Abstract
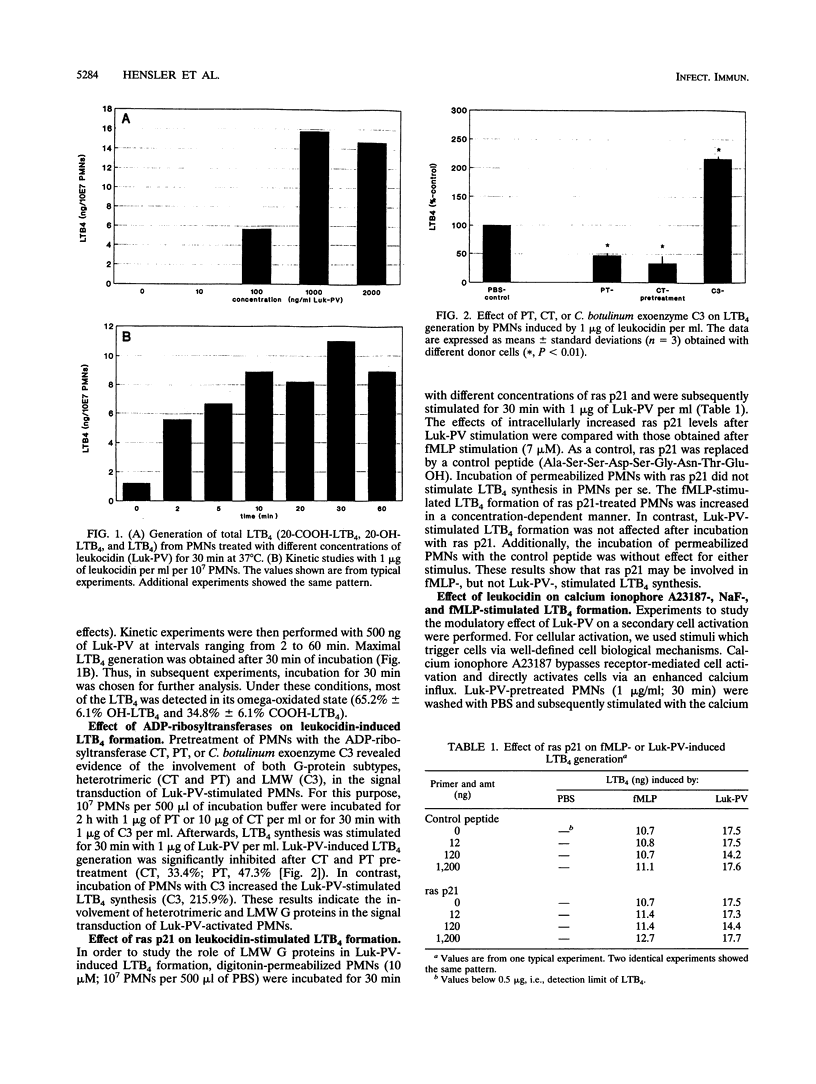
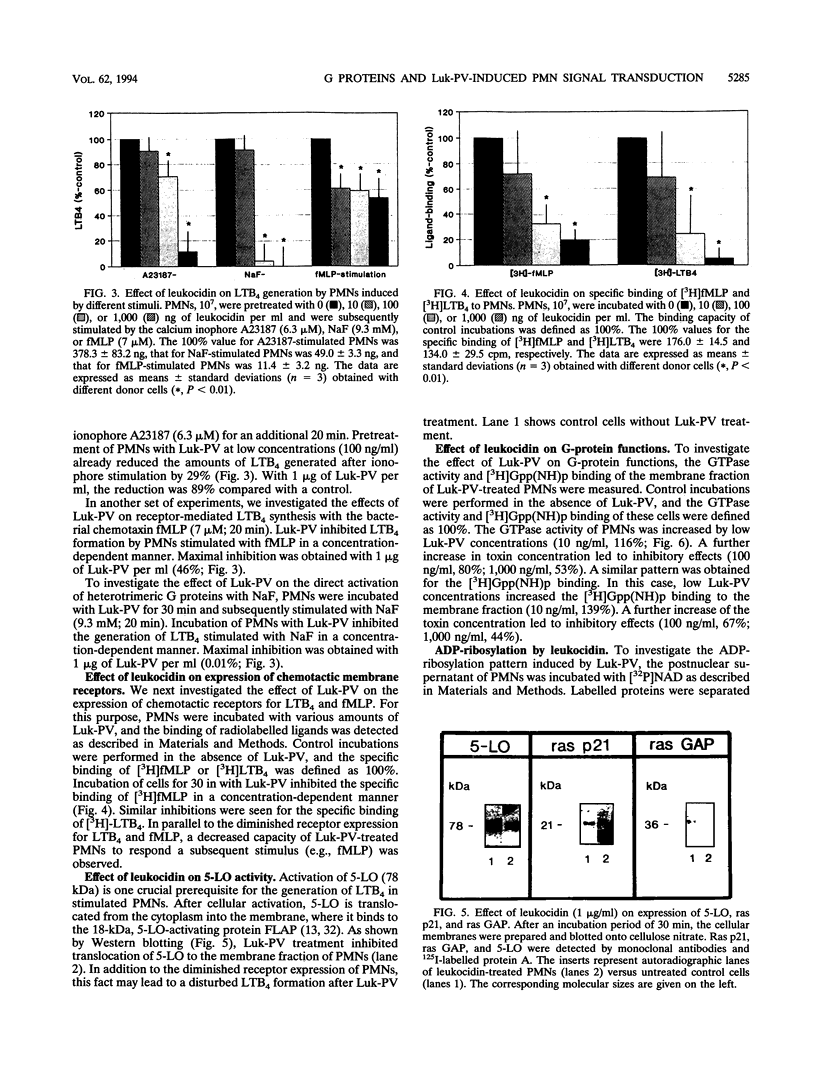
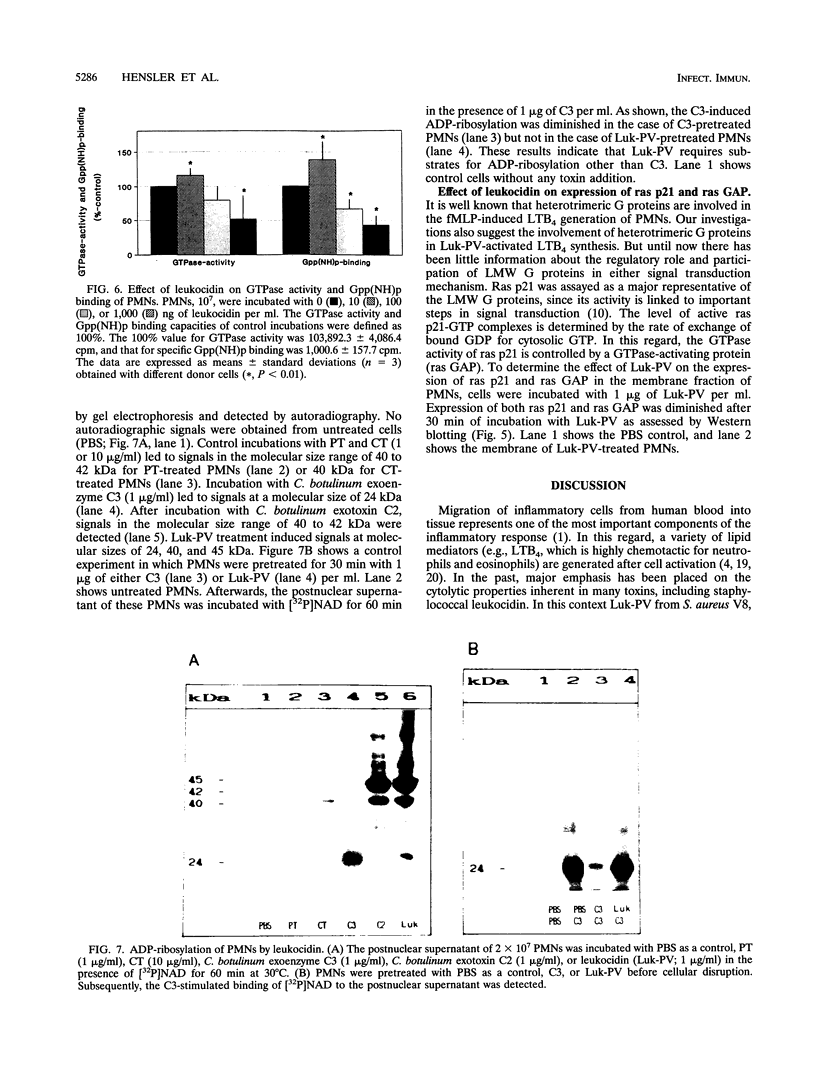
Significant amounts of leukotriene B4 (LTB4) are generated by human polymorphonuclear neutrophils (PMNs) after incubation with the Panton-Valentine leukocidin (Luk-PV) from Staphylococcus aureus V8 strains. We showed that GTP-binding proteins (G proteins) are involved in the Luk-PV-activated signal transduction of PMNs. ADP-ribosylation of heterotrimeric G proteins by cholera and pertussis toxins decreased the Luk-PV-induced LTB4-generation. In contrast, ADP-ribosylation of the low-molecular-weight G proteins rho and rac by Clostridium botulinum exoenzyme C3 increased the Luk-PV-induced LTB4 synthesis. The subsequent stimulation of Luk-PV-treated PMNs by either calcium ionophore A23187, sodium fluoride, or formylmethionyl-leucyl-phenylalanine was significantly inhibited. This decrease was paralleled by a loss of G-protein functions, including GTPase activity and GTP-binding capacity. An increase of G-protein functions was obtained with low amounts of Luk-PV. In addition to the modulated G-protein functions, ADP-ribosylation of 24-, 40-, and 45-kDa proteins by Luk-PV was detected. As shown in control experiments, the ADP-ribosylated 24-kDa proteins were not substrates for C. botulinum exoenzyme C3. Introduction of ras p21 into digitonin-permeabilized PMNs was without effect on subsequent Luk-PV stimulation. In addition, the translocation of ras p21, ras GAP, and 5-lipoxygenase into the membrane of Luk-PV-treated PMNs, as well as the expression of chemotactic membrane receptors for LTB4 and formylmethionyl leucyl phenylalanine, was significantly diminished.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray M. A. Leukotrienes in inflammation. Agents Actions. 1986 Oct;19(1-2):87–99. doi: 10.1007/BF01977263. [DOI] [PubMed] [Google Scholar]
