Abstract
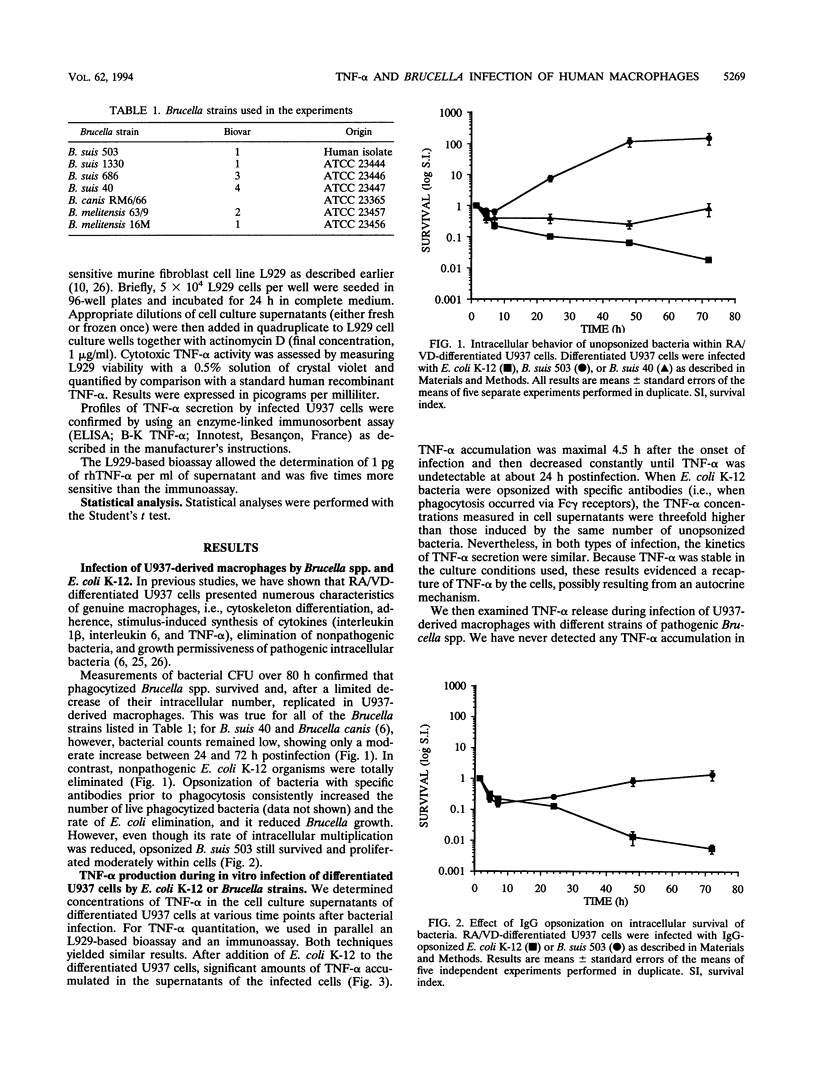
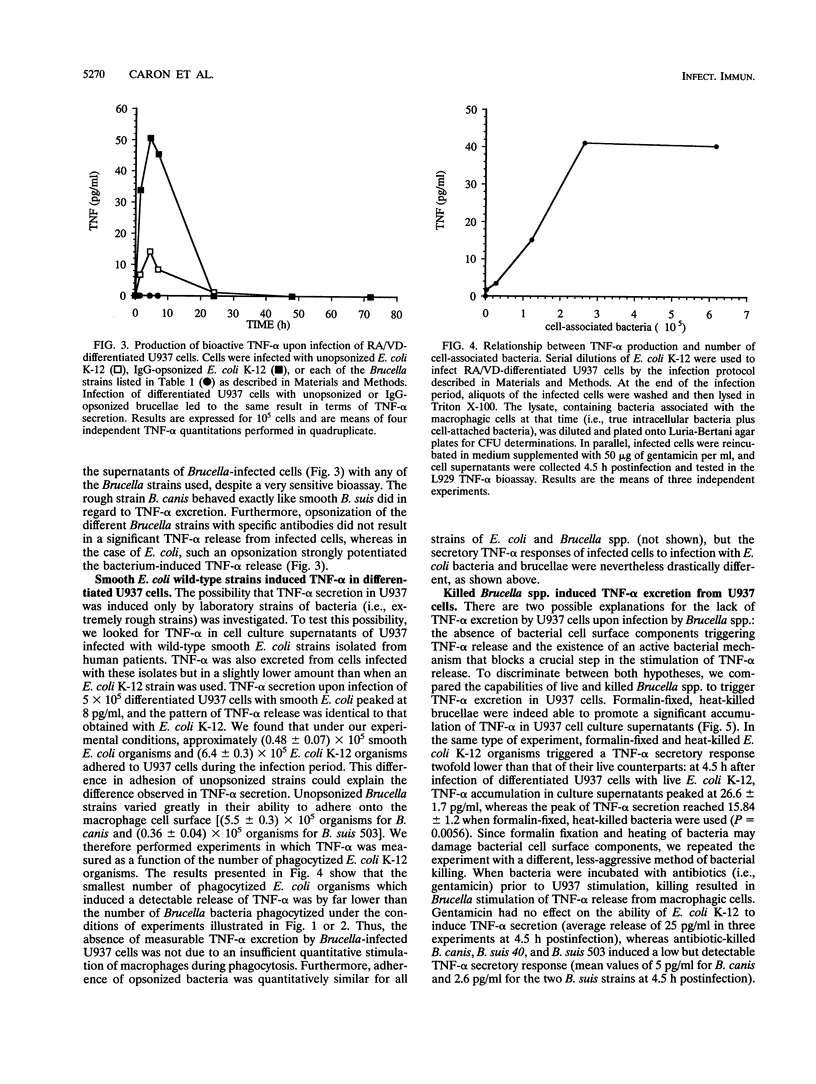
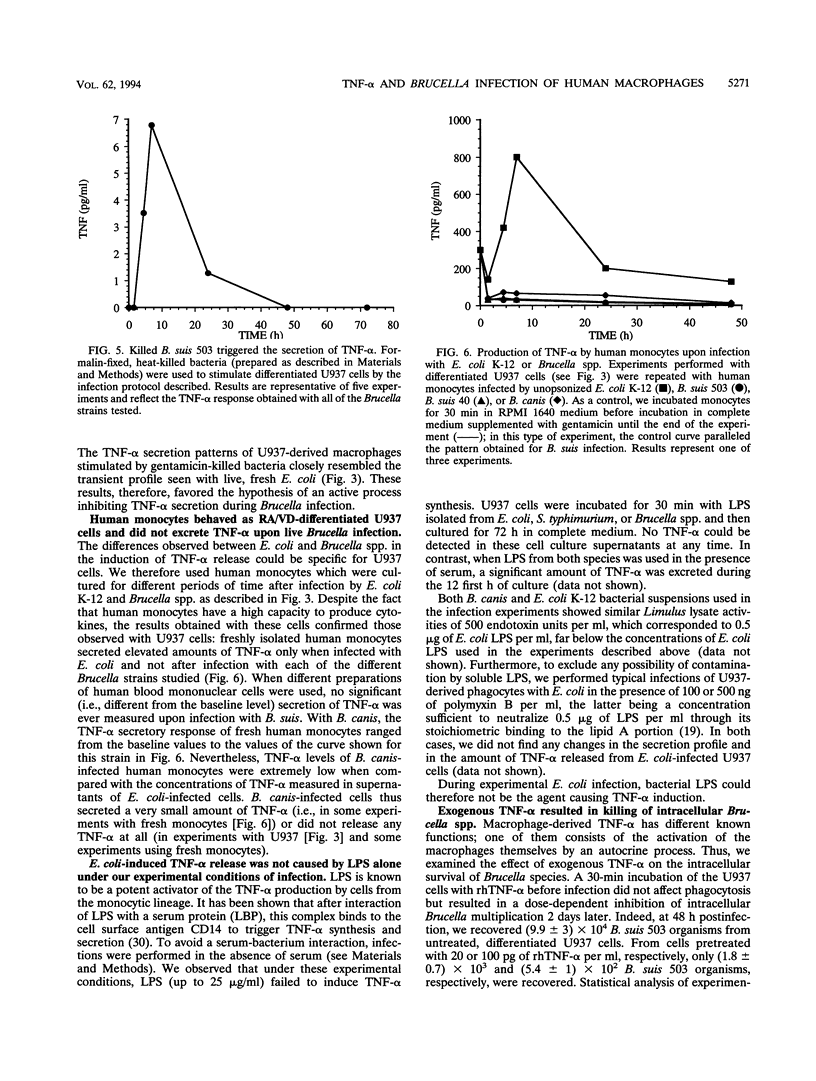
Tumor necrosis factor alpha (TNF-alpha) plays a central role in activation of first-line defenses of a host against foreign organisms. To determine whether Brucella infection modulated TNF-alpha production, we measured the biological activity of this cytokine in supernatants of U937 cell-derived macrophages and of fresh human monocytes infected with Brucella spp. Neither the smooth nor rough Brucella strains used induced any measurable TNF-alpha excretion upon infection. On the contrary, as reported before for other gram-negative bacteria, phagocytosis of nonpathogenic Escherichia coli was followed by a rapid and transient induction of TNF-alpha release, suggesting an involvement of this cytokine in some autocrine process. As expected, the Brucella strains tested survived and/or multiplied within U937-derived macrophages, whereas E. coli was rapidly eliminated after phagocytosis. Immunoglobulin G opsonization of E. coli strains enhanced their intracellular killing and strongly potentiated TNF-alpha secretion. Immunoglobulin G opsonization of Brucella strains, in contrast, did not lead to TNF-alpha production, although their rate of intracellular multiplication was reduced. Killed brucellae, however, promoted a significant excretion of TNF-alpha from U937-derived macrophages into cell culture supernatants. We finally demonstrated that pretreatment of U937-derived macrophages with exogenous TNF-alpha significantly inhibited intracellular multiplication of Brucella spp. These results and experiments performed on fresh human monocytes or with isolated lipopolysaccharide (LPS) showed that (i) differences in TNF-alpha production observed during macrophage infection by Brucella spp. and E. coli were not due to differences in LPS structure but resulted from active inhibition of TNF-alpha production by a specific process linked to Brucella spp. and (ii) the capacity of Brucella spp. to use pathways avoiding TNF-alpha production during infection may be considered a major attribute of virulence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman M. H., Wong S. Y., Remington J. S. Cytokines, Toxoplasma and intracellular parasitism. Immunol Rev. 1992 Jun;127:97–117. doi: 10.1111/j.1600-065x.1992.