calculated. The optical density was read at 405 nm with a reference wavelength at approximately 490 nm. T cells cultured in the absence of chlamydial antigen served as internal control.
2.8. Antibody and antibody isotype determinations
Blood samples were collected by retro-orbital plexus puncture and vaginal lavage was obtained by flushing the vaginal vault with 100 µl of PBS before immunization and at 2, 4, 8 and 16 weeks after the last boost. Also, serum samples were collected 14 days after the primary challenge and at 7, 14 and 28 days after the secondary challenge. The amounts of Chlamydia-specific antibodies (IgG, isotypes IgG1, IgG2a, and secretory IgA) in sera and vaginal washes were measured by a standard ELISA procedure described previously {Macmillan, 2007 #4488}. Briefly, 96-well microtiter plates (Nunc Life Technologies, Rochester, NY) were coated with 10 µg/ml of chlamydial antigen in 100 µl of PBS at 4°C overnight. Plates were blocked with 1% bovine serum albumin containing 5% goat serum in PBS and 100 µl of serum or 50 µl of vaginal wash in twofold serial dilutions was added per well. Plates were incubated with 100 µl of horseradish peroxidase-conjugated goat anti-mouse IgA or IgG isotypes (Southern Biotechnology Associates, Inc., Birmingham, Ala.) for 1 h and developed with 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS). The optical density was measured at 490 nm on a Microplate reader. Results, generated simultaneously with a standard curve, display data sets corresponding to absorbance values as mean concentrations (ng/ml) ± SD and represent the mean values from triplicate experiments.
2.8. Statistical analysis
Differences between the course of infection in groups of challenged immune mice and immune response, including antibody and cytokine production, were tested by Student’s 2-tailed t-test. The results were expressed as mean ± standard deviation (SD). Tests were performed using Sigma Stat software (SPSS Inc.). A value of p ≤ 0.05 was considered significant.
3. RESULTS
3.1. Magnitude of chlamydial-specific Th1/Th2 cytokine responses induced by the multisubunit vaccine candidate
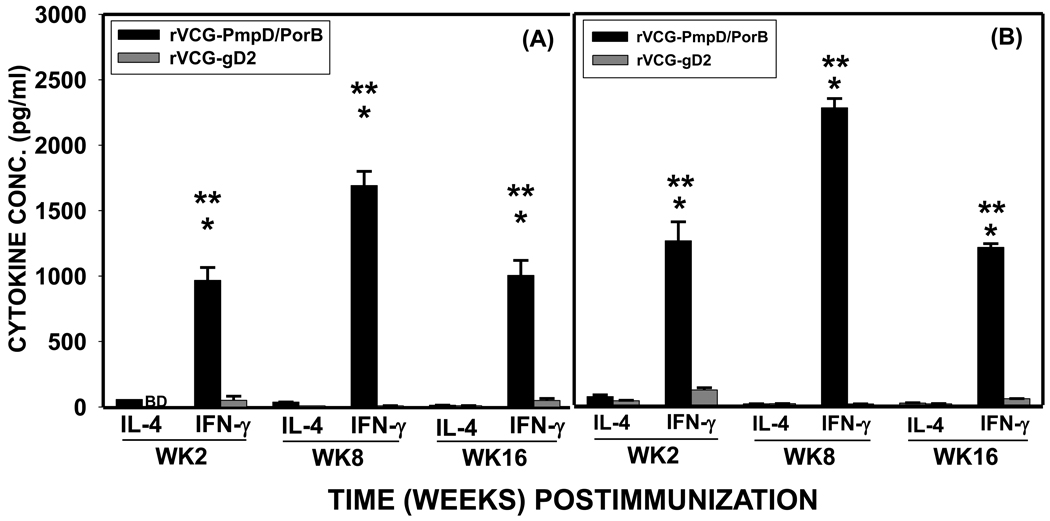
We have constructed a multisubunit vaccine expression vector and produced a candidate vaccine comprising rVCG simultaneously expressing the chlamydial PorB and PmpD antigens (rVCG-PmpD/PorB). To examine specific Th1/Th2 cell responses induced by the vaccine candidate, CD4+ T cells were purified at various time points from the ILN and spleens of immunized mice and analyzed for IFN-γ (Th1) and IL-4 (Th2) cytokine secretion upon restimulation with chlamydial antigen. Significantly higher (p< 0.05) amounts of Chlamydia-specific IFN-γ were produced by both mucosal (Fig. 1A) and systemic (Fig. 1B) immune T cells from rVCG-PmpD/PorB-immunized mice compared to rVCG-gD2 (control)-immunized mice, at all time points evaluated. The results also showed the secretion of significantly lower (p< 0.05) levels of IL-4 compared to IFN-γ by these T cells at the same time points tested, indicating the early recruitment and retention of antigen-specific Th1 cells in the genital mucosa.
Fig. 1.
The longevity of the immune response to immunization with the rVCG vaccine was evaluated by comparing the responses elicited 16 weeks after boosting with those elicited at earlier time points. Although the levels of chlamydial-specific Th1 response at week 16 were substantially lower than those induced 8 weeks after immunization, they remained appreciably high compared to non-vaccinated controls (Fig. 1). The high level of chlamydial-specific IFN-γ secreted by immune T cells from ILN and splenic tissues at week 16 post-immunization indicated that the multisubunit vaccine induced long lasting immune responses in both mucosal and systemic tissues.