- Bremm K. D., König W., Thelestam M., Alouf J. E. Modulation of granulocyte functions by bacterial exotoxin and endotoxins. Immunology. 1987 Nov;62(3):363–371. [PMC free article] [PubMed] [Google Scholar]
- Brom J., Köller M., Schönfeld W., Knöller J., Erbs G., Müller F. E., König W. Decreased expression of leukotriene B4 receptor sites on polymorphonuclear granulocytes of severely burned patients. Prostaglandins Leukot Essent Fatty Acids. 1988 Dec;34(3):153–159. doi: 10.1016/0952-3278(88)90139-1. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cribier B., Prévost G., Couppie P., Finck-Barbançon V., Grosshans E., Piémont Y. Staphylococcus aureus leukocidin: a new virulence factor in cutaneous infections? An epidemiological and experimental study. Dermatology. 1992;185(3):175–180. doi: 10.1159/000247443. [DOI] [PubMed] [Google Scholar]
- Finck-Barbançon V., Prévost G., Piémont Y. Improved purification of leukocidin from Staphylococcus aureus and toxin distribution among hospital strains. Res Microbiol. 1991 Jan;142(1):75–85. doi: 10.1016/0923-2508(91)90099-v. [DOI] [PubMed] [Google Scholar]
- GLADSTONE G. P., MUDD S., HOCHSTEIN H. D., LENHART N. A. The assay of anti-staphylococcal leucocidal components (F and S) in human serum. Br J Exp Pathol. 1962 Jun;43:295–312. [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Owen D. The biochemistry of ras p21. Biochem J. 1991 Nov 1;279(Pt 3):609–631. doi: 10.1042/bj2790609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler T., Köller M., König W. Regulation of leukotriene B4 generation from human polymorphonuclear granulocytes after stimulation with formyl-methionyl-leucyl phenylalanine: effects of pertussis and cholera toxins. Infect Immun. 1991 Sep;59(9):3046–3052. doi: 10.1128/iai.59.9.3046-3052.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler T., König B., Prévost G., Piémont Y., Köller M., König W. Leukotriene B4 generation and DNA fragmentation induced by leukocidin from Staphylococcus aureus: protective role of granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF for human neutrophils. Infect Immun. 