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E. Production of transforming growth factor-beta by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-gamma. J Immunol. 1993 Mar 1;150(5):1838–1845. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Blanchard D. K., Friedman H., Klein T. W., Djeu J. Y. Induction of interferon-gamma and tumor necrosis factor by Legionella pneumophila: augmentation of human neutrophil bactericidal activity. J Leukoc Biol. 1989 Jun;45(6):538–545. doi: 10.1002/jlb.45.6.538. [DOI] [PubMed] [Google Scholar]
- Canning P. C., Roth J. A., Deyoe B. L. Release of 5'-guanosine monophosphate and adenine by Brucella abortus and their role in the intracellular survival of the bacteria. J Infect Dis. 1986 Sep;154(3):464–470. doi: 10.1093/infdis/154.3.464. [DOI] [PubMed] [Google Scholar]
- Caron E., Liautard J. P., Köhler S. Differentiated U937 cells exhibit increased bactericidal activity upon LPS activation and discriminate between virulent and avirulent Listeria and Brucella species. J Leukoc Biol. 1994 Aug;56(2):174–181. doi: 10.1002/jlb.56.2.174. [DOI] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Gan H., Newman G., McCarthy P. L., Remold H. G. TNF-alpha response of human monocyte-derived macrophages to Mycobacterium avium, serovar 4, is of brief duration and protein kinase C dependent. J Immunol. 1993 Apr 1;150(7):2892–2900. [PubMed] [Google Scholar]
- Goldstein J., Hoffman T., Frasch C., Lizzio E. F., Beining P. R., Hochstein D., Lee Y. L., Angus R. D., Golding B. Lipopolysaccharide (LPS) from Brucella abortus is less toxic than that from Escherichia coli, suggesting the possible use of B. abortus or LPS from B. abortus as a carrier in vaccines. Infect Immun. 1992 Apr;60(4):1385–1389. doi: 10.1128/iai.60.4.1385-1389.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Baldwin C. L. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993 Jan;61(1):124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Leonard B., Benson R., Baldwin C. L. Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell Immunol. 1993 Oct 15;151(2):309–319. doi: 10.1006/cimm.1993.1241. [DOI] [PubMed] [Google Scholar]
