Abstract
The majority of bird species studied to date have molt schedules that are not concurrent with other energy demanding life history stages, an outcome assumed to arise from energetic trade-offs. Empirical studies reveal that molt is one of the most energetically demanding and perplexingly inefficient growth processes measured. Furthermore, small birds, which have the highest mass-specific basal metabolic rates (BMRm), have the highest costs of molt per gram of feathers produced. However, many small passerines, including white-plumed honeyeaters (WPHE; Lichenostomus penicillatus), breed in response to resource availability at any time of year, and do so without interrupting their annual molt. We examined the energetic cost of molt in WPHE by quantifying weekly changes in minimum resting metabolic rate (RMRmin) during a natural-molt period in 7 wild-caught birds. We also measured the energetic cost of feather replacement in a second group of WPHEs that we forced to replace an additional 25% of their plumage at the start of their natural molt period. Energy expenditure during natural molt revealed an energy conversion efficiency of just 6.9% (±0.57) close to values reported for similar-sized birds from more predictable north-temperate environments. Maximum increases in RMRmin during the molt of WPHE, at 82% (±5.59) above individual pre-molt levels, were some of the highest yet reported. Yet RMRmin maxima during molt were not coincident with the peak period of feather replacement in naturally molting or plucked birds. Given the tight relationship between molt efficiency and mass-specific metabolic rate in all species studied to date, regardless of life-history pattern (Efficiency (%) = 35.720•10−0.494BMRm; r2 = 0.944; p = <0.0001), there appears to be concomitant physiological costs entrained in the molt period that is not directly due to feather replacement. Despite these high total expenditures, the protracted molt period of WPHE significantly reduces these added costs on a daily basis.
Introduction
Variations in life-history patterns are thought to represent a given species' maximization of lifetime inclusive fitness within a particular environment [1], [2]. The channeling of available resources during a specific time period into a single, resource-demanding activity is the most widely recognized, and perhaps most common pattern of resource partitioning [2]. Accordingly, widespread theoretical and empirical investigation reveals that the annual cycle of birds living in north temperate and high latitudes is characterized by pronounced temporal separation of the most intense phases of molt, breeding and migration, with little or no overlap between these activities at the individual level [3]–[7]. Indeed, birds that initiate molt during their final stages of breeding (in either naturally or experimentally induced late breeding pairs) often experience considerable fitness costs as parents [8–11, but see 12], as do their offspring [13]–[15]. Partitioning of molt from other life history stages is therefore assumed to be an adaptation that minimizes physiological stress while maximizing the allocation of productive energy [16].
Maintenance of the aerodynamic, insulative and signaling functions of avian plumage is of paramount importance and requires annual feather replacement in most bird species. During a complete molt, a bird must synthesize almost one-quarter of its total body protein in the form of feathers and other epidermal structures [17], [18]. This places a high demand on energy and nutrients, especially protein [19]. Therefore, detailed knowledge of the relative energy requirements of molt is integral for gaining insight into avian life history strategies.
Feather production costs, estimated from measurement of increases in basal metabolic rate throughout the molt period, show up to ten-fold variation between species. Small passerines, such as bluethroats (Luscinia s. svecica; 17 g), and redpolls (Carduelis f. flammea; 13 g) expend between 862 and 709 kJ·g dry feathers–1 [20], respectively, compared with 69 and 116 kJ·g dry feathers–1 for the kookaburra (Dacelo novaeguineae; 335 g; [21]) and the long-eared owl (Asio otus; 280 g; [22]), respectively. Much of this size-related variation in molt cost is thought to be a consequence of mass-related differences in metabolic rate, which predicts that the smallest birds, with the highest mass-specific metabolic rates (BMRm), will have the highest molt costs and hence lowest energy conversion efficiencies in feather production [20], [23], [24].
It is difficult, however, to reconcile predictions of high molt costs for small passerines with the observation that molt and breeding are often coincident in opportunistically breeding small passerine species living in unpredictable habitats [7], [25]–[27]. This is especially true of Australia's old-endemic passerines living in arid zones. Because erratic rainfall and periodic droughts are characteristic of this habitat [28], resource availability does not correspond with annual variation in photoperiod, in marked contrast with many temperate locations [29]. Consequently, residents of arid zones initiate breeding opportunistically, in order to match finite periods of favorable environmental conditions [26], [30], [31]. Molt in these birds, however, shows surprisingly little inter-annual variation in its schedule [26], despite vast irregularities in available resources and reproductive status [32]. Clearly, in these birds, breeding and molt are not temporally incompatible.
The coincidence of molt/breeding overlap in individuals of some species, but not in others, provokes the question: Is molt an inherently costly and inefficient process, or are costs dependent upon other life history characteristics? We examined these questions by measuring molt costs in the White-plumed honeyeater (WPHE; Lichenostomus penicillatus), an old-endemic Australian species that includes populations inhabiting the arid zone. These populations display a flexible breeding schedule that corresponds with unpredictable flushes of lerps, the larvae of a psylid insect associated with the River Redgums (Eucalyptus camaldulensis) on which adults forage [33]. In stark contrast, molt follows a regular schedule regardless of environmental conditions, and molt/breeding overlap is a regular occurrence [26], [32]. We aimed to see if their ability to molt and breed concurrently was associated with lower costs of molt. We also investigated whether the reported energetic inefficiency of feather production during molt was due to feather replacement per se by measuring the energetic consequences of feather replacement following artificial removal of 25% of feathers by mass in a separate group of honeyeaters. We found that energetic expenditure during natural molt in WPHE was surprisingly similar to expectations based on birds from more predictable north-temperate environments, but that these increases in energy expenditure were not coincident with the peak period of feather replacement in naturally molting or plucked birds.
