Abstract
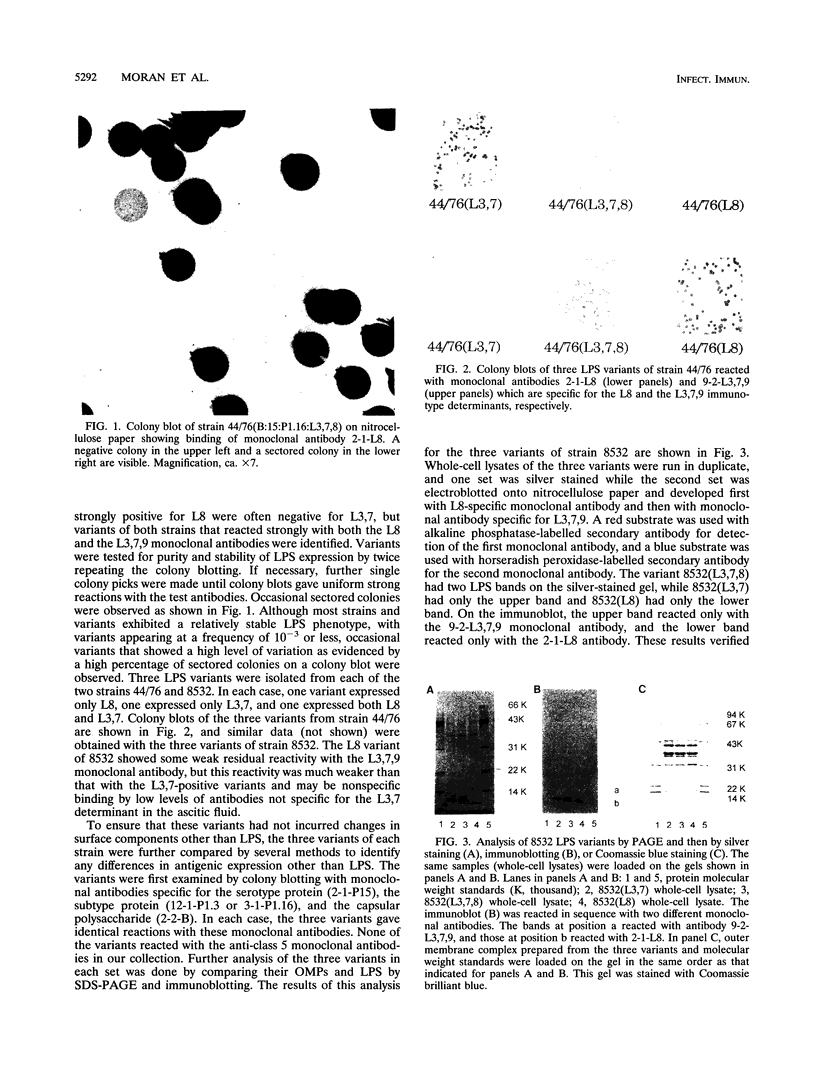
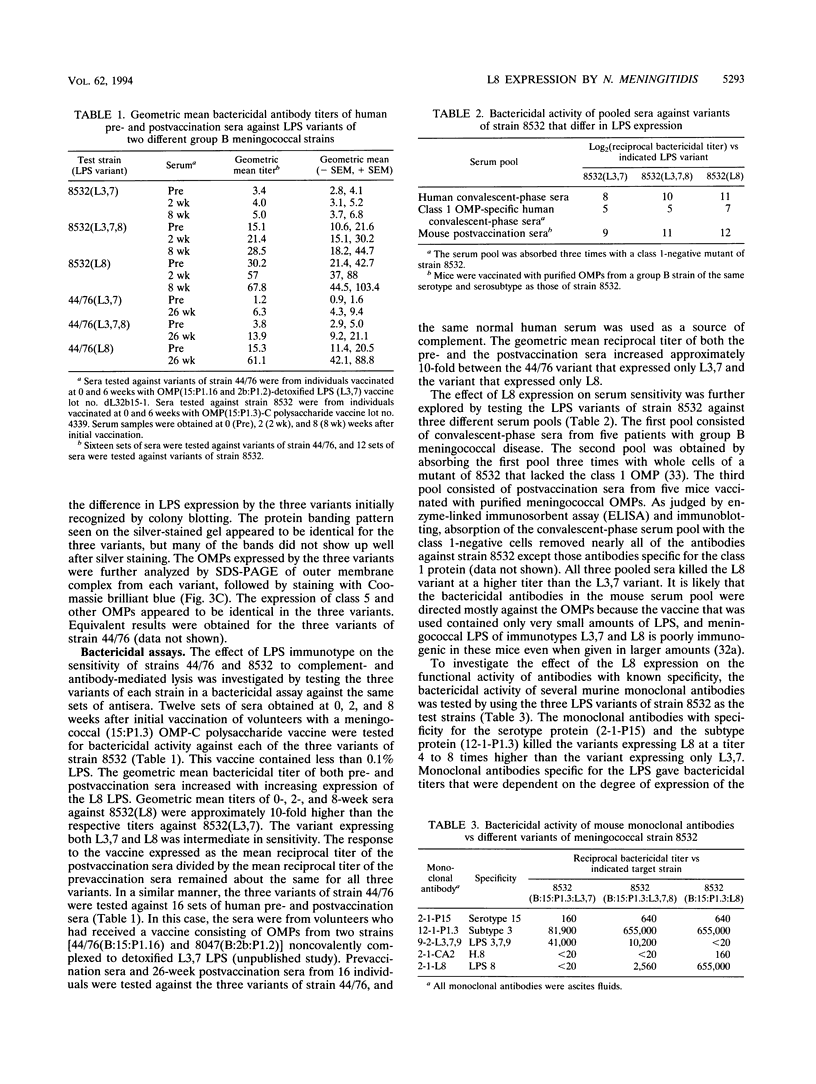
The influence of lipopolysaccharide (LPS) expression on the sensitivity of Neisseria meningitidis to serum bactericidal activity was investigated by using a series of variants that expressed different LPS determinants. For each of two different strains, a series of three LPS variants that expressed L3,7, L8, or both were analyzed. LPS variants were identified and monitored by colony blotting with murine monoclonal antibodies specific for the L8 or the L3,7,9 immunotype determinants. Differences in LPS expression were verified by analysis of proteinase K lysates of whole cells by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then by silver staining or immunoblotting. The variants were used as test strains in bactericidal assays with human sera and with murine sera and monoclonal antibodies. Expression of the L8 LPS epitope was correlated with increased sensitivity to serum bactericidal activity. The geometric mean bactericidal titers of 12 to 16 sets of pre- and postvaccination sera were determined for each variant. Mean serum titers increased progressively with increased expression of L8 on the target strain. The bactericidal titers of anti-outer membrane protein monoclonal antibodies also increased with increased L8 expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Neibert M., Crowe B. A., Strittmatter W., Kusecek B., Weyse E., Walsh M. J., Slawig B., Morelli G., Moll A. Purification and characterization of eight class 5 outer membrane protein variants from a clone of Neisseria meningitidis serogroup A. J Exp Med. 1988 Aug 1;168(2):507–525. doi: 10.1084/jem.168.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARILE M. F., YAGUCHI R., EVELAND W. C. A simplified medium for the cultivation of pleuropneumonia-like organisms and the L-forms of bacteria. Am J Clin Pathol. 1958 Aug;30(2):171–176. doi: 10.1093/ajcp/30.2_ts.171. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Moran E. E., Zollinger W. D. Antibodies to meningococcal H.8 (Lip) antigen fail to show bactericidal activity. Can J Microbiol. 1990 Feb;36(2):117–122. doi: 10.1139/m90-021. [DOI] [PubMed] [Google Scholar]
- Fourel D., Mizushima S., Pagès J. M. Dynamics of the exposure of epitopes on OmpF, an outer membrane protein of Escherichia coli. Eur J Biochem. 1992 May 15;206(1):109–114. doi: 10.1111/j.1432-1033.1992.tb16907.x. [DOI] [PubMed] [Google Scholar]
- Fox A. J., Jones D. M., Scotland S. M., Rowe B., Smith A., Brown M. R., Fitzgeorge R. G., Baskerville A., Parsons N. J., Cole J. A. Serum killing of meningococci and several other gram-negative bacterial species is not decreased by incubating them with cytidine 5'-monophospho-N-acetyl neuraminic acid. Microb Pathog. 1989 Oct;7(4):317–318. doi: 10.1016/0882-4010(89)90050-8. [DOI] [PubMed] [Google Scholar]