- Kaye P. M., Bancroft G. J. Leishmania donovani infection in scid mice: lack of tissue response and in vivo macrophage activation correlates with failure to trigger natural killer cell-derived gamma interferon production in vitro. Infect Immun. 1992 Oct;60(10):4335–4342. doi: 10.1128/iai.60.10.4335-4342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly N. M., Young L., Cross A. S. Differential induction of tumor necrosis factor by bacteria expressing rough and smooth lipopolysaccharide phenotypes. Infect Immun. 1991 Dec;59(12):4491–4496. doi: 10.1128/iai.59.12.4491-4496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsiota-Bernard P., Léfèbre C., Sedqui M., Cornillet P., Guenounou M. Involvement of tumor necrosis factor alpha in intracellular multiplication of Legionella pneumophila in human monocytes. Infect Immun. 1993 Dec;61(12):4980–4983. doi: 10.1128/iai.61.12.4980-4983.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. C., Isa S., Vannier E., Georgilis K., Steere A. C., Dinarello C. A. Live Borrelia burgdorferi preferentially activate interleukin-1 beta gene expression and protein synthesis over the interleukin-1 receptor antagonist. J Clin Invest. 1992 Sep;90(3):906–912. doi: 10.1172/JCI115966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Nash T. W., Libby D. M., Horwitz M. A. IFN-gamma-activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1988 Jun 1;140(11):3978–3981. [PubMed] [Google Scholar]
- Parmely M. J., Zhou W. W., Edwards C. K., 3rd, Borcherding D. R., Silverstein R., Morrison D. C. Adenosine and a related carbocyclic nucleoside analogue selectively inhibit tumor necrosis factor-alpha production and protect mice against endotoxin challenge. J Immunol. 1993 Jul 1;151(1):389–396. [PubMed] [Google Scholar]