Methods
Ethics statement
The animals used in this study were captured under license from the New South Wales National Parks and Wildlife service (S11320). All experimental procedures were carried out under approval from the University of Wollongong Animal Ethics Committee (AE04/12), in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Feathers were plucked while birds were under methoxyflurane anesthesia, and all efforts were made to minimize any suffering throughout the study. Birds were released at the site of capture following completion of the study.
Study animals
White-plumed honeyeaters (WPHE) were caught in mist nets at Fowler's Gap Arid-Zone Research Station, New South Wales (31°S, 142°E) in August, and brought to the University of Wollongong (34°25′S, 150°54′E), where they were held in constant temperature (25°C) rooms under natural photoperiod. Birds were housed two per cage (ca. 40×60×60 cm), and were provided with commercial honeyeater food (Wombaroo Pty. Ltd. – min crude protein 13%) and water ad libitum.
Metabolic measurements
Birds were held for two weeks to habituate to captive conditions before determining pre-molt basal metabolic rate (BMR). By definition, BMR represents minimum metabolic rates while animals are post-absorptive, in the rest-phase of their daily cycle; and exposed to thermoneutral temperatures. Furthermore, BMR requires there to be an absence of energetically demanding activities, such as growth, reproduction, or molt. We therefore refer to metabolic rate measurements taken under these conditions during molt as minimum resting metabolic rate (RMRmin). Each individual's RMRmin was then measured weekly until the completion of feather regrowth. Birds were fasted for approximately 2 h before being removed from cages between 16:30 and 18:00, weighed, and then placed in individual 4-L respirometers. Respirometers were held in a constant temperature cabinet overnight, set at 33°C (± 1°C), verified to be thermally neutral for this species [personal obs., 34]. Air provided to the respirometers passed through a desiccant (Drierite) and was regulated at 400 mL/min by calibrated mass-flow controllers (Tylan Corp.). Respirometers were removed from the cabinet between 08:00 and 09:00, at which time birds were reweighed and assessed for molt (see below). Oxygen consumption rates (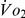 ; ml/min) were evaluated by comparing measurements of O2 content of the inlet and outlet air for each chamber using an Oxzilla oxygen analyzer (Sable Systems), sampled every 5 seconds. Each bird's BMR was measured sequentially for 15 min out of every 36 min throughout the night. Because basal metabolism is characterized by extended periods of stable
; ml/min) were evaluated by comparing measurements of O2 content of the inlet and outlet air for each chamber using an Oxzilla oxygen analyzer (Sable Systems), sampled every 5 seconds. Each bird's BMR was measured sequentially for 15 min out of every 36 min throughout the night. Because basal metabolism is characterized by extended periods of stable 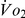 [35], RMRmin was calculated as the mean of the two lowest five-minute running averages of
[35], RMRmin was calculated as the mean of the two lowest five-minute running averages of 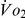 measured, usually between 02:00 and 05:00. Metabolic rates (
measured, usually between 02:00 and 05:00. Metabolic rates (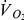 ) were converted to kJ·d−1 using the conversion 1 L O2 = 20.08 kJ [36]. All reported values of oxygen consumption represent STP conditions, and volume effects on gas concentrations due to inequalities in respiratory quotient have been addressed using an equation appropriate to the measurement system [37]:
) were converted to kJ·d−1 using the conversion 1 L O2 = 20.08 kJ [36]. All reported values of oxygen consumption represent STP conditions, and volume effects on gas concentrations due to inequalities in respiratory quotient have been addressed using an equation appropriate to the measurement system [37]:
where 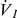 represents air flow rate corrected to STP conditions, FIO2 represents fractional O2 content in inlet air after removing CO2 and H2O, and FeO2 represents fractional O2 content in excurrent chamber air after removing CO2 and H2O.
represents air flow rate corrected to STP conditions, FIO2 represents fractional O2 content in inlet air after removing CO2 and H2O, and FeO2 represents fractional O2 content in excurrent chamber air after removing CO2 and H2O.
Energetic cost of molt
Five individuals were euthanized prior to the onset of molt and all feathers plucked, divided into 10 regions: head, neck, dorsal body, ventral body, dorsal covert, ventral covert, cloacal, primary, secondary and rectrices. The feathers were then dried overnight at 80°C and weighed to the nearest mg. The total energy content of the plumage was calculated assuming that dry feathers have an energy content of 26.4 kJ·g−1 [19]. The total cost of molt in each individual (below) was taken as the total increase in BMR above pre-molt levels over the molt period, plus the energy contained in the new plumage.
