Abstract
Background
Long QT syndrome (LQTS) is an inherited ion channel disorder manifesting with prolongation of the cardiac repolarization phase and severe ventricular arrhythmias. The common KCNE1 D85N potassium channel variant prolongs QT interval by inhibiting IKs (KCNQ1) and IKr (KCNH2) currents and is therefore a suitable candidate for a modifier gene in LQTS.
Methods
We studied the effect of D85N on age-, sex-, and heart rate-adjusted QT-interval duration by linear regression in LQTS patients carrying the Finnish founder mutations KCNQ1 G589D (n = 492), KCNQ1 IVS7-2A>G (n = 66), KCNH2 L552S (n = 73), and KCNH2 R176W (n = 88). We also investigated the association between D85N and clinical variables reflecting the severity of the disease.
Results
D85N was associated with a QT prolongation by 26 ms (SE 8.6, p = 0.003) in males with KCNQ1 G589D (n = 213), but not in females with G589D (n = 279). In linear regression, the interaction between D85N genotype and sex was significant (p = 0.028). Within the KCNQ1 G589D mutation group, KCNE1 D85N carriers were more often probands of the family (p = 0.042) and were more likely to use beta blocker medication (p = 0.010) than non-carriers. The number of D85N carriers in other founder mutation groups was too small to assess its effects.
Conclusions
We propose that KCNE1 D85N is a sex-specific QT-interval modifier in type 1 LQTS and may also associate with increased severity of disease. Our data warrant additional studies on the role of KCNE1 D85N in other genetically homogeneous groups of LQTS patients.
Background
Long QT syndrome (LQTS) is an inherited arrhythmia disorder associated with risk of torsades de pointes, ventricular fibrillation, and sudden death. LQTS is caused by mutations of the ion channel genes controlling the repolarization phase of the cardiac action potential cycle [1,2]. The potassium channel KCNE1, also known as minK, regulates both the voltage-gated slowly activating IKs potassium channel [3,4], encoded by the KCNQ1 gene, and the rapidly activating IKr potassium channel [5], encoded by the KCNH2 gene. Mutations in KCNE1 underlie the LQT5 subtype of LQTS [6] and account for approximately 3% of known LQTS mutations [7]. In homozygous form, KCNE1 mutations may cause sensorineural hearing loss in association with LQTS, or Jervell-Lange-Nielsen syndrome [8].
A common variant D85N of KCNE1 was originally detected by Tesson et al. [9]. This variant has subsequently been shown to slow IKs potassium channel, when studied in Xenopus oocytes [10], and to exhibit significant loss-of-function effects on both the KCNQ1- and KCNH2-mediated potassium currents, as measured in Chinese hamster ovarian cells [11]. In general population, KCNE1 D85N has a minor allele frequency of 0.8-1.4% [11-13] and is associated with a significant prolongation of the electrocardiographic QT interval [12-14]. Moreover, D85N has been detected in many LQTS patients as a second variant in addition to a more severe mutation [10,11], as well as in some individuals with drug-induced LQTS [15].
These findings prompted us to study whether KCNE1 D85N would also modify the QT interval and/or the clinical picture in patients with genetically homogeneous forms of LQTS in which the variability caused by the disease-causing mutation itself can be controlled for. To this end, we took advantage of the unique situation in Finland where four different mutations account for approximately 70% of the known spectrum of LQTS genes [16] and where the prevalence of molecularly defined LQTS appears to be the highest in the world [17]. Our results indicate a sex-specific QT-prolonging effect for D85N in KCNQ1 mutation carriers.
Methods
Patient cohort
The study sample consisted of all available (n = 712) carriers of the Finnish LQTS founder mutations, including 492 carriers of KCNQ1 G589D (5 of them also carried KCNH2 R176W, 1 KCNH2 L552S, and 1 a non-founder nonsense mutation KCNQ1 Y171X), 66 carriers of KCNQ1 IVS7-2A>G (1 of them also carried KCNH2 R176W), 73 carriers of KCNH2 L552S (2 of them homozygous), and 88 carriers of KCNH2 R176W. These four founder mutations account for 70%, and KCNQ1 G589D alone 50%, of all Finnish LQTS patients with an established molecular diagnosis of LQTS [16]. The collective prevalence of these four founder mutations in the general Finnish population is as high as 0.4% [17].
