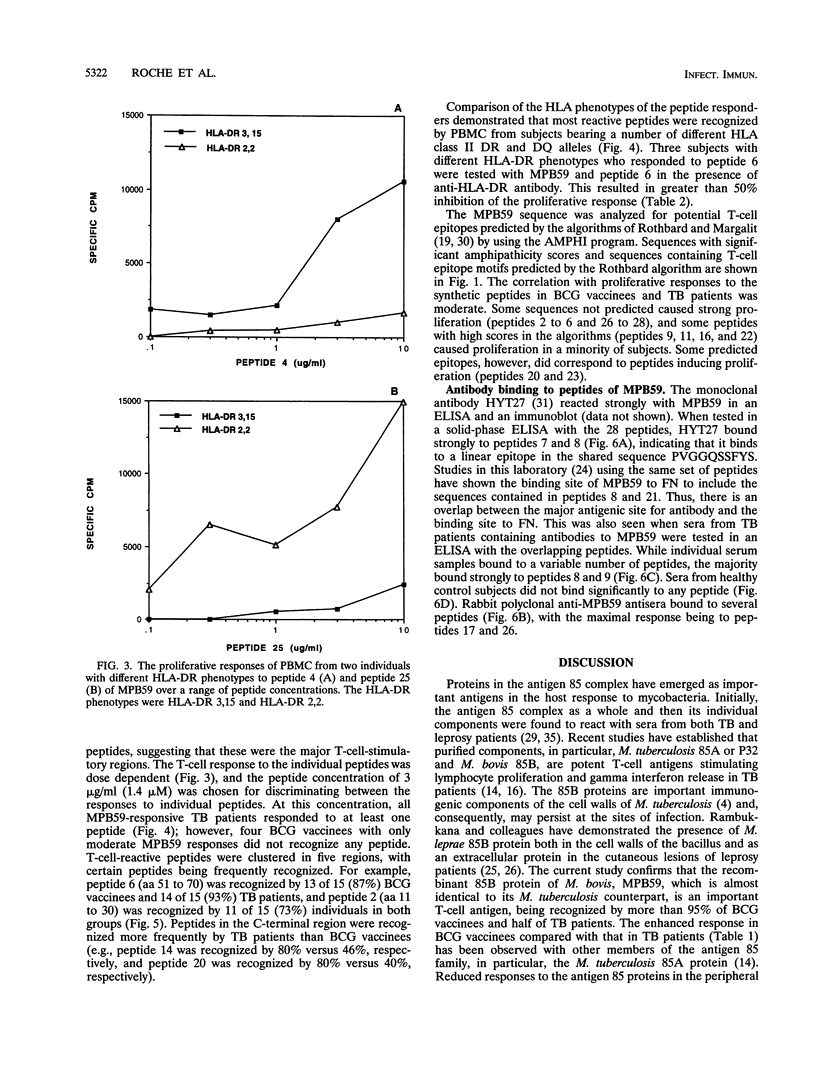
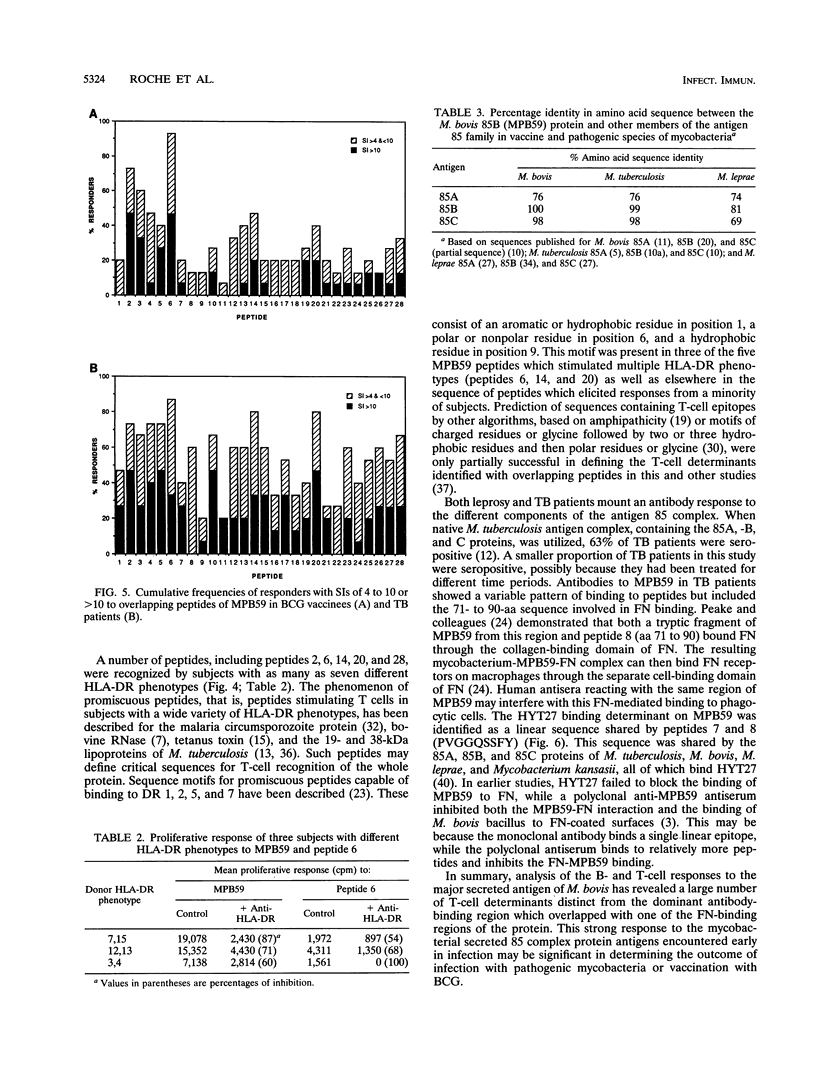
Abstract
Among the first proteins encountered by the host immune system upon infection or vaccination with mycobacteria are those secreted by the bacillus during growth. The antigen 85 complex of Mycobacterium bovis bacillus Calmette-Gúerin (BCG) is composed of three closely related members. The mature 85B protein of M. bovis (MPB59) has a high degree of amino acid identity with the M. bovis 85A protein (76%) and the Mycobacterium tuberculosis 85B (99%) and 85A (76%) proteins. We have examined the regions of MPB59 which stimulate human T- and B-cell responses by use of a set of 28 synthetic peptides, 20 amino acids (aa) in length and overlapping by 10 aa. Initial proliferative assays with recombinant MPB59 demonstrated that peripheral blood mononuclear cells from 95% of BCG vaccinees and 52% of tuberculosis patients responded to the whole mature protein. Peripheral blood mononuclear cells from MPB59 responders, but not nonresponders, were stimulated by peptides in a dose-dependent fashion. Five peptides were reactive in more than half of the MPB59 responders. The T-cell-reactive regions were essentially identical in the M. bovis and M. tuberculosis 85B proteins. Subjects with a variety of HLA-DR phenotypes responded to a number of these peptides. The dominant T-cell-reactive regions were distinct from the peptides recognized by sera from tuberculosis patients (aa 71 to 100) and the murine monoclonal antibody HYT27 (aa 61 to 90). The region reactive with antibodies overlapped part of the MPB59 sequence recently shown to participate in the binding of MPB59 to fibronectin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams E., Britton W. J., Morgan A., Goodsall A. L., Basten A. Identification of human T cell epitopes in the Mycobacterium leprae heat shock protein 70-kD antigen. Clin Exp Immunol. 1993 Dec;94(3):500–506. doi: 10.1111/j.1365-2249.1993.tb08225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams E., Britton W., Morgan A., Sergeantson S., Basten A. Individuals from different populations identify multiple and diverse T-cell determinants on mycobacterial HSP70. Scand J Immunol. 1994 Jun;39(6):588–596. doi: 10.1111/j.1365-3083.1994.tb03417.x. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Mehra V., Hirschfield G. R., Fong S. J., Abou-Zeid C., Rook G. A., Hunter S. W., Brennan P. J., Modlin R. L. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989 Oct 15;143(8):2656–2662. [PubMed] [Google Scholar]