Energetic cost of feather replacement
A further 24 birds were randomly assigned to one of two treatments. “Plucked” birds (n = 12), once anaesthetized (methoxyflurane), had 25% of their body feathers, by weight, removed from each of the seven body regions outlined above, along with two feathers each from the secondaries (s6 and s7) and primaries (p7 and p8) of each wing and two centre rectrices. Plucking took place in the first week of October. Control (“sham plucked”) birds (n = 12) were also anaesthetized, but then simply handled for one minute and allowed to recover. Metabolic rates and feather growth rates (mm·day–1 [38]) were recorded weekly for each individual until they had fully replaced their artificially removed feathers (6 weeks). These measurements were continued for the duration of natural molt in the sham-plucked birds. The natural molt data are based on measurements of seven control birds due to the death of three birds following an equipment failure, and the inability of two birds to fully adjust to captive conditions (as evidenced by large fluctuations in body mass).
Statistical Analyses
Differences in individual metabolic rates between different time periods were analyzed using repeated measures ANOVA using JMP 7 (SAS). Comparison of metabolic rates, metabolic increases and feather growth rates between natural molting and plucked individuals were conducted using one-way ANOVA, again using JMP 7. All values given below represent mean (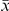 ) ± 1 S.E. unless otherwise stated.
) ± 1 S.E. unless otherwise stated.
Interspecific variation in molt cost
We compiled data on the cost of molt per gram of feathers replaced for all species studied using respirometry (indirect calorimetry), and used these to calculate conversion efficiency (energy contained within the plumage as a percentage of energy expended during its growth; Table S1). Using a tree pruned from Hackett et al. [39] and the regressionv2.m package [40] in MATLAB (R2007b), we first fitted the best of several models of evolution to the efficiency of feather production data, including species values (ordinary least squares, no phylogenetic signal), Brownian motion (phylogenetic generalized least squares), Ornstein–Uhlenbeck process (OU, drift about a fitness peak) or with branch lengths transformed using Pagel's λ parameter. We then tested for a relationship between these traits and metabolic intensity (BMRm). The OU and Pagel's λ fitting procedures calculate branch length transformation parameters, d for the OU process that is a function of time and λ for Pagel's transformation, which is constant across the tree, by restricted maximum likelihood (REML). Values close to 1 indicate a significant phylogenetic signal, whereas values close to 0 suggest the trait in question is independent of phylogeny.
Results
Molt phenology
White-plumed honeyeaters initiated molt on 13 October (±6.0 days, range 27 September - 14 November; n = 7). This appeared similar to the onset of molt in the wild, with birds at Fowler's Gap Research Station displaying the same range of molt stage as those in captivity in Wollongong in early January (personal obs.). Feather replacement lasted for 168 days (± 5.21; range = 143–196 days), from the loss of the innermost primary until the completion of feather replacement in all tracts.
Energetic cost of molt
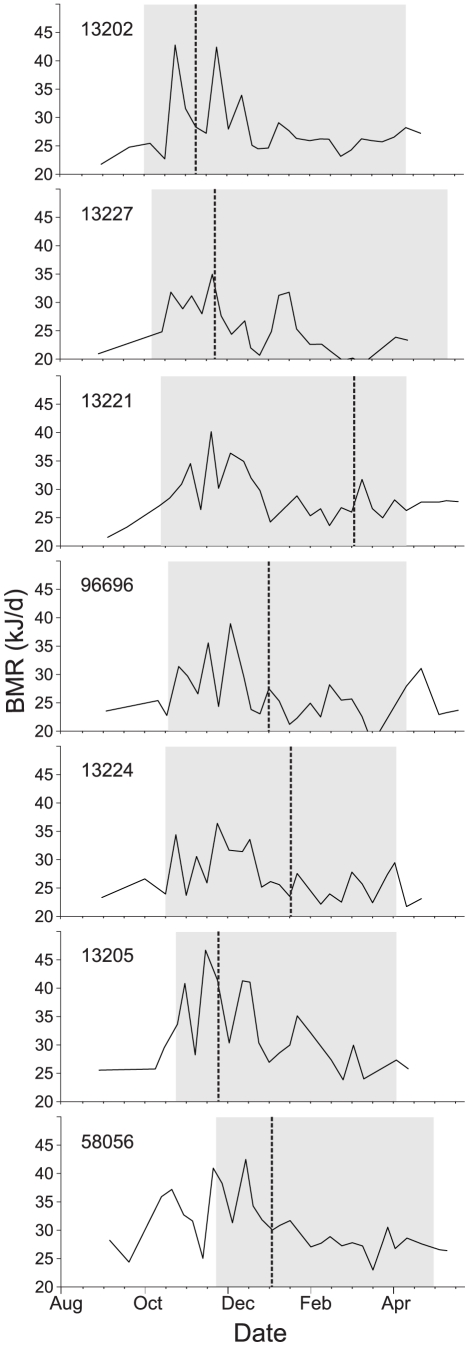
Pre-molt BMR, measured at least 30 days prior to the loss of the first primary, averaged 23.0 kJ·d−1 (± 0.65, n = 7). An individual's highest RMRmin during the molt period was recorded 30 days (± 3.7) after molt onset, and was 18.43 kJ·day−1 (± 1.14) above their pre-molt BMR, representing an 82% (± 5.59) increase. Each individual's highest RMRmin value during molt was significantly greater than both pre-molt BMR and RMRmin measured at the time of their most intense feather replacement (F2 ,5 = 97.41; P<0.0005; Table 1). The pattern of RMRmin throughout the molt period, however, did not gradually rise preceding an individual's maxima, nor show a graded decline thereafter. Instead, each bird's RMRmin, both during and outside the molt period, appeared to oscillate stochastically, with multiple peaks and troughs evident. Importantly, the pattern and size of these oscillations did not directly correspond with molt stage (Figure 1).