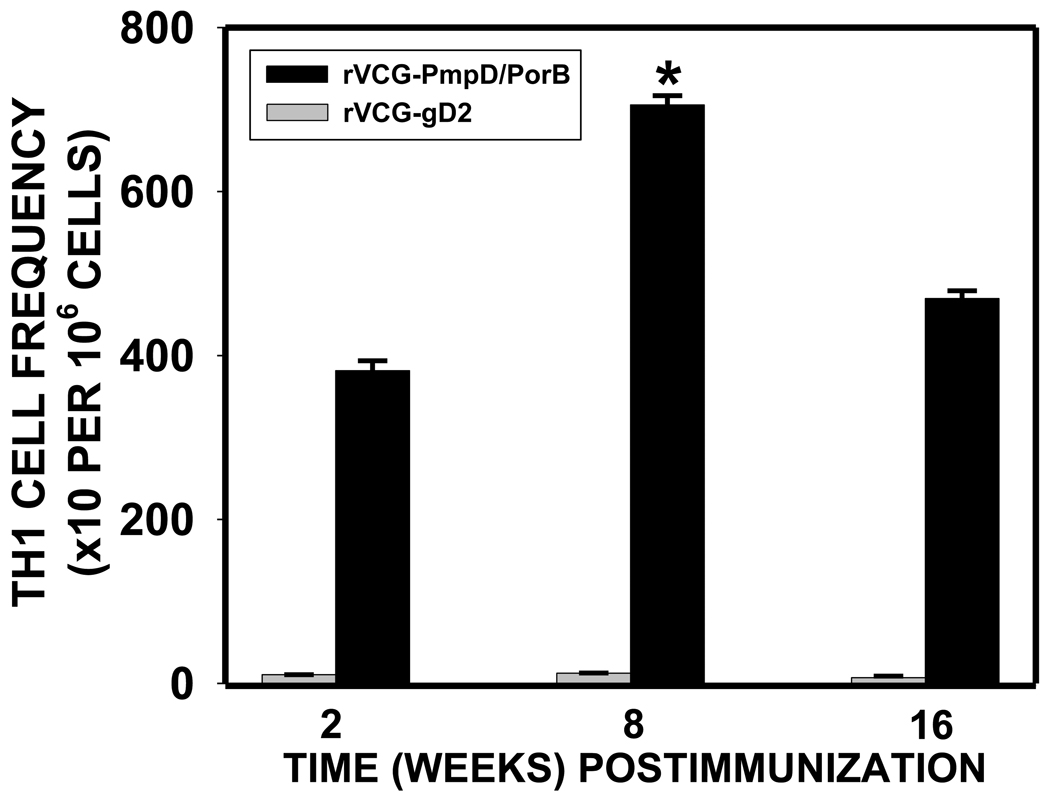
3.2. Frequency of chlamydial antigen–specific IFN-γ-secreting Th1 cells
We hypothesized that antichlamydial immune effectors will be elicited and sustained in the genital mucosa of rVCG multisubunit vaccine-immunized animals. To test this hypothesis, the frequency of chlamydial-specific Th1 cells in the genital mucosa was monitored by measuring the antigen-specific IFN-γ-secretion using the Bio-Plex cytokine assay kit in combination with the limiting dilution technique at different time points after immunization. The results presented in Fig. 2 show that 2 weeks post-immunization, a high frequency of chlamydial-specific Th1 cells was recruited into the genital mucosa of vaccinated mice that significantly increased at week 8, and persisted for up to 16 weeks. On the other hand, the frequency of Th1 cells in the rVCG-gD2 control-immunized mice was similar to that of naive wild type mice which have a Th1 frequency of 15 (range, 9–21) per 106 cells.
Fig. 2.
3.3. Induction of Chlamydia-specific memory T cells during the recall of immunity
To determine the establishment of adequate immunological memory after vaccination with the multisubunit vaccine candidate, vaccine-immune mice were rested for 14 weeks after primary infection, a time period during which non-vaccinated mice become susceptible to reinfection, and rechallenged with live chlamydiae. T cells from the rVCG-PmpD/PorB-immunized and rechallenged mice showed significantly higher amounts of IFN-γ secretion upon stimulation with chlamydial antigen as compared to T cells from rVCG-gD2 control mice at the time of chlamydial challenge. The enhanced local infiltration of chlamydial-specific IFN-γ-secreting T cells resulted in the high Th1 cell frequency in the genital tract 7 days after rechallenge (Table 1).
Table 1.
Correlation of local mucosal Chlamydia-specific Th1 cell-mediated immunity with levels of secretary IgA and Th1-associated IgG2a memory responses
| Immunization Group | Th1 response (IFN-γ) |
Th1 Frequency per 106 cells |
Genital IgA (ng/ml) |
Genital IgG1 (ng/ml) |
Genital IgG2a (ng/ml) |
|---|---|---|---|---|---|
| rVCG-PmpD/PorBa | 966.87 ± 98.58 | 381.45 ± 12.18 | 93.5 ± 2.0 | 18.65 ± 1.2 | 453.0 ± 46.0 |
| rVCG-gD2a | 50.64 ± 31.93 | 12.24 ± 0.26 | 7.4 ± 1.5 | 10.48 ± 1.0 | 36.10 ± 5.0 |
| rVCG-PmpD/PorBb | 1141.53 ± 41.54 | 428.5 ± 12.0 | 185.0 ± 10.0 | 17.42 ± 1.0 | 472.0 ± 32.0 |
| rVCG-gD2b | 31.10 ± 1.5 | 16.0 ± 0.50 | 12.20 ± 2.0 | 12.82 ± 1.4 | 26.40 ± 4.0 |
| rVCG-PmpD/PorBc | 1841.53 ±44.47 | 528.0 ± 17.2 | 197.0 ± 22.0 | 17.42 ± 1.0 | 505.0 ± 18.0 |
| rVCG-gD2c | 31.10 ± 1.50 | 16.0 ± 0.50 | 12.20 ± 2.0 | 12.82 ± 1.4 | 16.0 ± 1.0 |
Iliac lymph node (ILN) immune T cells and genital lavage samples were obtained on Day 14 post immunization;
immune animals were challenged intravaginally 2 weeks after immunization and samples were collected on Day 14 post challenge (PC);
immune mice were challenged 98 days after the primary challenge infection and samples were collected on Day 14 PC.
The ILN drains the genital tract in mice.