Subjects taking any known QT-prolonging medication at the moment of electrocardiogram (ECG) recording were excluded from the study. QT intervals were measured manually by a single cardiologist (H.S.) using the mean of two consecutive QT intervals in lead II in standard 12-lead ECG. Heart rate-corrected QT (QTc) was calculated using the Bazett's formula (QT/√RR). The study was performed in accordance with the Declaration of Helsinki and written informed consent was obtained from all participants. The Ethics Review Committee of the Department of Medicine, University of Helsinki, approved the study.
Molecular genetic analyses
KCNE1 253G>A (D85N, rs1805128) was genotyped in DNA from venous blood samples using polymerase chain reaction with primers 5'-GAGATTGGAGTGGTGGATGGA-3' and 5'-CACCCCTTACAACAGCCAAAA-3' followed by Lwe I digestion (Fermentas, Ontario, Canada) and agarose gel electrophoresis. Previously identified heterozygous and major and minor homozygous samples served as controls in the assay.
Statistical analyses
Normality of continuous distributions was reviewed visually. Non-normally distributed age was normalized by Blom's method. The effect of KCNE1 D85N on age-, sex-, and heart rate (RR interval) -adjusted QT interval was studied by linear regression analysis using 1-df additive model (genotype coded as D85D = 0, D85N = 1, N85N = 2). Because gender differences were detected, an additional model included also a multiplicative interaction term between KCNE1 D85N genotype and sex. In KCNQ1 G589D carriers, association of D85N with proband/family member status (information available for all 492 G589D carriers), appearance of syncope (information available, n = 488), use of beta blocker medication (information available, n = 347), and occurrence of pacemaker or implantable cardioverter-defibrillator (information available, n = 349) was studied by Fisher exact test. Statistical analyses were performed with SPSS 17.0 (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL, USA). A p value < 0.05 was considered statistically significant.
Results
Effect of KCNE1 D85N on QT interval in LQTS founder mutation carriers
Of the 712 LQTS founder mutation carriers, 689 had the KCNE1 genotype D85D, 21 had the heterozygous genotype D85N, and 2 were minor homozygotes (N85N) (Table 1). The frequency of the N85 allele was 1.8%, which is slightly higher than the corresponding frequency in the general Finnish population (1.4%) [12] and in other reported populations (0.8-1.0%) [11,13]. Of the 21 D85N heterozygotes, 15 had the KCNQ1 G589D mutation, 2 had KCNH2 L552S, 3 had KCNH2 R176W, and 1 was a double heterozygote for the KCNQ1 G589D and KCNH2 R176W mutations. None of the KCNQ1 IVS7-2A>G mutation carriers had the minor N85 allele. The two N85N minor homozygotes (boys aged 4 and 10) were KCNQ1 G589D carriers and were siblings from the same family. As a whole, the founder mutation carriers were derived from 126 LQTS families. The number of KCNE1 D85N carriers in type 2 LQTS was too small for statistical comparisons and therefore the analyses were confined to KCNQ1 G589D carriers and the combined group of founder mutation carriers.
Table 1.
The clinical characteristics of the study sample
| D85D major homozygotes | D85N heterozygotes | N85N minor homozygotes | |
|---|---|---|---|
| All subjects | |||
| n (%) | 689 (96.8) | 21 (2.9) | 2 (0.3) |
| Age (years) | 29.7 ± 20.0 | 28.7 ± 20.2 | 7.0 ± 4.2 |
| Males (%) | 280 (40.6) | 8 (38.1) | 2 (100) |
| QTc (ms) | 458 ± 36.8 | 472 ± 35.2 | 468 ± 34.6 |
| Founder mutation status | |||
| KCNQ1 G589D | |||
| n | 474a | 16b | 2 |
| QTc | 459 ± 36.2 | 479 ± 31.0 | 468 ± 34.6 |
| KCNQ1 IVS7-2A>G | |||
| n | 66b | 0 | 0 |
| QTc | 465 ± 30.0 | - | - |
| KCNH2 L552S | |||
| n | 71c | 2 | 0 |
| QTc | 464 ± 48.3 | 490 ± 7.1 | - |
| KCNH2 R176W | |||
| n | 84d | 4e | 0 |
| QTc | 445 ± 30.6 | 442 ± 46.5 | - |
The figures represent the mean ± SD. SD, standard deviation; QTc, heart rate-corrected QT interval.
aFour subjects carrying also KCNH2 R176W, one KCNH2 L552S, and one KCNQ1 Y171X.
bOne subject carrying also KCNH2 R176W.
cIncludes two KCNH2 L552S homozygotes and one subject carrying also KCNQ1 G589D.
dFour subjects carrying also KCNQ1 G589D and one KCNQ1 IVS7-2A>G.
eOne subject carrying also KCNQ1 G589D.