Table 1. Minimum resting metabolic rates during molt and replacement of plucked feathers.
| Natural molt | Plucked | |||
| Pre molt | 23.04 | ±0.65 | 23.41 | ±0.54 |
| Peak feather replacement | 30.77 | ±5.41 | 29.51 | ±1.22 |
| Maximum RMRmin | 40.84∧ | ±4.22 | 39.26 | ±1.14 |
Minimum resting metabolic rates (kJ·d−1) at various stages of feather replacement in captive White-plumed honeyeaters undergoing natural molt (n = 7) compared to conspecifics undergoing natural molt while simultaneously replacing an additional 25% of their plumage that was plucked during the experiment (n = 12; means ± SE).
indicates significantly higher values within treatments (p<0.05); there were no significant differences between treatments at any stage.
Figure 1. Minimum resting metabolic rate of individual White-plumed honeyeaters during molt.
Minimum resting metabolic rate (RMRmin in kJ·d−1) of 7 captive White-plumed Honeyeaters preceding, during, and following the period of natural molt. Numbers indicate individual identifiers. Grey shaded areas represent a given individual's molt period, dashed vertical lines represent the individual's period of peak feather replacement.
The most intense period of feather replacement occurred 56 (± 13.7) days after molt onset, at which time metabolic rates were not significantly higher than an individual's pre-molt BMR (Table 1). Furthermore, individuals yet to molt displayed very similar RMRmin profiles with respect to calendar date to their molting conspecifics, irrespective of their molt schedule (Figure 1).
Because the post-molt BMR averaged 13% (±5.7) higher than pre-molt levels (t = 2.46; d.f. = 6; P = 0.049), the BMR “baseline” used to judge molt costs on a given date was based on an interpolation between pre- and post-molt BMR measurements for each individual. These values were not adjusted for body mass as variation in individual mass accounted for less than 5% of the overall variation in RMRmin during molt (F1 ,186 = 8.93; r2 = 0.046; P = 0.0032). Integrating the daily increases in metabolic rate above this baseline throughout each bird's molt period produced an average energetic cost of feather synthesis of 453.4 kJ·bird−1 (±66.6). The energetic cost of molt (Cf; kJ.g−1 dry feathers) represents the sum of synthesis costs and the energy contained in the replacement plumage. Given an average total plumage mass of 1.28 g measured in non-molting birds and an energy content of 26.4 kJ·g−1 [19], the Cf in WPHE averaged 380.6 kJ·g dry feathers−1 (±49.3). Thus, the energy content of the newly synthesized plumage represented just 6.9% (±0.57) of the energy expended for feather renewal.
Energetic cost of feather replacement
There was no significant difference in natural molt initiation date between plucked and sham-plucked (control) WPHE (F1 ,21 = 0.713; P = 0.682). Primary feather growth rates were linear until feathers approached their full length, after which time growth rates slowed. Growth rates for the linear part of plucked feather re-growth were indistinguishable from those of corresponding primaries in sham-plucked (naturally molting) WPHEs (P>0.200; Table 2). Plucked birds also grew their naturally molted primaries (one and two) at rates that were statistically indistinguishable from sham-plucked birds (P>0.200; Table 2).
Table 2. Primary feather growth rates during molt and replacement of plucked feathers.
| Feather | Natural molt | Plucked | t | d.f. | p | ||
| Primary 1 | 2.70 | ±0.13 | 2.74 | ±0.16 | −0.732 | 10 | 0.732 |
| Primary 2 | 2.83 | ±0.13 | 2.97 | ±0.18 | −1.087 | 9 | 0.293 |
| Primary 7 | 2.65 | ±0.07 | 2.57 | ±0.07 | 0.876 | 12 | 0.393 |
| Primary 8 | 2.60 | ±0.19 | 2.35 | ±0.18 | 1.277 | 12 | 0.232 |
Feather growth rates (mm/d ± S.E.) of selected primaries in captive White-plumed honeyeaters undergoing natural molt compared to conspecifics undergoing natural molt while simultaneously replacing an additional 25% of their plumage that was plucked during the experiment.
The metabolic rate profiles of plucked birds did not differ significantly from those of naturally molting conspecifics in relation to calendar date, despite the overlap of naturally molted and plucked feather regrowth in the 10 plucked individuals. The RMRmin of plucked birds prior to molt, during the peak phase of feather regrowth, and at the individual maxima were not significantly different to RMRmin measured contemporaneously in sham plucked (naturally molting) birds (P>0.05 Table 1).
Interspecific variation in molt cost
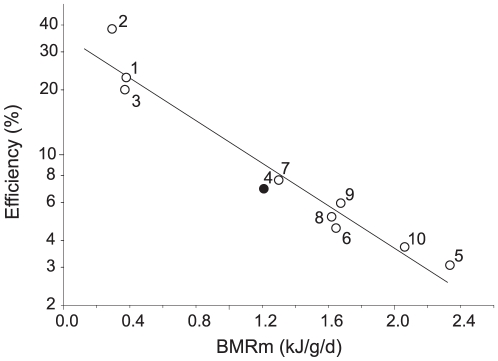
Our estimate of the energetic cost (and hence efficiency) of molt in WPHE adds to a growing body of evidence suggesting that these traits differ markedly between certain species (Table S1). The efficiency of energy conversion to new feathers during molt showed no relationship to either relative feather mass (P = 0.36) or molt rate (P = 0.06). Efficiency was, however, highly correlated with metabolic intensity (mass-specific basal metabolic rate, BMRm, kJ.g−1.d−1; r2 = 0.944, P = <0.0001; Figure 2) such that:
Figure 2. Feather production efficiency during molt.