- Borremans M., de Wit L., Volckaert G., Ooms J., de Bruyn J., Huygen K., van Vooren J. P., Stelandre M., Verhofstadt R., Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989 Oct;57(10):3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Raison R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J Immunol. 1985 Dec;135(6):4171–4177. [PubMed] [Google Scholar]
- Chen J. S., Lorenz R. G., Goldberg J., Allen P. M. Identification and characterization of a T cell-inducing epitope of bovine ribonuclease that can be restricted by multiple class II molecules. J Immunol. 1991 Dec 1;147(11):3672–3678. [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Lamb J. R., Young D. B. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect Immun. 1988 May;56(5):1260–1266. doi: 10.1128/iai.56.5.1260-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Content J., de la Cuvellerie A., De Wit L., Vincent-Levy-Frébault V., Ooms J., De Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect Immun. 1991 Sep;59(9):3205–3212. doi: 10.1128/iai.59.9.3205-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit L., de la Cuvellerie A., Ooms J., Content J. Nucleotide sequence of the 32 kDa-protein gene (antigen 85 A) of Mycobacterium bovis BCG. Nucleic Acids Res. 1990 Jul 11;18(13):3995–3995. doi: 10.1093/nar/18.13.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espitia C., Sciutto E., Bottasso O., González-Amaro R., Hernández-Pando R., Mancilla R. High antibody levels to the mycobacterial fibronectin-binding antigen of 30-31 kD in tuberculosis and lepromatous leprosy. Clin Exp Immunol. 1992 Mar;87(3):362–367. doi: 10.1111/j.1365-2249.1992.tb03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith A., Moreno C., Lathigra R., Roman E., Fernandez M., Brett S., Mitchell D. M., Ivanyi J., Rees A. D. Analysis of human T-cell epitopes in the 19,000 MW antigen of Mycobacterium tuberculosis: influence of HLA-DR. Immunology. 1991 Sep;74(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Havlir D. V., Wallis R. S., Boom W. H., Daniel T. M., Chervenak K., Ellner J. J. Human immune response to Mycobacterium tuberculosis antigens. Infect Immun. 1991 Feb;59(2):665–670. doi: 10.1128/iai.59.2.665-670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. C., Mutch D. A., Winkel K. D., Saul A. J., Jones G. L., Doran T. J., Rzepczyk C. M. Identification of two promiscuous T cell epitopes from tetanus toxin. Eur J Immunol. 