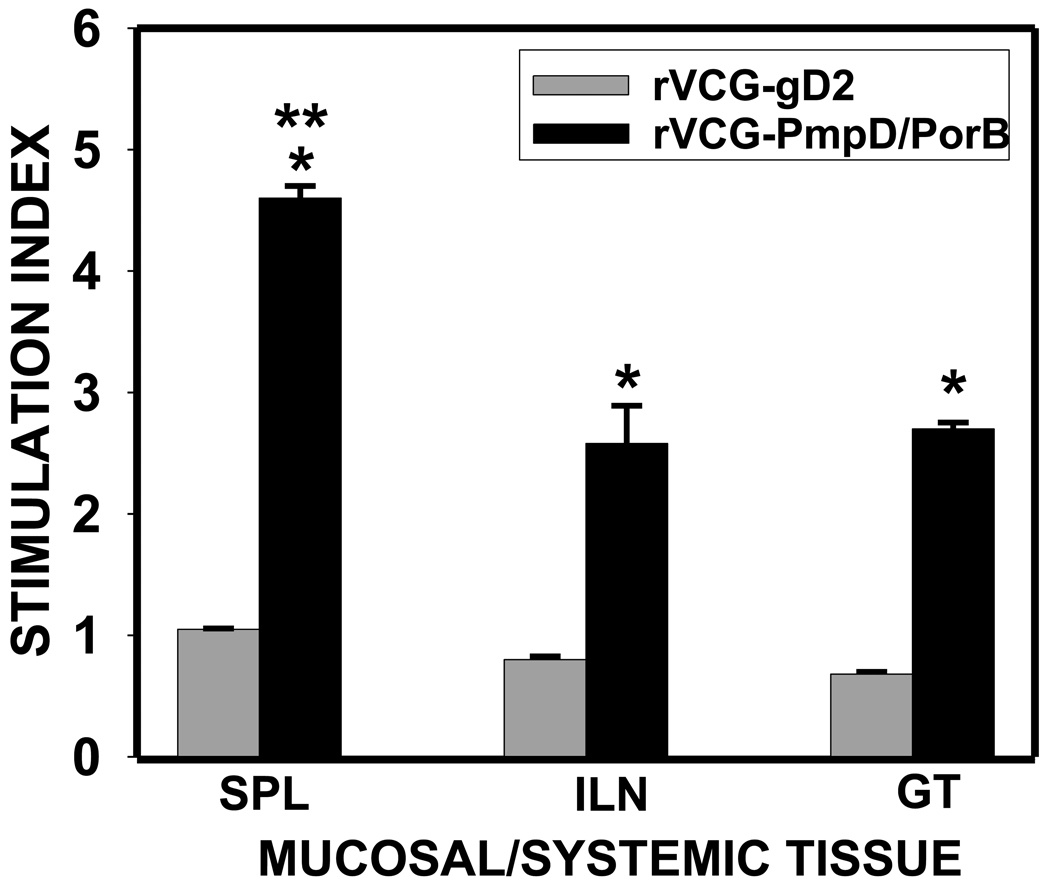
3.4. Proliferative response
Purified immune T cells from rVCG-PmpD/PorB or rVCG-gD2 immunized mice were assessed for their ability to proliferate in response to in vitro restimulation in culture with chlamydial antigen by the BrdU incorporation assay. To compare the antigen-specific proliferative responses elicited between the systemic and mucosal compartments, we analyzed stimulation index (SI) values (the ratio between absorbance values of stimulated and non-stimulated cells) of the IFN-γ levels obtained after stimulation of T cells purified from the spleen, ILN and GT tissues of vaccine-immunized mice. Fig. 3 shows that mice immunized with rVCG-PmpD/PorB had significantly higher (p< 0.05) T cell proliferative responses in all compartments evaluated compared to the rVCG-gD2 control [mean experimental absorbance values were 0.299 ± 0.029 (SPL), 0.199 ± 0.019 (ILN) and 0.141 ± 0.0 (GT)]. Also, the magnitude of IFN-γ response to chlamydial antigen in ILN and GT tissues was statistically indistinct but was significantly lower (p<0.05) than the corresponding response to antigen in the spleen, which indicates a potentially greater concentration of C. trachomatis IFN-γ responsive cells in systemic compared to mucosal tissues at the indicated time after immunization.
Fig. 3.
3.5. Induction of chlamydial-specific antibody responses
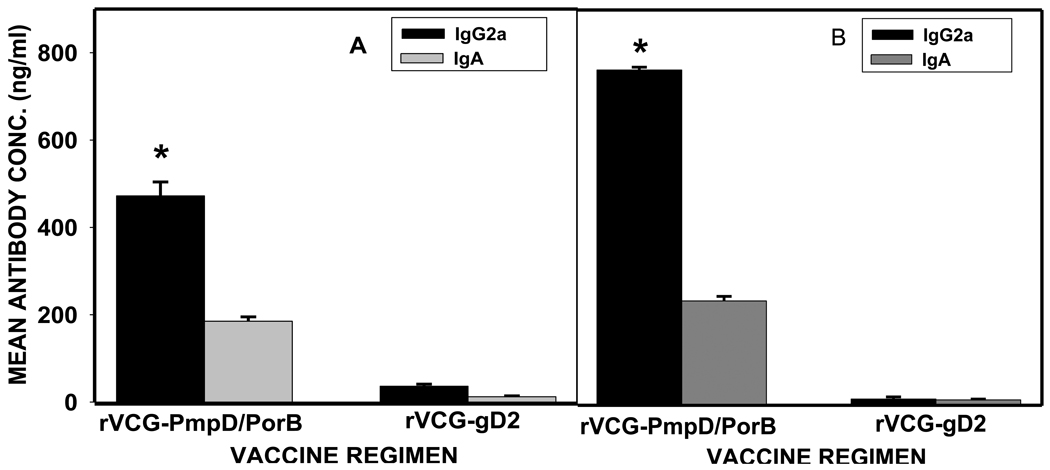
Specific antibody responses elicited at different time points following immunization with rVCG-PmpD/PorB were measured by titrating the serum and vaginal secretions of vaccinated and control mice against chlamydial antigen, using an ELISA assay. The results (Fig. 4) showed that mice immunized with rVCG-PmpD/PorB developed significant (P< 0.05) antigen-specific secretory IgA and IgG2a antibodies in both vaginal secretions and serum, compared with controls (Fig. 4). Also, there was early (week 2) induction of significant amounts of antibody by immunized mice that remained high even at week 16 post-immunization.
Fig. 4.
3.6. Induction of long-term memory antibody response following genital chlamydial reinfection
Two weeks after immunization, mice were challenged with 105 IFU/mouse of live chlamydiae and the level of mucosal and systemic antibody responses was measured. Prior to challenge, immunized mice had persisting high levels of chlamydial-specific antibodies in both genital mucosal and systemic tissues (Fig. 4). Following challenge, mucosal and systemic IgA and IgG2a antibody levels increased above the week 2 pre-challenge levels with high concentrations (450 ng/ml) of IgG2a antibody being recalled into the local vaginal mucosa (Fig. 5). The results also showed that the Th1-associated IgG2a antibodies secreted in serum 2 weeks after the primary challenge was significantly higher (p< 0.05) than that secreted into the vaginal mucosa.
Fig. 5.