Feather production efficiency (%; 100·kJ content·[kJ expended + content]−1) during natural molt in relation to mass specific BMR (kJ·g−1·d−1) measured using indirect calorimetry for different species of birds. Filled circle represents our white-plumed honeyeater (Lichenostomus penicillatus; 4), unfilled circles represent long-eared owl (Asio otus; 1), kookaburra (Dacelo novaeguineae; 2), European kestrel (Falco tinnunculus; 3), bluethroat (Luscinia s. Svecica; 5), European stonechat (Saxicola torquata rubicula; 6), East African stonechat (Saxicola torquata axillaries; 7), white-crowned sparrow (Zonotrichia leucophrys gambelii; 8), chaffinch (Fringilla coelebs; 9), and redpoll (Carduelis f. Flammea; 10). Values and sources in Table S1.
Phylogenetic regression revealed a strong phylogenetic signal in the relationship between BMRm and conversion efficiency during molt (Table S2), yet the relationship between BMRm and efficiency remained highly significant (r2 = 0.884). Models based on phylogenetic relationships alone (null model) were equally parsimonious as the metabolic intensity model, however this is not unexpected given that efficiency was almost synonymous with BMRm (r2 = 0.944).
The average daily cost of molt (total cost (kJ)/duration (days)) of WPHE was equivalent to 13% of their pre-molt BMR; the lowest daily molt cost of all species studied (Table S1). There was no apparent phylogenetic signal in the interspecies variation in these average daily costs (3).
Discussion
White-plumed honeyeaters experience a considerable increase in RMRmin during the population's molt period, despite living in an environment with unpredictable resource availability and often experiencing molt/breeding overlap. Maximum increases in RMRmin during a molt cycle have been shown to be as little as 10% of pre-molt levels in large birds such as the 280 g long-eared owl [22], and as high as 111% and 106% in bluethroats (17 g) and redpolls (13 g), respectively [20]. White-plumed honeyeaters showed some of the highest reported increases in metabolic rate, averaging 82% above individual pre-molt levels (Table S1).
Increases in RMRmin were not, however, coincident with the temporal stage of feather replacement in either naturally molting or plucked birds. Surprisingly, individual WPHE reached their metabolic maxima relatively synchronously with respect to calendar date, regardless of their molt stage (Figure 1). Further, RMRmin maxima were no higher in the plucked birds than in naturally molting conspecifics (Table 1), despite replacing an additional 25% of their plumage at the same rate as naturally molting birds (Table 2). White-crowned sparrows have also been shown to replace substantial amounts of plucked feathers without experiencing a rise in RMRmin [41], demonstrating that feather replacement per se can not explain the increased energy demands measured during the molt period. Moreover, weekly measures of RMRmin in individual WPHE did not follow a graded rise and a steady decline about the period of feather replacement maxima. Large RMRmin fluctuations were measured periodically, suggesting that coincident physiological processes are of a cyclical nature. Importantly, such week-to-week fluctuations would be overlooked in studies with less frequent metabolic measurements.
Efficiency of energy conversion during molt (energy contained in the feathers produced as a proportion of energy expended) is enigmatically low in all birds studied and, with the exception of kookaburras [21], remains the most inefficient form of protein conversion described in vertebrates [42]. The energetic cost of the molt period in WPHE was remarkably similar to values reported for other small passerines from more predictable north-temperate environments [e.g. 23]. Furthermore, the WPHE values are consistent with accumulating evidence that metabolic rate increases during molt scale to a species' metabolic intensity (BMRm: kJ·g−1·d−1) consistently across all species examined [20], [23], [24]. This predicts that the smallest birds with the highest mass-specific metabolic rates will have the highest costs, and hence the lowest conversion efficiencies (Figure 2). Although there appears to be a phylogenetic component to this relationship, interpretation of the influence of phylogeny on the costs entrained in the molt period is hindered by the complete lack of data from small (i.e. high metabolic intensity) non-passerine species and large (i.e. low metabolic intensity) passerine species. Given the tight relationship between molt efficiency and mass-specific metabolic rate in all species studied to date, regardless of life-history pattern, there appears to be underlying costs entrained in the molt period that are proportional to metabolic intensity, but are not due to feather replacement per se. Efficiency may, therefore, be an artifact of the assumption that metabolic increases during molt are solely fueling feather synthesis.