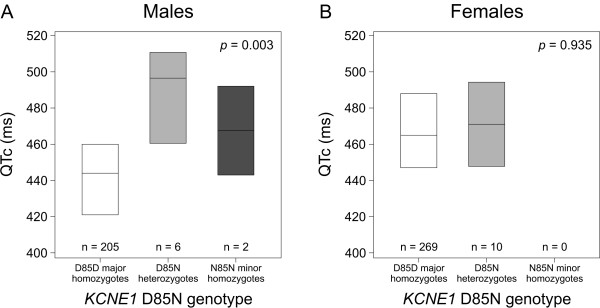
Patients with KCNQ1 G589D (n = 492) was the largest group of founder mutation carriers. A QT-prolonging effect of 26 ms (SE 8.6, p = 0.003) was detected in males with G589D (n = 213), whereas D85N did not prolong QT interval in females with G589D (n = 279) (Table 2, Figure 1). In our cohort, all male KCNQ1 G589D carriers with KCNE1 N85 (n = 8) were of age ≤16. To account for the possible confounding effect of age, male G589D carriers ≤16 years were analyzed also as a separate group. Even in this analysis, the effect of N85 allele on QT interval remained essentially the same (+23 ms, SE 8.8, p = 0.010). The majority (9 out of 10) of the female KCNQ1 G589D carriers with KCNE1 D85N were adults ≥18 years (median age 34 years, range 0-59 years). In order to further study the gender specificity of D85N, a multiplicative interaction term for D85N genotype and sex was introduced into the linear model. This interaction term received a significance of p = 0.028, indicating that D85N indeed has a sex-specific effect for QT interval in KCNQ1 G589D carriers.
Table 2.
Effect of KCNE1 D85N on age-, sex-, and heart rate-adjusted QT interval
| Total n | n with D85N | Effect (ms) | SE (ms) | p value | |
|---|---|---|---|---|---|
| All founder mutation carriers | 712 | 23 | +13.1 | 6.0 | 0.028 |
| Malesa | 290 | 10 | +20.1 | 7.7 | 0.010 |
| Femalesa | 422 | 13 | +1.6 | 9.1 | 0.857 |
| KCNQ1 G589D carriers | 492 | 18 | +16.9 | 6.3 | 0.007 |
| Malesa | 213 | 8 | +25.7 | 8.6 | 0.003 |
| Femalesa | 279 | 10 | +0.7 | 9.2 | 0.935 |
SE, standard error.
aQT interval adjusted by age and heart rate.
Figure 1.
QTc-interval duration in different KCNE1 D85N genotype classes in males and females with KCNQ1 G589D. Box plots show medians and interquartile ranges. The p values in linear regression analyses using additive genotypic model are shown for (A) males and (B) females separately. For interaction between D85N genotype and sex, p = 0.028. QTc, heart rate-corrected QT-interval.
When all founder mutation carriers were considered together, KCNE1 D85N was associated with a 13 ms (SE 6.0) prolongation of QT interval per each N85 minor allele (p = 0.028, Table 2). A similar gender-specific analysis revealed that this association was confined to males (Table 2).
Association of KCNE1 D85N with selected clinical variables
The clinical importance of the LQTS-modifying effect of KCNE1 D85N was studied in the largest founder mutation group of KCNQ1 G589D carriers. In these subjects, the proband/family member status, occurrence of syncope, use of beta blocker medication, and use of pacemaker or implantable cardioverter-defibrillator were compared between the D85N heterozygotes and non-carriers (Figure 2). Of the 16 D85N heterozygotes, 5 (31%) were probands, compared to 58 (12%) of the 474 D85D homozygotes (p = 0.042). The proportion of subjects using beta blocker medication was also higher in D85N heterozygotes (81%) than in D85D homozygotes (47%, p = 0.010). The median age at which the medication was started was 15 years (range 0-58 years) for the D85N heterozygotes and 8 years (range 5-11 years) for the N85N minor homozygotes. A similar but non-significant trend for difference between non-carriers and heterozygotes was observed in the occurrence of syncope and use of pacemaker or implantable-cardioverter defibrillator (Figure 2).