- Gamian A., Beurret M., Michon F., Brisson J. R., Jennings H. J. Structure of the L2 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J Biol Chem. 1992 Jan 15;267(2):922–925. [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis G. A., Vedros N. A. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun. 1987 Jan;55(1):174–180. doi: 10.1128/iai.55.1.174-180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Beurret M., Gamian A., Michon F. Structure and immunochemistry of meningococcal lipopolysaccharides. Antonie Van Leeuwenhoek. 1987;53(6):519–522. doi: 10.1007/BF00415511. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Johnson K. G., Kenne L. The structure of an R-type oligosaccharide core obtained from some lipopolysaccharides of Neisseria meningitidis. Carbohydr Res. 1983 Sep 16;121:233–241. doi: 10.1016/0008-6215(83)84020-8. [DOI] [PubMed] [Google Scholar]
- Jones D. M., Borrow R., Fox A. J., Gray S., Cartwright K. A., Poolman J. T. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb Pathog. 1992 Sep;13(3):219–224. doi: 10.1016/0882-4010(92)90022-g. [DOI] [PubMed] [Google Scholar]
- Kerwood D. E., Schneider H., Yamasaki R. Structural analysis of lipooligosaccharide produced by Neisseria gonorrhoeae, strain MS11mk (variant A): a precursor for a gonococcal lipooligosaccharide associated with virulence. Biochemistry. 1992 Dec 29;31(51):12760–12768. doi: 10.1021/bi00166a008. [DOI] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Hu Z., Westerink M. A., Poolman J. T., Griffiss J. M. Electromorphic characterization and description of conserved epitopes of the lipooligosaccharides of group A Neisseria meningitidis. Infect Immun. 1988 Oct;56(10):2631–2638. doi: 10.1128/iai.56.10.2631-2638.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindman C. R., Frøholm L. O., Halstensen A. I., Holten E. Untreated meningococcemia in two siblings. NIPH Ann. 1983 Dec;6(2):191–201. [PubMed] [Google Scholar]
- Mandrell R. E., Kim J. J., John C. M., Gibson B. W., Sugai J. V., Apicella M. A., Griffiss J. M., Yamasaki R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991 May;173(9):2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen M., Butcher S., Idänpän-Heikkilä I., Wahlström E., Muttilainen S., Runeberg-Nyman K., Sarvas M., Mäkelä P. H. The class 1 outer membrane protein of Neisseria meningitidis produced in Bacillus subtilis can give rise to protective immunity. Mol Microbiol. 1992 Sep;6(17):2499–2506. doi: 10.1111/j.1365-2958.1992.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Pages J. M., Bolla J. M., Bernadac A., Fourel D. Immunological approach of assembly and topology of OmpF, an outer membrane protein of Escherichia coli. Biochimie. 1990 Feb-Mar;72(2-3):169–176. doi: 10.1016/0300-9084(90)90142-4. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Colony variants of Neisseria meningitidis strain 2996 (B:2b:P1.2): influence of class-5 outer membrane proteins and lipopolysaccharides. J Med Microbiol. 1985 Apr;19(2):203–209. doi: 10.1099/00222615-19-2-203. [DOI] [PubMed] [Google Scholar]