There is ample evidence that molting birds undergo extensive physiological changes beyond the production of feathers alone, including plasma volume expansion [43], [44], and organ hypertrophy [45]. In addition, there is increasing evidence that immune processes are altered during the molt period, with evidence of energetically- and nutritionally-demanding acute-phase and inflammatory responses being down-regulated during this period of the annual cycle (Table 3; [46]–[48]). This would reduce the competition between immunity and molt for nutritional resources, thus permitting birds to maintain consistent feather growth rates when immune challenged [49] and, perhaps more importantly, to ensure feathers of a consistently high quality are grown [50]. Indeed, it could be hypothesized that the high cost of molt is not primarily due to the quantity of nutrients/protein directly needed for feather synthesis, but instead represents the cost of ensuring a constant supply of these materials during feather replacement, even when confronting immune challenges. There is experimental evidence that protein turnover during molt is 3.5 times that expected for peak rates of feather synthesis in white-crowned sparrows [51]. Given the consistent relation between rates of protein turnover and metabolism in endotherms of very different sizes [52], if molt provokes similar proportionate increases in protein turnover in all birds, it follows logically that molt costs will scale with metabolic intensity rather than the actual amount of feathers being replaced. Furthermore, such protein turnover may well follow a cyclic pattern, which would be in accordance with the metabolic profiles observed in molting WPHE and their lack of correspondence with feather growth (Figure 1). Additional indirect evidence for the high cost of such quality assurance mechanisms comes from studies of molting waterfowl. These birds characteristically show a brief period of simultaneous flight feather replacement, at a purportedly high cost when considering the relatively small mass of feathers being replaced [53]. Such costs may be necessary to ensure high quality, particularly for flight feathers. Unfortunately we did not compare the quality of feathers formed following plucking to those formed during natural molt.
Table 3. Published accounts of immune system alterations measured during the molt period.
| Tissue | Change | Species | Reference |
| Spleen mass | increase | Willow tit | [54] |
| Monocytes | increase | House sparrow | [55] |
| Red Knot | [47] | ||
| Total immunoglobulins | increase | King penguin | [56] |
| Great tit | [57] | ||
| Humoral immunity | decrease | Red knot | [47] |
| Domestic Chicken | [46] | ||
| Inflammatory response | decrease | House sparrow | [48] |
| Red knot | [47] | ||
| Domestic chicken | [46] |
Regardless of the physiological mechanisms underpinning the metabolic costs entrained in the molt period, a key factor that modulates the physiological burden on the individual is the rate at which these costs are incurred [38], [42]. Decreased rates of molt should decrease the daily costs to an individual while also minimizing any potential disparity between resource requirements and the availability of these substrates in the diet. As such, relative molt costs, in the form of average daily costs as a proportion of pre-molt BMR, provide a more appropriate conceptual framework when considering both the physiological burden placed on an individual, and how this may differ between species. Molt duration appears to contract with increasing latitude in parallel with the decreasing summer duration. For example, bluethroats breeding and molting in the arctic undergo a complete molt in 62 days, practically twice as fast as East African stonechats (123 d), and almost three times as fast as WPHE (168 d, Table S1). Consequently, although the total cost of molt in WPHE is differs little from same-sized birds with much faster molt rates, their protracted molt period substantially reduces these molt costs on a daily basis to an extent that their daily molt costs are the lowest for all species studied. Interestingly, their prolonged molt appears to be mediated through a direct decrease in the rate at which each feather is grown, rather than a reduced frequency of feather shedding compared to north-temperate species [38]. These patterns are particularly beneficial for species living in environments with unpredictable resource availability, where breeding and molt schedules are likely to overlap.
Supporting Information
Estimates of feather production cost based on indirect calorimetry for different species of birds.
(DOCX)
Evaluation of evolutionary models for the efficiency of feather production.
(DOCX)
Evaluation of null evolutionary models for the average daily cost of molt.
(DOCX)
Acknowledgments
We would like to thank Terence O'Dwyer, Malsha Kitulagodage, Jodie Dunn, Jodie McGill and Tracy Maddocks for help in maintaining the animals. Lee Astheimer and Terence O'Dwyer helped with metabolic measurements, molt assessment, and plucking. BriAnne Addison provided valuable assistance in conducting the phylogenetic regressions, and two anonymous referees provided valuable comments on an earlier version of this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The Australian Research Council (DP0453021) and the Institute for Conservation Biology at the University of Wollongong contributed funds for this research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ricklefs RE. Density dependence, evolutionary optimization, and the diversification of avian life histories. Condor. 2000;102:9–22. [Google Scholar]