Figure 2.
Association of the KCNE1 D85N variant with selected clinical variables in KCNQ1 G589D carriers. Percentage of (A) probands, (B) patients having experienced syncope, (C) patients with beta blocker medication, and (D) patients with pacemaker or ICD, in different KCNE1 D85N genotype classes. The p value between D85N heterozygotes and D85D major homozygotes in Fisher exact test is shown above the columns and the number of cases in each genotype group inside the columns. ICD, implantable cardioverter-defibrillator.
Discussion
The KCNE1 D85N variant has been shown to lead to a substantial QT-interval prolongation in the general population [12-14]. In fact, we are not aware of any other gene variant showing a population-based frequency of >1% and an equally large effect on QT interval. In order to test the effect of this variant on cardiac repolarization in patients with LQTS, we studied the association of KCNE1 D85N to QT interval in a homogeneous group of Finnish LQTS founder mutation carriers. We found that the minor N85 allele significantly prolongs age-, sex-, and heart rate-adjusted QT interval in type 1 LQTS patients with the mutation G589D. An interaction between D85N genotype and sex was detected (p = 0.028), and a sex-specific analysis revealed that the N85 allele prolongs QT interval by 26 ms in males but not in females (Table 2). Our material is too small to draw conclusions of the role of D85N in LQTS type 2 caused by KCNH2 mutations.
In this study, we detected a gender difference in the effect of KCNE1 D85N on QT interval in type 1 LQTS. We reported previously that the occurrence of the N85 minor allele was associated with a 10 ms prolongation of the QT interval in the general Finnish population [12]. We carried out a retrospect analysis of our population sample [12] and found that the effect of D85N on QT interval was slightly larger in males (+11.3 ms) than in females (+10.0 ms). Gender is known to influence QT interval at the population level, with females in general showing longer QT intervals than males [18], and there are sex- and age-specific differences in risk of arrhythmias in the different subtypes of LQTS [19,20]. It is of note that also KCNQ1 G589D presents a gender difference since the female carriers have >20 ms longer QTc compared to the male carriers [21], a finding replicated in the present study (Figure 1, D85D homozygotes). KCNE1 D85N appears to invert this sex difference. Interestingly, Friedlander et al. [22] found that G38S, another polymorphism of KCNE1, was associated with QT-interval variation in healthy males but not in females.
Sex differences in cardiac repolarization appear to result from both transcriptional and non-transcriptional regulatory mechanisms. In female mice hearts, KCNE1 mRNA is more abundant compared to males [23]. Differences in KCNE1 protein levels may therefore influence the sex-specific sensitivity to KCNE1 variants. Sex hormones have also been reported to alter the gating kinetics of cardiac ion channels and to modulate the effects of channel blocking agents [24,25]. For example, 17β-oestradiol inhibits IKs [24] and IKr [25] channels and enhances the effect of a KCNH2 blocker [25]. It is therefore possible that KCNE1 D85N interferes with the binding of sex-specific IKs channel regulators, but the exact mechanisms underlying the sex difference reported in the present study remain to be explored.
In previous studies, KCNE1 D85N has been found to associate with congenital LQTS [10,11]. Compound carriers of two or more LQTS mutations have a longer heart rate-corrected QT interval (QTc) and a more severe phenotype than carriers of only one mutation [10,26]. KCNE1 D85N has also been proposed to cause LQTS in the absence of any documented mutation in known LQTS genes, but the age at onset may be higher and QTc shorter than in patients with a more severe LQTS mutation [11]. In vitro experiments indicate that KCNE1 N85 significantly reduces both IKs and IKr currents, thus delaying repolarization in mammalian cells [11]. This gene variant also contributes to loss of IKs function together with a KCNQ1 mutation in Xenopus oocytes [10]. In another study of mammalian cells, however, D85N did not affect IKs current when co-expressed with KCNQ1 [27].