1994 Jun;62(6):2529–2535. doi: 10.1128/iai.62.6.2529-2535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E., Maclouf J., Murphy R. C., Henson P. M. Reversible membrane association of neutrophil 5-lipoxygenase is accompanied by retention of activity and a change in substrate specificity. J Biol Chem. 1992 Nov 5;267(31):22048–22053. [PubMed] [Google Scholar]
- Iiri T., Ohoka Y., Ui M., Katada T. Modification of the function of pertussis toxin substrate GTP-binding protein by cholera toxin-catalyzed ADP-ribosylation. J Biol Chem. 1992 Jan 15;267(2):1020–1026. [PubMed] [Google Scholar]
- Kahn R. A. Fluoride is not an activator of the smaller (20-25 kDa) GTP-binding proteins. J Biol Chem. 1991 Aug 25;266(24):15595–15597. [PubMed] [Google Scholar]
- Kato I., Noda M. ADP-ribosylation of cell membrane proteins by staphylococcal alpha-toxin and leukocidin in rabbit erythrocytes and polymorphonuclear leukocytes. FEBS Lett. 1989 Sep 11;255(1):59–62. doi: 10.1016/0014-5793(89)81060-9. [DOI] [PubMed] [Google Scholar]
- Köller M., Hensler T., König B., Prévost G., Alouf J., König W. Induction of heat-shock proteins by bacterial toxins, lipid mediators and cytokines in human leukocytes. Zentralbl Bakteriol. 1993 Apr;278(2-3):365–376. doi: 10.1016/s0934-8840(11)80853-4. [DOI] [PubMed] [Google Scholar]
- Köller M., König W., Brom J., Erbs G., Müller F. E. Studies on the mechanisms of granulocyte dysfunctions in severely burned patients--evidence for altered leukotriene generation. J Trauma. 1989 Apr;29(4):435–445. doi: 10.1097/00005373-198904000-00004. [DOI] [PubMed] [Google Scholar]