- Ried G., Hindennach I., Henning U. Role of lipopolysaccharide in assembly of Escherichia coli outer membrane proteins OmpA, OmpC, and OmpF. J Bacteriol. 1990 Oct;172(10):6048–6053. doi: 10.1128/jb.172.10.6048-6053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenqvist E., Høiby E. A., Wedege E., Kusecek B., Achtman M. The 5C protein of Neisseria meningitidis is highly immunogenic in humans and induces bactericidal antibodies. J Infect Dis. 1993 May;167(5):1065–1073. doi: 10.1093/infdis/167.5.1065. [DOI] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Hammack C. A., Apicella M. A., Griffiss J. M. Instability of expression of lipooligosaccharides and their epitopes in Neisseria gonorrhoeae. Infect Immun. 1988 Apr;56(4):942–946. doi: 10.1128/iai.56.4.942-946.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen K., Nikaido H. Lipopolysaccharide structure required for in vitro trimerization of Escherichia coli OmpF porin. J Bacteriol. 1991 Jan;173(2):926–928. doi: 10.1128/jb.173.2.926-928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., Cole J. A., Parsons N. J. The sialylation of gonococcal lipopolysaccharide by host factors: a major impact on pathogenicity. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):287–292. doi: 10.1111/j.1574-6968.1992.tb14054.x. [DOI] [PubMed] [Google Scholar]
- Tinsley C. R., Heckels J. E. Variation in the expression of pili and outer membrane protein by Neisseria meningitidis during the course of meningococcal infection. J Gen Microbiol. 1986 Sep;132(9):2483–2490. doi: 10.1099/00221287-132-9-2483. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Boslego J., Moran E., Garcia J., Cruz C., Ruiz S., Brandt B., Martinez M., Arthur J., Underwood P. Meningococcal serogroup B vaccine protection trial and follow-up studies in Chile. The Chilean National Committee for Meningococcal Disease. NIPH Ann. 1991 Dec;14(2):211–213. [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983 Apr;40(1):257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Type-specific antigens of group A Neisseria meningitidis: lipopolysaccharide and heat-modifiable outer membrane proteins. Infect Immun. 1980 May;28(2):451–458. doi: 10.1128/iai.28.2.451-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Moran E. Meningococcal vaccines--present and future. Trans R Soc Trop Med Hyg. 1991;85 (Suppl 1):37–43. doi: 10.1016/0035-9203(91)90339-z. [DOI] [PubMed] [Google Scholar]