1990 Mar;20(3):477–483. doi: 10.1002/eji.1830200304. [DOI] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Young D. B. A novel approach to the identification of T-cell epitopes in Mycobacterium tuberculosis using human T-lymphocyte clones. Immunology. 1987 Jan;60(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988 Sep;170(9):3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie K. R., Adams E., Britton W. J., Garsia R. J., Basten A. Sequence and immunogenicity of the 70-kDa heat shock protein of Mycobacterium leprae. J Immunol. 1991 Jul 1;147(1):312–319. [PubMed] [Google Scholar]
- Nagai S., Wiker H. G., Harboe M., Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991 Jan;59(1):372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan D., Arrhenius T., Sidney J., Del Guercio M. F., Albertson M., Wall M., Oseroff C., Southwood S., Colón S. M., Gaeta F. C. On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol. 1991 Oct 15;147(8):2663–2669. [PubMed] [Google Scholar]
- Peake P., Gooley A., Britton W. J. Mechanism of interaction of the 85B secreted protein of Mycobacterium bovis with fibronectin. Infect Immun. 1993 Nov;61(11):4828–4834. doi: 10.1128/iai.61.11.4828-4834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Burggraaf J. D., Yong S., Faber W. R., Thole J. E., Harboe M. Heterogeneity of monoclonal antibody-reactive epitopes on mycobacterial 30-kilodalton-region proteins and the secreted antigen 85 complex and demonstration of antigen 85B on the Mycobacterium leprae cell wall surface. Infect Immun. 1992 Dec;60(12):5172–5181. doi: 10.1128/iai.60.12.5172-5181.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Krieg S., Faber W. R. Association of the mycobacterial 30-kDa region proteins with the cutaneous infiltrates of leprosy lesions. Evidence for the involvement of the major mycobacterial secreted proteins in the local immune response of leprosy. Scand J Immunol. 1992 Jul;36(1):35–48. doi: 10.1111/j.1365-3083.1992.tb02938.x. [DOI] [PubMed] [Google Scholar]
- Rinke de Wit T. F., Bekelie S., Osland A., Wieles B., Janson A. A., Thole J. E. The Mycobacterium leprae antigen 85 complex gene family: identification of the genes for the 85A, 85C, and related MPT51 proteins. Infect Immun. 