- 2.Jacobs JD, Wingfield JC. Endocrine control of life-cycle stages: a constraint on response to the environment? Condor. 2000;102:35–51. [Google Scholar]
- 3.Barta Z, Houston AI, McNamara JM, Welham RK, Hedenstrom A, et al. Annual routines of non-migratory birds: optimal moult strategies. Oikos. 2006;112:580–593. [Google Scholar]
- 4.Newton I, Rothery P. The timing, duration and pattern of moult and its relationship to breeding in a population of the European Greenfinch Carduelis chloris. Ibis. 2005;147:667–679. [Google Scholar]
- 5.Hemborg C, Sanz JJ, Lundberg A. Effects of latitude on the trade-off between reproduction and moult: a long-term study with pied flycatcher. Oecologia. 2001;129:206–212. doi: 10.1007/s004420100710. [DOI] [PubMed] [Google Scholar]
- 6.Hahn TP. Reproductive seasonality in an opportunistic breeder, the red cross-bill (Loxia curvirostra). Ecology. 1998;79:2365–2375. [Google Scholar]
- 7.Craig A. The timing of breeding and wing-moult of 4 African Sturnidae. Ibis. 1983;125:346–352. [Google Scholar]
- 8.Hemborg C, Merilä J. Reproductive investment and moult-breeding overlap in the collared flycatcher Ficedula albicollis: an experimental approach. Annales Zoologici Fennici. 1999;36:1–9. [Google Scholar]
- 9.Nilsson JA, Svensson E. The cost of reproduction: A new link between current reproductive effort and future reproductive success. Proceedings of the Royal Society B-Biological Sciences. 1996;263:711–714. [Google Scholar]
- 10.Slagsvold T, Dale S. Disappearance of female pied flycatchers in relation to breeding stage and experimentally induced molt. Ecology. 1996;77:461–472. [Google Scholar]
- 11.Siikamaki P, Hovi M, Ratti O. A trade-off between current reproduction and moult in the Pied Flycatcher - an experiment. Functional Ecology. 1994;8:587–593. [Google Scholar]
- 12.Sanz JJ. Clutch size manipulation in the pied flycatcher: Effects on nestling growth, parental care and moult. Journal of Avian Biology. 1997;28:157–162. [Google Scholar]
- 13.Hemborg C, Lundberg A. Costs of overlapping reproduction and moult in passerine birds: an experiment with the pied flycatcher. Behavioral Ecology and Sociobiology. 1998;43:19–23. [Google Scholar]
- 14.Siikamaki P. Limitation of reproductive success by food availability and breeding time in pied flycatchers. Ecology. 1998;79:1789–1796. [Google Scholar]
- 15.Svensson E, Nilsson JA. The trade-off between molt and parental care: A sexual conflict in the blue tit? Behavioral Ecology. 1997;8:92–98. [Google Scholar]
- 16.Wingfield JC. Organisation of vertebrate annual cycles: implications for control mechanisms. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2008;363:425–441. doi: 10.1098/rstb.2007.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chilgren JD. Body composition of captive White-crowned Sparrows during postnuptial moult. Auk. 1977;94:677–688. [Google Scholar]
- 18.Newton I. The temperatures, weights and body composition of moulting bullfinches. Condor. 1968;70:323–332. [Google Scholar]
- 19.Murphy ME. Energetics and nutrition of molt. In: Carey C, editor. Avian energetics and nutritional ecology. New York: Plenum Press; 1996. pp. 158–198. [Google Scholar]
- 20.Lindström A, Visser GH, Daan S. The energetic cost of feather synthesis in proportional to basal metabolic rate. Physiological Zoology. 1993;66:490–510. [Google Scholar]
- 21.Buttemer WA, Nicol SC, Sharman A. Thermoenergetics of pre-moulting and moulting kookaburras (Dacelo novaeguineae): they're laughing. Journal of Comparative Physiology B. 2003;173:223–230. doi: 10.1007/s00360-003-0326-z. [DOI] [PubMed] [Google Scholar]
- 22.Wijandts H. Ecological energetics of the long-eared owl (Asio otus). Ardea. 1984;72:1–92. [Google Scholar]
- 23.Klaassen M. Moult and basal metabolic costs in males of two species of stonechats: the European Saxicola torquata ribicula and the East African S. t. axillaris. Oecologia. 1995;104:424–432. doi: 10.1007/BF00341339. [DOI] [PubMed] [Google Scholar]
- 24.Dietz MW, Daan S, Masman D. Energy requirements for molt in the Kestrel Falco tinnunculus. Physiological Zoology. 1992;65:1217–1235. [Google Scholar]
- 25.Foster MS. The overlap of molting and breeding in some tropical birds. Condor. 1975;77:304–314. [Google Scholar]
- 26.Keast A. Moult in birds of the Australian dry country relative to rainfall and breeding. Journal of the Zoological Society of London. 1968;155:185–200. [Google Scholar]
- 27.Chapman A. Breeding and moult of four bird species in tropical West Africa. Tropical Zoology. 1995;8:227–238. [Google Scholar]
- 28.Macdonald BCT. University of New South Wales Fowlers Gap arid zone research station - nearly 50 years of research. Rangeland Journal. 2000;22:5–31. [Google Scholar]
- 29.Wikelski M, Martin LB, Scheuerlein A, Robinson MT, Robinson ND, et al. Avian circannual clocks: adaptive significance and possible involvement of energy turnover in their proximate control. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2008;363:411–423. doi: 10.1098/rstb.2007.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farner DS, Serventy DL. The timing of reproduction in birds in the arid regions of Australia. Anatomical Record. 1960;137:354. [Google Scholar]