- König W., Schönfeld W., Raulf M., Köller M., Knöller J., Scheffer J., Brom J. The neutrophil and leukotrienes--role in health and disease. Eicosanoids. 1990;3(1):1–22. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Molski T. F., Kanaho Y., Becker E. L., Sha'afi R. I. G-protein dissociation, GTP-GDP exchange and GTPase activity in control and PMA treated neutrophils stimulated by fMet-Leu-Phe. Biochem Biophys Res Commun. 1987 Mar 13;143(2):489–498. doi: 10.1016/0006-291x(87)91380-5. [DOI] [PubMed] [Google Scholar]
- McDonald P. P., McColl S. R., Naccache P. H., Borgeat P. Activation of the human neutrophil 5-lipoxygenase by leukotriene B4. Br J Pharmacol. 1992 Sep;107(1):226–232. doi: 10.1111/j.1476-5381.1992.tb14491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr C., Koch G., Just I., Aktories K. ADP-ribosylation by Clostridium botulinum C3 exoenzyme increases steady-state GTPase activities of recombinant rhoA and rhoB proteins. FEBS Lett. 1992 Feb 3;297(1-2):95–99. doi: 10.1016/0014-5793(92)80335-e. [DOI] [PubMed] [Google Scholar]
- Noda M., Kato I., Hirayama T., Matsuda F. Fixation and inactivation of staphylococcal leukocidin by phosphatidylcholine and ganglioside GM1 in rabbit polymorphonuclear leukocytes. Infect Immun. 1980 Aug;29(2):678–684. doi: 10.1128/iai.29.2.678-684.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Kato I., Hirayama T., Matsuda F. Mode of action of staphylococcal leukocidin: effects of the S and F components on the activities of membrane-associated enzymes of rabbit polymorphonuclear leukocytes. Infect Immun. 1982 Jan;35(1):38–45. doi: 10.1128/iai.35.1.38-45.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Polakis P. G., Evans T., Snyderman R. Multiple chromatographic forms of the formylpeptide chemoattractant receptor and their relationship to GTP-binding proteins. Biochem Biophys Res Commun. 1989 May 30;161(1):276–283. doi: 10.1016/0006-291x(89)91592-1. [DOI] [PubMed] [Google Scholar]
- Prentki M., Wollheim C. B., Lew P. D. Ca2+ homeostasis in permeabilized human neutrophils. Characterization of Ca2+-sequestering pools and the action of inositol 1,4,5-triphosphate. J Biol Chem. 1984 Nov 25;259(22):13777–13782. [PubMed] [Google Scholar]