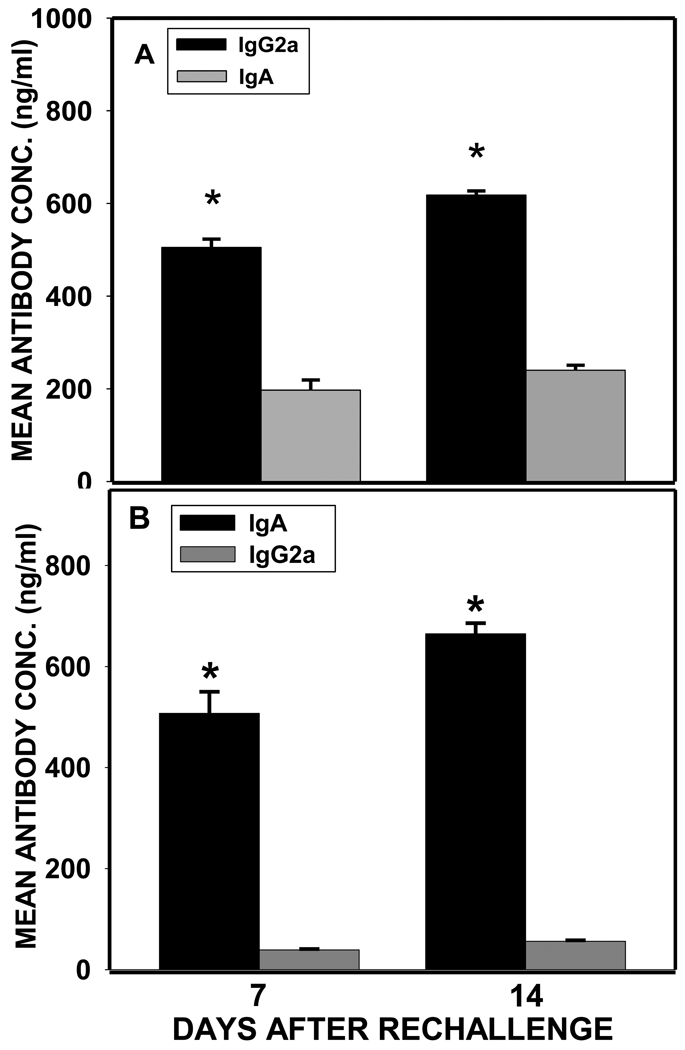
To determine the long-term recall responses of antibody in vivo, serum and vaginal secretions obtained from immune mice rechallenged 98 days after the initial primary challenge were evaluated for the magnitude of antibodies elicited at various time points. The results showed that high levels of IgA and IgG2a antibodies were elicited and sustained in both mucosal and systemic tissues following chlamydial rechallenge (Fig. 6). Also, IgG2a levels were significantly higher (p< 0.05) than IgA levels in both serum and genital tract mucosa (Fig. 6).
Fig. 6.
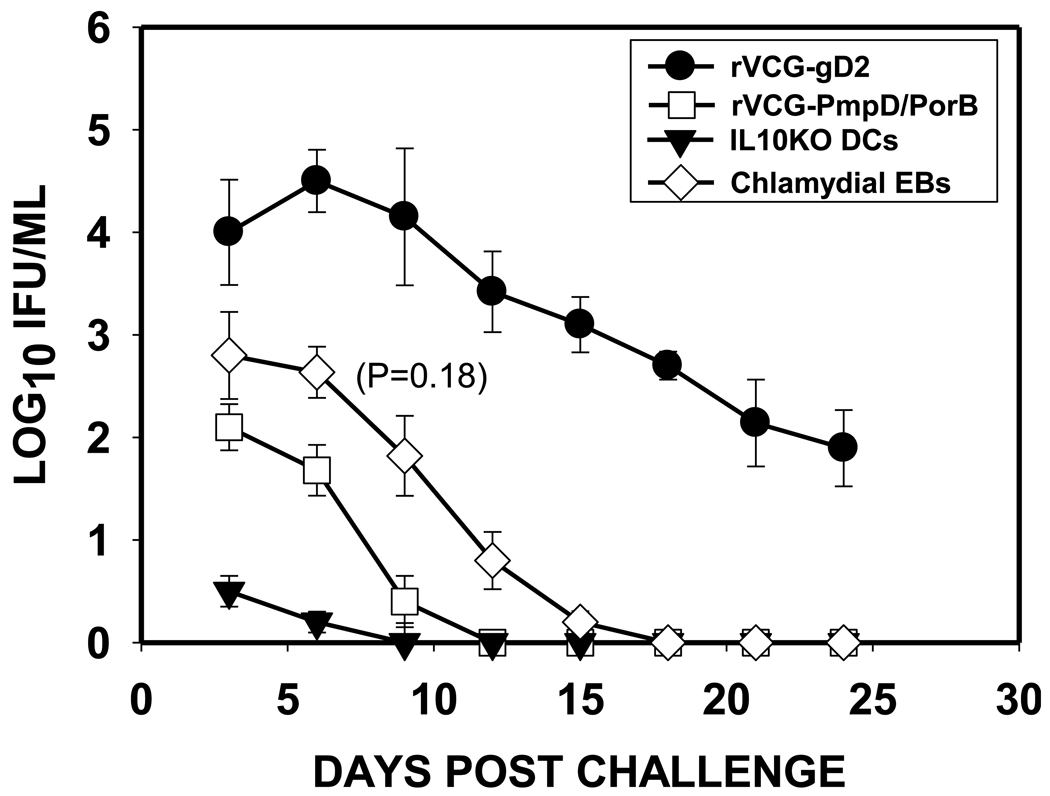
3.7. Intramuscular immunization with rVCG-PmpD/PorB confers protection against genital chlamydial infection
To evaluate vaccine efficacy, animals were challenged intravaginally with live chlamydiae two weeks after the last immunization and periodically monitored for clearance. As a positive control, the level of protection conferred by the candidate vaccine was compared to the sterile immunity conferred by an IL-10 deficient dendritic cell (DC)-based cellular vaccine {Igietseme, 2000 #1516}. The results showed that within one week, mice immunized with the multisubunit candidate vaccine shed approximately 3-log lower chlamydial IFUs than the controls (rVCG-gD2) and at least 1-log lower IFUs than the infected mice (Figure 7). In addition, vaccinated mice cleared the infection within 12 days post challenge, which was superior to the EB-infected group although the level of protection lagged behind the IL-10 deficient DC-based immunotherapy by 3 days. The result indicated that the multisubunit candidate vaccine conferred a level of protective immunity that is moderately better (though not statistically significant, p=1.8) than that of the live infection although less than the level afforded by the IL-10 deficient DC-based immunotherapy.