- 31.Zann RA, Morton SR, Jones KR, Burley NT. The timing of breeding by Zebra finches in relation to rainfall in central Australia. Emu. 1995;95:208–222. [Google Scholar]
- 32.Astheimer LB, Buttemer WA. Gonadal and hormonal patterns in the annual cycle of an Australian honeyeater. In: Adams NJ, Slotow, R.H, editors. Proc 22 Int Ornithol Congr, Durban. Johannesburg: BirdLife South Africa; 1999. pp. 1768–1783. [Google Scholar]
- 33.Astheimer LB, Buttemer WA. Changes in latitude, change in attitude: a perspecive on ecophysiological studies of Australian birds. Emu. 2002;102:19–27. [Google Scholar]
- 34.Buttemer WA, Astheimer LB. Testosterone does not affect basal metabolic rate or blood parasite load in captive male white-plumed honeyeaters Lichenostomus penicillatus. Journal of Avian Biology. 2000;31:479–488. [Google Scholar]
- 35.Buttemer WA, Astheimer LB, Wingfield JC. The effect of corticosterone on standard metabolic rates of small passerine birds. Journal of Comparative Physiology B. 1991;161:427–431. doi: 10.1007/BF00260804. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Nielsen K. Cambridge: Cambridge University Press; 1979. Animal Physiology. [Google Scholar]
- 37.Withers PC. Design, calibration and calculation for flow-through respirometry systems. Australian Journal of Zoology. 2001;49:445–461. [Google Scholar]
- 38.Dawson A. A detailed analysis of primary feather moult in the Common Starling Sturnus vulgaris - new feather mass increases at a constant rate. Ibis. 2003;145:E69–E76. [Google Scholar]
- 39.Hackett SJ, Kimball RT, Reddy S, Bowie RCK, Braun EL, et al. A phylogenomic study of birds reveals their evolutionary history. Science. 2008;320:1763–1768. doi: 10.1126/science.1157704. [DOI] [PubMed] [Google Scholar]
- 40.Lavin SR, Karasov WH, Ives AR, Middleton KM, Garland T. Morphometrics of the avian small intestine compared with that of nonflying mammals: A phylogenetic approach. Physiological and Biochemical Zoology. 2008;81:526–550. doi: 10.1086/590395. [DOI] [PubMed] [Google Scholar]
- 41.Schieltz PC, Murphy ME. Diurnal variation in oxygen consumption by molting and nonmolting sparrows. Comparative Biochemistry and Physiology. 1995;112A:265–272. [Google Scholar]
- 42.Murphy ME, King JR. Energy and nutrient use during moult by White-crowned Sparrows Zonotrichia leucophrys gambelii. Ornis Scandinavica. 1992;23:304–313. [Google Scholar]
- 43.Chilgren JD, DeGraw WA. Some blood characterisitcs of White-crowned sparrows during molt. Auk. 1977;94:169–171. [Google Scholar]
- 44.DeGraw WA, Kern MD. Changes in the blood and plasma-volume of Harris sparrows during postnuptial moult. Comparative Biochemistry and Physiology. 1985;81A:889–893. doi: 10.1016/0300-9629(85)90925-9. [DOI] [PubMed] [Google Scholar]
- 45.Ward P, D'Cruz D. Seasonal changes in thymus gland of a tropical bird. Ibis. 1968;110:203. [Google Scholar]
- 46.Alodan MA, Mashaly MM. Effect of induced molting in laying hens on production and immune parameters. Poultry Science. 1999;78:171–177. doi: 10.1093/ps/78.2.171. [DOI] [PubMed] [Google Scholar]
- 47.Buehler DM, Piersma T, Matson K, Tieleman BI. Seasonal redistribution of immune function in a migrant shorebird: annual-cycle effects override adjustments to thermal regime. American Naturalist. 2008;172:783–796. doi: 10.1086/592865. [DOI] [PubMed] [Google Scholar]
- 48.Martin LB. Trade-offs between molt and immune activity in two populations of house sparrows (Passer domesticus). Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2005;83:780–787. [Google Scholar]
- 49.Ilmonen P, Taarna T, Hasselquist D. Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proceedings of the Royal Society B-Biological Sciences. 2000;267:665–670. doi: 10.1098/rspb.2000.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freed LA, Cann RL, Goff ML, Kuntz WA, Bodner GR. Increase in avian malaria at upper elevation in Hawaii. Condor. 2005;107:753–764. [Google Scholar]
- 51.Murphy ME, Taruscio TG. Sparrows increase their rates of tissue and whole-body protein synthesis during the annual molt. Comparative Biochemistry and Physiology. 1995;111A:385–396. [Google Scholar]
- 52.Young VR, Yu YM, Fukagawa NK. Energy and protein turnover. In: J.M K, Tucker HG, editors. Energy Metabolism: Tissue Determinants and Cellular Corollaries. New York: Raven Press; 1992. pp. 439–466. [Google Scholar]
- 53.Portugal SJ, Green JA, Butler PJ. Annual changes in body mass and resting metabolism in captive barnacle geese (Branta leucopsis): the importance of wing moult. Journal of Experimental Biology. 2007;210:1391–1397. doi: 10.1242/jeb.004598. [DOI] [PubMed] [Google Scholar]
- 54.Silverin B, Fange R, Viebke PA, Westin J. Seasonal changes in mass and histology of the spleen in Willow Tits Parus montanus. Journal of Avian Biology. 1999;30:255–262. [Google Scholar]
- 56.Bourgeon S, Viera VM, Raclot T, Groscolas R. Hormones and immunoglobulin levels in king penguins during moulting and breeding fasts. Ecoscience. 2007;14:519–528. [Google Scholar]
- 57.Pap PL, Vagasi CI, Tokolyi J, Czirjak GA, Barta Z. Variation in haematological indices and immune function during the annual cycle in the Great Tit Parus major. Ardea. 2010;98:105–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Estimates of feather production cost based on indirect calorimetry for different species of birds.
(DOCX)
Evaluation of evolutionary models for the efficiency of feather production.
(DOCX)
Evaluation of null evolutionary models for the average daily cost of molt.
(DOCX)