1993 Sep;61(9):3642–3647. doi: 10.1128/iai.61.9.3642-3647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Rumschlag H. S., Shinnick T. M., Cohen M. L. Serological responses of patients with lepromatous and tuberculoid leprosy to 30-, 31-, and 32-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol. 1988 Oct;26(10):2200–2202. doi: 10.1128/jcm.26.10.2200-2202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou C., Yuan Z. L., Andersen A. B., Bennedsen J. Production and partial characterization of monoclonal hybridoma antibodies to Mycobacterium tuberculosis. Acta Pathol Microbiol Immunol Scand C. 1985 Dec;93(6):265–272. doi: 10.1111/j.1699-0463.1985.tb02955.x. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Schöningh R., Janson A. A., Garbe T., Cornelisse Y. E., Clark-Curtiss J. E., Kolk A. H., Ottenhoff T. H., De Vries R. R., Abou-Zeid C. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol Microbiol. 1992 Jan;6(2):153–163. doi: 10.1111/j.1365-2958.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- Turneer M., Van Vooren J. P., De Bruyn J., Serruys E., Dierckx P., Yernault J. C. Humoral immune response in human tuberculosis: immunoglobulins G, A, and M directed against the purified P32 protein antigen of Mycobacterium bovis bacillus Calmette-Guérin. J Clin Microbiol. 1988 Sep;26(9):1714–1719. doi: 10.1128/jcm.26.9.1714-1719.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordermeier H. M., Harris D. P., Friscia G., Román E., Surcel H. M., Moreno C., Pasvol G., Ivanyi J. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38-kDa antigen in patients with active disease. Eur J Immunol. 1992 Oct;22(10):2631–2637. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- Wiertz E. J., van Gaans-van den Brink J. A., Gausepohl H., Prochnicka-Chalufour A., Hoogerhout P., Poolman J. T. Identification of T cell epitopes occurring in a meningococcal class 1 outer membrane protein using overlapping peptides assembled with simultaneous multiple peptide synthesis. J Exp Med. 1992 Jul 1;176(1):79–88. doi: 10.1084/jem.176.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Bennedsen J. Quantitative and qualitative studies on the major extracellular antigen of Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):830–838. doi: 10.1164/ajrccm/141.4_Pt_1.830. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Patarroyo M. E., Ramirez C., Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Nagai S., Harboe M., Ljungqvist L. A family of cross-reacting proteins secreted by Mycobacterium tuberculosis. Scand J Immunol. 1992 Aug;36(2):307–319. doi: 10.1111/j.1365-3083.1992.tb03104.x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]