- Saha A. K., Mukhopadhyay N. K., Dowling J. N., Ficht T. A., Adams L. G., Glew R. H. Characterization of a phosphomonoesterase from Brucella abortus. Infect Immun. 1990 May;58(5):1153–1158. doi: 10.1128/iai.58.5.1153-1158.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staugas R. E., Harvey D. P., Ferrante A., Nandoskar M., Allison A. C. Induction of tumor necrosis factor (TNF) and interleukin-1 (IL-1) by Pseudomonas aeruginosa and exotoxin A-induced suppression of lymphoproliferation and TNF, lymphotoxin, gamma interferon, and IL-1 production in human leukocytes. Infect Immun. 1992 Aug;60(8):3162–3168. doi: 10.1128/iai.60.8.3162-3168.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Taimi M., Chateau M. T., Cabane S., Marti J. Synergistic effect of retinoic acid and 1,25-dihydroxyvitamin D3 on the differentiation of the human monocytic cell line U937. Leuk Res. 1991;15(12):1145–1152. doi: 10.1016/0145-2126(91)90183-t. [DOI] [PubMed] [Google Scholar]
- Taimi M., Defacque H., Commes T., Favero J., Caron E., Marti J., Dornand J. Effect of retinoic acid and vitamin D on the expression of interleukin-1 beta, tumour necrosis factor-alpha and interleukin-6 in the human monocytic cell line U937. Immunology. 1993 Jun;79(2):229–235. [PMC free article] [PubMed] [Google Scholar]
- Wewers M. D., Herzyk D. J. Alveolar macrophages differ from blood monocytes in human IL-1 beta release. Quantitation by enzyme-linked immunoassay. J Immunol. 1989 Sep 1;143(5):1635–1641. [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zheng L., Nibbering P. H., van Furth R. Stimulation of the intracellular killing of Staphylococcus aureus by human monocytes mediated by Fc gamma receptors I and II. Eur J Immunol. 1993 Nov;23(11):2826–2833. doi: 10.1002/eji.1830231116. [DOI] [PubMed] [Google Scholar]
- van Heeckeren A. M., Rikihisa Y., Park J., Fertel R. Tumor necrosis factor alpha, interleukin-1 alpha, interleukin-6, and prostaglandin E2 production in murine peritoneal macrophages infected with Ehrlichia risticii. Infect Immun. 1993 Oct;61(10):4333–4337. doi: 10.1128/iai.61.10.4333-4337.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]