Fig. 7.
3.8. Immune correlates of long-term protective chlamydial immunity
The ability of rVCG-PmpD/PorB candidate vaccine to induce adequate immunological memory was determined by correlating the magnitude and frequency of local mucosal Chlamydia-specific Th1 cell-mediated immunity with levels of secretary IgA and Th1-associated IgG2a memory responses. Following resolution of the primary genital tract infection, immunized and non-immunized mice were rechallenged intravaginally after 98 days and the cellular and humoral immune responses were evaluated 7 days after. The results presented in Table 1 shows the correlation of the cellular immunological parameters with the level of humoral responses of vaccinated animals. The magnitude and frequency of specific Th1 cells recruited into the genital mucosa of vaccinated mice 7 days after reinfection are approximately 30–60-fold higher than the control group. Also, the genital mucosal chlamydial-specific IgA and the Th1-associated IgG2a concentrations in the vaccinated mice were 16–30-fold higher than in the control mice on day 7 after rechallenge. On the other hand, the chlamydial-specific Th2-associated IgG1 response was non-remarkably low and indistinguishable between the vaccinated and control mice. Notably, the frequency of Th1 cells recruited into the genital mucosa correlated with the level of the Th1-associated IgG2a antibody elicited in vaginal lavage following both challenge and reinfection. The results indicate that the ability of the multisubunit vaccine to induce chlamydial-specific immune memory is due to the retention of a high frequency of chlamydial-specific Th1 cells and the associated IgG2a response in the genital mucosa.
4. Discussion
It is generally accepted that an efficacious chlamydial vaccine would be a pivotal strategy for effective control of infection and significant reduction in healthcare costs associated with management of Chlamydia-induced complications. The finding that protection against chlamydial infection correlates with a high frequency of Th1 cells and the associated antibodies {Igietseme, 1998 #1512} implies that a vaccine candidate inducing such responses will be potentially efficacious. In this respect, a number of delivery vehicles and adjuvants, including recombinant viral and bacterial vectors {Murdin, 1995 #2343;Starnbach, 2003 #4436}, and mucosal adjuvants {Singh, 2006 #4356} have been used to deliver Chlamydia antigens. So far, only the IL-10 deficient dendritic cell (DC)-based cellular vaccine has produced a sterilizing long-term immunity in a mouse genital infection model {Igietseme, 2000 #1516}. While the cellular vaccine approach is of limited practical application, the efficacy of the system indicates that given an effective delivery vehicle, an efficacious Chlamydia vaccine is possible.
The novel recombinant VCG delivery platform has been show to be an effective carrier and delivery system for multiple C. trachomatis proteins, inducing significant immune responses and protection in the absence of supplementary adjuvants {Eko, 2004 #4135;Ifere, 2007 #4500}. The results of these studies established the vaccine advantage of a multisubunit approach over the use of single subunits. The present study was undertaken to investigate whether immunization with an rVCG-based multisubunit chlamydial vaccine will induce immunological memory against a genital chlamydial infection. Because both cell-mediated and humoral immune effectors may control C. trachomatis immunity, we simultaneously investigated specific cellular and antibody responses in the serum and genital mucosa of immunized mice. Intramuscular immunization with the rVCG-PmpD/PorB vaccine construct resulted in the rapid and sustained recruitment of a high frequency of specific Th1 cells into the genital mucosa, which increased albeit marginally after challenge infection. The early development of specific immunity after infection may have been due to the presence of memory T lymphocytes indicating that rVCG vaccine immunization of mice elicits chlamydial-specific IFN-γ-secreting T cells, which can be recalled to respond to chlamydial exposure. The importance of a T helper type 1 (Th1) immune response during Chlamydia infection has previously been demonstrated in human clinical studies and studies in experimental animal models of genital and ocular infection {Igietseme, 2003 #4101;Morrison, 2002 #4100;Igietseme, 2004 #4225;Loomis, 2002 #4228;Murthy, 2007 #4518}.
Our results also show that immunization with rVCG-PmpD/PorB elicited significant systemic and local mucosal IgA and IgG2a antibody responses, detectable in serum and vaginal secretions that persisted for 112 days (the study period). These findings are consistent with the report of a recent study showing that subcutaneous immunization of koalas with a multisubunit chlamydial vaccine, adjuvanted with either immune stimulating complex or Alhydrogel elicited both humoral and cell-mediated immune responses that lasted >270 days {Carey, 2010 #4775}. The results confirm our prediction that the induction of long-lasting chlamydial immunity is possible and may require the appropriate delivery of a multisubunit vaccine formulation, even in the absence of external adjuvants. The high magnitude of both mucosal and systemic IgA and IgG2a antibodies secreted 2 weeks after primary challenge and the rapid elicitation of high levels of these antibodies as early as 7 days after rechallenge indicates the development of B cell memory that could be recalled 98 days after primary challenge. However, it has been shown that high titers of C. trachomatis-specific antibodies do not correlate with resolution of infection in humans and in fact, are more strongly correlated with increased severity of sequelae of infection, such as tubal infertility in women {Punnonen, 1979 #2720}. It has also been observed that even the antibodies that neutralize chlamydial infectivity in vitro do not necessarily correlate with protection in vivo {Chaganty, 2010 #4777; Igietseme, 2004 #4225}. Thus, although likely important, the precise role that antibodies play in protection against Chlamydia, as well as whether they are involved in the early initiation of protection from infection in the genital tract, remains unclear. Although the relative contribution of antibody in the resolution of a primary infection is not completely understood, recent studies suggest that the predominant role of antibodies in chlamydial clearance is in resistance to re-infection by enhancing rapid Th1 activation {Moore, 2002 #4226;Igietseme, 2004 #4225;Morrison, 2005 #4771}. Also, antibody may mediate protection by blocking the initial attachment of Chlamydia to epithelial cells thereby limiting dissemination to distant sites and enhancing chlamydial clearance. In this regard, recent reports indicate that IgA and IgG antibodies to nonlinear epitopes of MOMP can protect mice against both mucosal and systemic primary chlamydial infections in the presence or absence of T and B cells {Pal, 2008 #4779;Pal, 1997 #4780}. Consequently, the functional ability of vaccine-induced antibodies may be more appropriately defined by the quality and not necessarily the quantity of the elicited antibodies. It has thus been suggested that a future anti-chlamydial vaccine should elicit both antibody and T cell-mediated immune responses for optimal memory response and vaccine efficacy {Eko, 2004 #4135;Pal, 2008 #4779}. The induction of the Th1-associated IgG2a isotype has been previously shown to be associated with protection against Chlamydia {Igietseme, 1998 #1512}. The higher magnitude of chlamydial-specific IFN-γ and IgG2a responses in the spleen and serum, respectively compared to the genital tract, suggests a higher frequency of antigen-responsive immune effectors in the systemic rather than the mucosal compartment. This result is consistent with findings in humans showing that the dominant immunoglobulin isotype found in the cervico-vaginal fluid of the female genital tract is IgG rather than secretory IgA {Mestecky, 2005 #4703; Agrawal, 2009 #4785}. This implies that the immune effectors that mediate chlamydial clearance in the genital tract originate from immune inductive sites outside the genital tract.