In the present study, we found that the QT-prolonging effect of KCNE1 D85N is substantially larger in males with the LQTS mutation KCNQ1 G589D (26 ms) than in the general population (10 ms). This result suggests that KCNQ1 and KCNE1 mutations and variants may interact with each other, at least in males, to produce an even more pronounced QT-prolonging effect than when occurring separately. This interaction could be caused by the coassembly of the two proteins to form a functional IKs channel. However, due to small number of subjects with the KCNE1 N85 allele and LQT1 mutations other than KCNQ1 G589D, we cannot draw conclusions on the general applicability of this effect in LQT1, nor provide sufficient data on a possible similar relation in LQT2. It should be emphasized that the association between D85N and QT interval in type 1 LQTS should be replicated in another material. However, we realize that this is not an easy task considering the population prevalence of D85N. Thus, any replication material should contain hundreds of genetically uniform patients with type 1 LQTS.
Our study also provides evidence that the KCNE1 D85N variant may be associated with a more severe phenotype of type 1 LQTS, as the proportions of probands and users of beta blocker medication were significantly higher in D85N heterozygotes than in non-carriers (Figure 2). Indirectly, these associations suggest that D85N carriers may seek medical help more often than non-carriers. A similar but non-significant trend was detected in the occurrence of syncope and use of pacemaker or implantable cardioverter-defibrillator. Paulussen et al. [15] identified 2 carriers of the N85 allele among 32 patients with drug-induced LQTS, while no such carriers were observed in the series of 34 patients with torsades de pointes studied by Mank-Seymour et al. [28]. Sotoodehnia et al. [29] suggested that D85N could be associated with a higher death rate in males but not in females. Clearly, more extensive population-based studies are required in which the role KCNE1 D85N variation as determinant of life-threatening and fatal arrhythmias will be explored. Polymorphisms in another gene, NOS1AP, have also been associated with QT prolongation and cardiac events in LQTS patients [30].
Conclusions
Our study suggests that KCNE1 D85N variation has a gender-dependent QT-prolonging effect in KCNQ1 G589D mutation carriers and could thus complicate the symptoms of LQTS. Previously, this variant has not been studied in vivo in a large cohort of LQTS mutation carriers. As KCNE1 D85N is relatively frequent occurring in 2-3% of the general population, this interaction between KCNQ1 and KCNE1 could ultimately have direct implications in counseling of LQTS patients. However, future studies are required to assess the direct risks of arrhythmias and/or sudden death in LQTS patients carrying KCNE1 N85.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AML participated in the design of the study, performed the molecular genetic and statistical analyses, and drafted the manuscript. AM participated in the design of the study, data analysis, and writing of the manuscript. HS collected the patient material and participated in the design of the study, data analysis, and writing of the manuscript. KK participated in the design of the study, data analysis, and writing of the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Annukka M Lahtinen, Email: annukka.lahtinen@helsinki.fi.
Annukka Marjamaa, Email: annukka.marjamaa@helsinki.fi.
Heikki Swan, Email: heikki.swan@hus.fi.
Kimmo Kontula, Email: kimmo.kontula@hus.fi.
Acknowledgements
The authors acknowledge Susanna Saarinen, Hanna Nieminen, and Hanna Ranne for their expert technical assistance. Financial support was received from The Sigrid Jusélius Foundation, The Academy of Finland, The Finnish Foundation for Cardiovascular Research, The Finnish Cultural Foundation (to AML), and The Emil Aaltonen Foundation (to AM).