- Rouzer C. A., Samuelsson B. Reversible, calcium-dependent membrane association of human leukocyte 5-lipoxygenase. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7393–7397. doi: 10.1073/pnas.84.21.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari H., Braquet P., Naccache P., Borgeat P. Characterization of effect of N-formyl-methionyl-leucyl-phenylalanine on leukotriene synthesis in human polymorphonuclear leukocytes. Inflammation. 1985 Jun;9(2):127–138. doi: 10.1007/BF00917585. [DOI] [PubMed] [Google Scholar]
- Scheffer J., König W., Braun V., Goebel W. Comparison of four hemolysin-producing organisms (Escherichia coli, Serratia marcescens, Aeromonas hydrophila, and Listeria monocytogenes) for release of inflammatory mediators from various cells. J Clin Microbiol. 1988 Mar;26(3):544–551. doi: 10.1128/jcm.26.3.544-551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Bokoch G. M., Button D., Smolen J. E. Regulation of ligand-receptor dynamics by guanine nucleotides. Real-time analysis of interconverting states for the neutrophil formyl peptide receptor. J Biol Chem. 1987 Jan 5;262(1):135–139. [PubMed] [Google Scholar]
- WOODIN A. M. Assay of the two components of staphylococcal leucocidin and their antibodies. J Pathol Bacteriol. 1961 Jan;81:63–68. doi: 10.1002/path.1700810108. [DOI] [PubMed] [Google Scholar]
- WOODIN A. M. Purification of the two components of leucocidin from Staphylococcus aureus. Biochem J. 1960 Apr;75:158–165. doi: 10.1042/bj0750158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodin A. M., Wieneke A. A. The participation of phospholipids in the interaction of leucocidin and the cell membrane of the polymorphonuclear leucocyte. Biochem J. 1967 Dec;105(3):1029–1038. doi: 10.1042/bj1051029. [DOI] [PMC free article] [PubMed] [Google Scholar]