Of the various formulations of chlamydial vaccine antigens tested in the mouse model of genital infection, the chlamydial MOMP has been the leading candidate to date and has been delivered by various delivery systems and routes {Berry, 2004 #4327;Eko, 2003 #4099;Pal, 2002 #4144}. However, experience with purified or recombinant MOMP as a protective antigen in several animal models {Su, 1995 #3366;Eko, 2003 #4099;Pal, 2001 #4145} indicated that MOMP alone may be inadequate, suggesting a need for a multisubunit approach or a more effective delivery system that will optimize the protective immune response. We have previously shown that intramuscular immunization of mice with an rVCG multisubunit vaccine construct co-expressing MOMP and PorB induced significant (p<0.05) protective immunity three weeks after immunization compared to controls {Ifere, 2007 #4500}. Furthermore, immunized mice challenged with a high dose of live chlamydiae were protected from Chlamydia induced infertility. In the present study, we demonstrated that the rVCG-PmpD/PorB-immunized animals successfully resolved a genital challenge infection by day 12 after challenge. In this study, we compared the level of protection of the rVCG multisubunit candidate vaccine with that conferred by the IL-10 deficient (IL-10KO) dendritic cell (DC)-based cellular vaccine {Igietseme, 2000 #1516}, the only vaccine to produce a sterilizing long-term immunity as well as a live C. muridarum (MoPn) infection in a mouse genital infection model. Although the IL-10KO DC vaccine provided the best protection, the level of protection afforded by the rVCG-based multisubunit vaccine was remarkable compared to controls and moderately better than that generated by a live chlamydial infection. The significant reduction in the number of recoverable chlamydial IFUs and shortening of the time taken to clear the challenge infection further underlines the vaccine potential of rVCG. The primary challenge infection stimulated a sustained chlamydial-specific anamnestic response to the vaccine antigens that could be recalled 14 weeks later, and capable of inducing high levels of humoral and cell-mediated responses. These results further support our hypothesis that rVCG-based multisubunit chlamydial vaccines could potentially induce long lasting protection against genital chlamydial infection. A previous study reported that long-term protection in the genital tract against C. trachomatis infection is conveyed by IFN-γ-producing CD4+ memory T cells, which appear to be maintained in the absence of antibodies and local antigen deposition {Johansson, 2001 #4786}. If this multisubunit chlamydial vaccine is subsequently confirmed to afford long-term protection against live chlamydial infection, it would likely be due to a combination of antibody-producing B cells and IFN-γ-producing CD4+ T cell memory, among other factors.
In conclusion, this study clearly demonstrates that the rVCG multisubunit candidate vaccine effectively stimulates specific anamnestic mucosal and systemic immune responses after genital chlamydial reinfection. Furthermore, the vaccine antigens are expressed in the bacterial cell envelop in their native form and induction of high levels of immune responses and protection does not require addition of supplementary adjuvants. These properties are invaluable for the rapid development and production of a cost-effective chlamydial vaccine.
Acknowledgements
This work was supported by a Public Health Service grant AI41231 from the National Institutes of Health. The investigation was conducted in a facility constructed with support from Research Facilities Improvement Grant #1 C06 RR18386 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schachter J, Grayston JT. Epidemiology of Human Chlamydial Infections. In: Stephens RS, Byrne GI, Christiansen G, Clarke IN, Grayston JT, Rank RG, et al., editors. Chlamydial Infections. San Francisco, CA: Berkeley; 1998. pp. 3–10. [Google Scholar]
- 2.Brunham RC, Zhang DJ. Transgene as vaccine for Chlamydia. Am Heart J. 1999;138:S519–S522. doi: 10.1016/s0002-8703(99)70291-7. [DOI] [PubMed] [Google Scholar]
- 3.Igietseme JU, Eko FO, Black CM. Contemporary approaches to designing and evaluating vaccines against Chlamydia. Expert Rev Vaccines. 2003;2(1):129–146. doi: 10.1586/14760584.2.1.129. [DOI] [PubMed] [Google Scholar]
- 4.Stagg AJ. Vaccines against Chlamydia: approaches and progress. Mol Med Today. 1998;4(4):166–173. doi: 10.1016/s1357-4310(98)01232-5. [DOI] [PubMed] [Google Scholar]
- 5.Igietseme JU, Black CM, Caldwell HD. Chlamydia vaccines: strategies and status. BioDrugs. 2002;16(1):19–35. doi: 10.2165/00063030-200216010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Morrison R, Caldwell H. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70(6):2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect Immun. 2000;68(12):6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore T, Ananaba GA, Bolier J, Bowers S, Belay T, Eko FO, et al. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology. 2002;105(2):213–221. doi: 10.1046/j.0019-2805.2001.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of T-cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Review of Vaccines. 2004;3(1):23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 10.Brunham RC, Peeling RW. Chlamydia trachomatis antigens: role in immunity and pathogenesis. Infect Agents Dis. 1994;3:218–233. [PubMed] [Google Scholar]
- 11.Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, et al. Genome sequence of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000;28(6):1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282(5389):754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 13.Stephens RS. Chlamydial Genomics and Vaccine Antigen Discovery. J Infect Dis. 2000;181 Suppl 3:S521–S523. doi: 10.1086/315631. [DOI] [PubMed] [Google Scholar]