References
- Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet. 2008;372:750–763. doi: 10.1016/S0140-6736(08)61307-0. [DOI] [PubMed] [Google Scholar]
- Hedley PL, Jørgensen P, Schlamowitz S, Wangari R, Moolman-Smook J, Brink PA, Kanters JK, Corfield VA, Christiansen M. The genetic basis of long QT and short QT syndromes: a mutation update. Hum Mutat. 2009;30:1486–1511. doi: 10.1002/humu.21106. [DOI] [PubMed] [Google Scholar]
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- McDonald TV, Yu Z, Ming Z, Palma E, Meyers MB, Wang KW, Goldstein SA, Fishman GI. A minK-HERG complex regulates the cardiac potassium current I(Kr) Nature. 1997;388:289–292. doi: 10.1038/40882. [DOI] [PubMed] [Google Scholar]
- Splawski I, Tristani-Firouzi M, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- Tyson J, Tranebjaerg L, Bellman S, Wren C, Taylor JF, Bathen J, Aslaksen B, Sørland SJ, Lund O, Malcolm S, Pembrey M, Bhattacharya S, Bitner-Glindzicz M. IsK and KvLQT1: mutation in either of the two subunits of the slow component of the delayed rectifier potassium channel can cause Jervell and Lange-Nielsen syndrome. Hum Mol Genet. 1997;6:2179–2185. doi: 10.1093/hmg/6.12.2179. [DOI] [PubMed] [Google Scholar]
- Tesson F, Donger C, Denjoy I, Berthet M, Bennaceur M, Petit C, Coumel P, Schwarts K, Guicheney P. Exclusion of KCNE1 (IsK) as a candidate gene for Jervell and Lange-Nielsen syndrome. J Mol Cell Cardiol. 1996;28:2051–2055. doi: 10.1006/jmcc.1996.0198. [DOI] [PubMed] [Google Scholar]
- Westenskow P, Splawski I, Timothy KW, Keating MT, Sanguinetti MC. Compound mutations: a common cause of severe long-QT syndrome. Circulation. 2004;109:1834–1841. doi: 10.1161/01.CIR.0000125524.34234.13. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Makiyama T, Itoh H, Sakaguchi T, Ohno S, Gong YZ, Yamamoto S, Ozawa T, Ding WG, Toyoda F, Kawamura M, Akao M, Matsuura H, Kimura T, Kita T, Horie M. D85N, a KCNE1 polymorphism, is a disease-causing gene variant in long QT syndrome. J Am Coll Cardiol. 2009;54:812–819. doi: 10.1016/j.jacc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Marjamaa A, Newton-Cheh C, Porthan K, Reunanen A, Lahermo P, Väänänen H, Jula A, Karanko H, Swan H, Toivonen L, Nieminen MS, Viitasalo M, Peltonen L, Oikarinen L, Palotie A, Kontula K, Salomaa V. Common candidate gene variants are associated with QT interval duration in the general population. J Intern Med. 2009;265:448–458. doi: 10.1111/j.1365-2796.2008.02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton-Cheh C, Eijgelsheim M, Rice KM, de Bakker PI, Yin X, Estrada K, Bis JC, Marciante K, Rivadeneira F, Noseworthy PA, Sotoodehnia N, Smith NL, Rotter JI, Kors JA, Witteman JC, Hofman A, Heckbert SR, O'Donnell CJ, Uitterlinden AG, Psaty BM, Lumley T, Larson MG, Stricker BH. Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat Genet. 2009;41:399–406. doi: 10.1038/ng.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouas L, Nicaud V, Berthet M, Forhan A, Tiret L, Balkau B, Guicheney P. Association of KCNQ1, KCNE1, KCNH2 and SCN5A polymorphisms with QTc interval length in a healthy population. Eur J Hum Genet. 2005;13:1213–1222. doi: 10.1038/sj.ejhg.5201489. [DOI] [PubMed] [Google Scholar]
- Paulussen AD, Gilissen RA, Armstrong M, Doevendans PA, Verhasselt P, Smeets HJ, Schulze-Bahr E, Haverkamp W, Breithardt G, Cohen N, Aerssens J. Genetic variations of KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 in drug-induced long QT syndrome patients. J Mol Med. 2004;82:182–188. doi: 10.1007/s00109-003-0522-z. [DOI] [PubMed] [Google Scholar]
- Fodstad H, Swan H, Laitinen P, Piippo K, Paavonen K, Viitasalo M, Toivonen L, Kontula K. Four potassium channel mutations account for 73% of the genetic spectrum underlying long-QT syndrome (LQTS) and provide evidence for a strong founder effect in Finland. Ann Med. 2004;36(Suppl 1):53–63. doi: 10.1080/17431380410032689. [DOI] [PubMed] [Google Scholar]