- 14.Longbottom D, Russell M, Dunbar SM, Jones GE, Herring AJ. Molecular Cloning and Characterization of the Genes Coding for the Highly Immunogenic Cluster of 90-Kilodalton Envelope Proteins from the Chlamydia psittaci Subtype That Causes Abortion in Sheep. Infect Immun. 1998 April 1;66(4):1317–1324. doi: 10.1128/iai.66.4.1317-1324.1998. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimwood J, Olinger L, Stephens RS. Expression of Chlamydia pneumoniae Polymorphic Membrane Protein Family Genes. Infect Immun. 2001 April 1;69(4):2383–2389. doi: 10.1128/IAI.69.4.2383-2389.2001. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niessner A, Kaun C, Zorn G, Speidl W, Türel Z, Christiansen G, et al. Polymorphic membrane protein (PMP) 20 and PMP 21 of Chlamydia pneumoniae induce proinflammatory mediators in human endothelial cells in vitro by activation of the nuclear factor-kappaB pathway. J Infect Dis. 2003;188(1):108–113. doi: 10.1086/375827. [DOI] [PubMed] [Google Scholar]
- 17.Kubo A, Stephens RS. Characterization and functional analysis of PorB, a Chlamydia porin and neutralizing target. Molecular Microbiology. 2000;38(4):772–780. doi: 10.1046/j.1365-2958.2000.02167.x. [DOI] [PubMed] [Google Scholar]
- 18.Kubo A, Stephens RS. Substrate-specific diffusion of select dicarboxylates through Chlamydia trachomatis PorB. Microbiology. 2001 Nov 1;147(11):3135–3140. doi: 10.1099/00221287-147-11-3135. [DOI] [PubMed] [Google Scholar]
- 19.Kawa DE, Stephens RS. Antigenic Topology of Chlamydial PorB Protein and Identification of Targets for Immune Neutralization of Infectivity. J Immunol. 2002 May 15;168(10):5184–5191. doi: 10.4049/jimmunol.168.10.5184. 2002. [DOI] [PubMed] [Google Scholar]
- 20.Crane DD, Carlson JH, Fischer ER, Bavoil P, Hsia R-c, Tan C, et al. From the Cover: Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. PNAS. 2006 February 7;103(6):1894–1899. doi: 10.1073/pnas.0508983103. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wehrl W, Brinkmann V, Jungblut PR, Meyer TF, Szczepek AJ. From the inside out - processing of the Chlamydial autotransporter qPmpD and its role in bacterial adhesion and activation of human host cells. Molecular Microbiology. 2004;51(2):319–334. doi: 10.1046/j.1365-2958.2003.03838.x. [DOI] [PubMed] [Google Scholar]
- 22.Kawa DE, Schachter J, Stephens RS. Immune response to the Chlamydia trachomatis outer membrane protein PorB. Vaccine. 2004 Oct 22;22(31–32):4282–4286. doi: 10.1016/j.vaccine.2004.04.035. 2004. [DOI] [PubMed] [Google Scholar]
- 23.Ifere G, He Q, Ananaba G, Lyn D, Lubitz W, Kellar K, et al. Immunogenicity and protection against genital Chlamydia infection and its complications by a multisubunit candidate vaccine. J Microbiol Immunol Infect. 2007;40:188–200. [PubMed] [Google Scholar]
- 24.Eko F, Lubitz W, McMillan L, Ramey K, Moore T, Ananaba GA, et al. Recombinant Vibrio cholerae ghosts as a delivery vehicle for vaccinating against Chlamydia trachomatis. Vaccine. 2003;21:1694–1703. doi: 10.1016/s0264-410x(02)00677-1. [DOI] [PubMed] [Google Scholar]
- 25.Eko FO, He Q, Brown T, McMillan L, Ifere GO, Ananaba GA, et al. A Novel Recombinant Multisubunit Vaccine against Chlamydia. J Immunol. 2004 September 1;173(5):3375–3382. doi: 10.4049/jimmunol.173.5.3375. 2004. [DOI] [PubMed] [Google Scholar]
- 26.Ekong EE, Okenu D, Mania-Pramanik J, He Q, Igietseme J, Ananaba G, et al. A Vibrio cholerae ghost-based subunit vaccine induces cross-protective chlamydial immunity that is enhanced by CTA2B, the nontoxic derivative of cholera toxin. FEMS Immunology & Medical Microbiology. 2009;55(2):280–291. doi: 10.1111/j.1574-695X.2008.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsey KH, Soderberg LSF, Rank RG. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. InfectImmun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eko F, Ekong E, Okenu D, He Q, Ananaba G, Black C, et al. Annu Meet Am Soc Microbiol. San Diego: 2010. May 23–27;, A multisubunit chlamydial vaccine generates broad-based protective immunity. 2010. [Google Scholar]
- 29.Eko FO, Mayr UB, Attridge SR, Lubitz W. Characterization and immunogenicity of Vibrio cholerae ghosts expressing toxin-coregulated pili. JBiotechnol. 2000;83:115–123. doi: 10.1016/s0168-1656(00)00315-1. [DOI] [PubMed] [Google Scholar]
- 30.Igietseme JU, Uriri IM, Kumar SN, Ananaba GA, Ojior OO, Momodu IA, et al. Route of Infection That Induces a High Intensity of Gamma Interferon-Secreting T Cells in the Genital Tract Produces Optimal Protection Against Chlamydia trachomatis Infection in Mice. Infect Immun. 1998;66(9):4030–4035. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macmillan L, Ifere G, He Q, Igietseme J, Kellar K, Okenu D, et al. A recombinant multivalent combination vaccine protects against Chlamydia and genital herpes. FEMS Immunol Med Microbiol. 2007;49:46–55. doi: 10.1111/j.1574-695X.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 32.Igietseme JU, Rank RG. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. InfectImmun. 1991;59:1346–1351. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kees U, Kynast G, Weber E, Krammer PH. A method for testing the specificity of influenz A virus-reactive memory cytotoxic T lymphocytes (CTL) clones in limiting dilution cultures. JImmunolMethods. 1984;69:215–227. doi: 10.1016/0022-1759(84)90320-x. [DOI] [PubMed] [Google Scholar]
- 34.Igietseme JU, Ananaba GA, Bolier J, Bowers S, Moore T, Belay T, et al. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for enhanced specific Th1 induction: potential for cellular vaccine development. J Immunol. 2000;164(4):4212–4219. doi: 10.4049/jimmunol.164.8.4212. [DOI] [PubMed] [Google Scholar]
- 35.Murdin AD, Su H, Klein MH, Caldwell HD. Poliovirus hybrids expressing neutralization epitopes from variable domains I and IV of the major outer membrane protein of Chlamydia trachomatis elicit broadly cross-reactive C. trachomatis-neutralizing antibodies. Infect Immun. 1995;63(3):1116–1121. doi: 10.1128/iai.63.3.1116-1121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starnbach MN, Loomis WP, Ovendale P, Regan D, Hess B, Alderson MR, et al. An Inclusion Membrane Protein from Chlamydia trachomatis Enters the MHC Class I Pathway and Stimulates a CD8+ T Cell Response. J Immunol. 2003 November 1;171(9):4742–4749. doi: 10.4049/jimmunol.171.9.4742. 2003. [DOI] [PubMed] [Google Scholar]
- 37.Singh J, Pandit S, Bramwell VW, Alpar HO. Diphtheria toxoid loaded poly-([epsilon]-caprolactone) nanoparticles as mucosal vaccine delivery systems. Methods. 2006;38(2):96. doi: 10.1016/j.ymeth.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Loomis WP, Starnbach MN. T cell responses to Chlamydia trachomatis. Current Opinion in Microbiology. 2002;5(1):87. doi: 10.1016/s1369-5274(02)00291-6. [DOI] [PubMed] [Google Scholar]
- 39.Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal Vaccination with a Secreted Chlamydial Protein Enhances Resolution of Genital Chlamydia muridarum Infection, Protects against Oviduct Pathology, and Is Highly Dependent upon Endogenous Gamma Interferon Production. Infect Immun. 2007 February 1;75(2):666–676. doi: 10.1128/IAI.01280-06. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carey AJ, Peter T, Galit R, Jacqui B, Karen N, Jonathon MH, et al. A Multi-Subunit Chlamydial Vaccine Induces Antibody and Cell-Mediated Immunity in Immunized Koalas (Phascolarctos cinereus): Comparison of Three Different Adjuvants. American Journal of Reproductive Immunology. 2010;63(2):161–172. doi: 10.1111/j.1600-0897.2009.00776.x. [DOI] [PubMed] [Google Scholar]
- 41.Punnonen R, Terho P, Nikkanen V, Meurman O. Chlamydial serology in infertile women by immunofluorescence. FertilSteril. 1979;31:656–659. doi: 10.1016/s0015-0282(16)44056-2. [DOI] [PubMed] [Google Scholar]
- 42.Chaganty BKR, Murthy AK, Evani SJ, Li W, Guentzel MN, Chambers JP, et al. Heat denatured enzymatically inactive recombinant chlamydial protease-like activity factor induces robust protective immunity against genital chlamydial challenge. Vaccine. 2010;28(11):2323. doi: 10.1016/j.vaccine.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison S, Morrison R. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005 Dec 1;175(11):7536–7542. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pal S, Bravo J, Peterson EM, de la Maza LM. Protection of Wild-Type and Severe Combined Immunodeficiency Mice against an Intranasal Challenge by Passive Immunization with Monoclonal Antibodies to the Chlamydia trachomatis Mouse Pneumonitis Major Outer Membrane Protein. Infect Immun. 2008 December 1;76(12):5581–5587. doi: 10.1128/IAI.00574-08. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal S, Theodor I, Peterson EM, de la Maza LM. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chalamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997;15(5):575. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 46.Barry M, Johnston S. Biological features of genetic immunization. Vaccine. 1997;15:788–791. doi: 10.1016/s0264-410x(96)00265-4. [DOI] [PubMed] [Google Scholar]
- 47.Mestecky J, Moldoveanu Z, Russell MW. Immunologic Uniqueness of the Genital Tract: Challenge for Vaccine Development. American Journal of Reproductive Immunology. 2005;53(5):208. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 48.Agrawal T, Vats V, Salhan S, Mittal A. The mucosal immune response to Chlamydia trachomatis infection of the reproductive tract in women. J R Immunol. 2009 doi: 10.1016/j.jri.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 49.He Q, M-Sobrido L, Eko FO, Palese P, Garcia-Sastre A, Lyn D, Okenu D, Bandea C, Ananaba GA, Black CM, Igietseme JU. Live-attenuated influenza viruses as delivery vectors for Chlamydia vaccines. Immunology. 2007;122(1):28–37. doi: 10.1111/j.1365-2567.2007.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng C, Bettahi I, Cruz-Fisher MI, Pal S, Jain P, Jia Z, et al. Induction of protective immunity by vaccination against Chlamydia trachomatis using the major outer membrane protein adjuvanted with CpG oligodeoxynucleotide coupled to the nontoxic B subunit of cholera toxin. Vaccine. 2009;27(44):6239. doi: 10.1016/j.vaccine.2009.07.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pal S, Davis HL, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis Mouse Pneumonitis Major Outer Membrane Protein by Use of CpG Oligodeoxynucleotides as an Adjuvant Induces a Protective Immune Response against an Intranasal Chlamydial Challenge. Infect Immun. 2002 September 1;70(9):4812–4817. doi: 10.1128/IAI.70.9.4812-4817.2002. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berry LJ, Hickey DK, Skelding KA, Bao S, Rendina AM, Hansbro PM, et al. Transcutaneous Immunization with Combined Cholera Toxin and CpG Adjuvant Protects against Chlamydia muridarum Genital Tract Infection. Infect Immun. 2004 February 1;72(2):1019–1028. doi: 10.1128/IAI.72.2.1019-1028.2004. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su H, Parnell M, Caldwell HD. Protective efficacy of a parenterally administered MOMP-derived synthetic oligopeptide vaccine in a murine model of Chlamydia trachomatis genital tract infection: serum neutralizing IgG antibodies do not protect against genital tract infection. Vaccine. 1995;13(11):1023–1032. doi: 10.1016/0264-410x(95)00017-u. [DOI] [PubMed] [Google Scholar]
- 54.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis Mouse Pneumonitis Major Outer Membrane Protein Can Elicit a Protective Immune Response against a Genital Challenge. Infect Immun. 2001 October 1;69(10):6240–6247. doi: 10.1128/IAI.69.10.6240-6247.2001. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansson M, Lycke N. Immunological memory in B-cell-deficient mice conveys long-lasting protection against genital tract infection with Chlamydia trachomatis by rapid recruitment of T cells. Immunology. 2001 Dec;102(2):199–208. doi: 10.1046/j.1365-2567.2001.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]