- Marjamaa A, Salomaa V, Newton-Cheh C, Porthan K, Reunanen A, Karanko H, Jula A, Lahermo P, Väänänen H, Toivonen L, Swan H, Viitasalo M, Nieminen MS, Peltonen L, Oikarinen L, Palotie A, Kontula K. High prevalence of four long QT syndrome founder mutations in the Finnish population. Ann Med. 2009;41:234–240. doi: 10.1080/07853890802668530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, Davignon A. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690–695. [PubMed] [Google Scholar]
- Lehmann MH, Timothy KW, Frankovich D, Fromm BS, Keating M, Locati EH, Taggart RT, Towbin JA, Moss AJ, Schwartz PJ, Vincent GM. Age-gender influence on the rate-corrected QT interval and the QT-heart rate relation in families with genotypically characterized long QT syndrome. J Am Coll Cardiol. 1997;29:93–99. doi: 10.1016/S0735-1097(96)00454-8. [DOI] [PubMed] [Google Scholar]
- Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, Folli R, Cappelletti D. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- Piippo K, Swan H, Pasternack M, Chapman H, Paavonen K, Viitasalo M, Toivonen L, Kontula K. A founder mutation of the potassium channel KCNQ1 in long QT syndrome: implications for estimation of disease prevalence and molecular diagnostics. J Am Coll Cardiol. 2001;37:562–568. doi: 10.1016/S0735-1097(00)01124-4. [DOI] [PubMed] [Google Scholar]
- Friedlander Y, Vatta M, Sotoodehnia N, Sinnreich R, Li H, Manor O, Towbin JA, Siscovick DS, Kark JD. Possible association of the human KCNE1 (minK) gene and QT interval in healthy subjects: evidence from association and linkage analyses in Israeli families. Ann Hum Genet. 2005;69:645–656. doi: 10.1046/j.1529-8817.2005.00182.x. [DOI] [PubMed] [Google Scholar]
- Drici MD, Baker L, Plan P, Barhanin J, Romey G, Salama G. Mice display sex differences in halothane-induced polymorphic ventricular tachycardia. Circulation. 2002;106:497–503. doi: 10.1161/01.CIR.0000023629.72479.24. [DOI] [PubMed] [Google Scholar]
- Busch AE, Busch GL, Ford E, Suessbrich H, Lang HJ, Greger R, Kunzelmann K, Attali B, Stühmer W. The role of the IsK protein in the specific pharmacological properties of the IKs channel complex. Br J Pharmacol. 1997;122:187–189. doi: 10.1038/sj.bjp.0701434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa J, Tamagawa M, Harada N, Honda S, Bai CX, Nakaya H, Furukawa T. Acute effects of oestrogen on the guinea pig and human IKr channels and drug-induced prolongation of cardiac repolarization. J Physiol. 2008;586:2961–2973. doi: 10.1113/jphysiol.2007.150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodstad H, Bendahhou S, Rougier JS, Laitinen-Forsblom PJ, Barhanin J, Abriel H, Schild L, Kontula K, Swan H. Molecular characterization of two founder mutations causing long QT syndrome and identification of compound heterozygous patients. Ann Med. 2006;38:294–304. doi: 10.1080/07853890600756065. [DOI] [PubMed] [Google Scholar]
- Nielsen NH, Winkel BG, Kanters JK, Schmitt N, Hofman-Bang J, Jensen HS, Bentzen BH, Sigurd B, Larsen LA, Andersen PS, Haunsø S, Kjeldsen K, Grunnet M, Christiansen M, Olesen SP. Mutations in the Kv1.5 channel gene KCNA5 in cardiac arrest patients. Biochem Biophys Res Commun. 2007;354:776–782. doi: 10.1016/j.bbrc.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Mank-Seymour AR, Richmond JL, Wood LS, Reynolds JM, Fan YT, Warnes GR, Milos PM, Thompson JF. Association of torsades de pointes with novel and known single nucleotide polymorphisms in long QT syndrome genes. Am Heart J. 2006;152:1116–1122. doi: 10.1016/j.ahj.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Sotoodehnia N, Vatta M, Lemaitre RN, Rautaharju P, Durda P, Towbin JA, Friedlander Y, Tracy RP, Manolio T, Burke GL, Kuller LH, Siscovick DS. KCNE1 Gene D85N Variant, QT Interval, and Risk of Mortality [abstract] Circulation. 2005;111:e231. [Google Scholar]
- Tomás M, Napolitano C, De Giuli L, Bloise R, Subirana I, Malovini A, Bellazzi R, Arking DE, Marban E, Chakravarti A, Spooner PM, Priori SG. Polymorphisms in the NOS1AP gene modulate QT interval duration and risk of arrhythmias in the long QT syndrome. J Am Coll Cardiol. 2010;55:2745–2752. doi: 10.1016/j.jacc.2009.12.065. [DOI] [PubMed] [Google Scholar]