Abstract
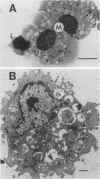
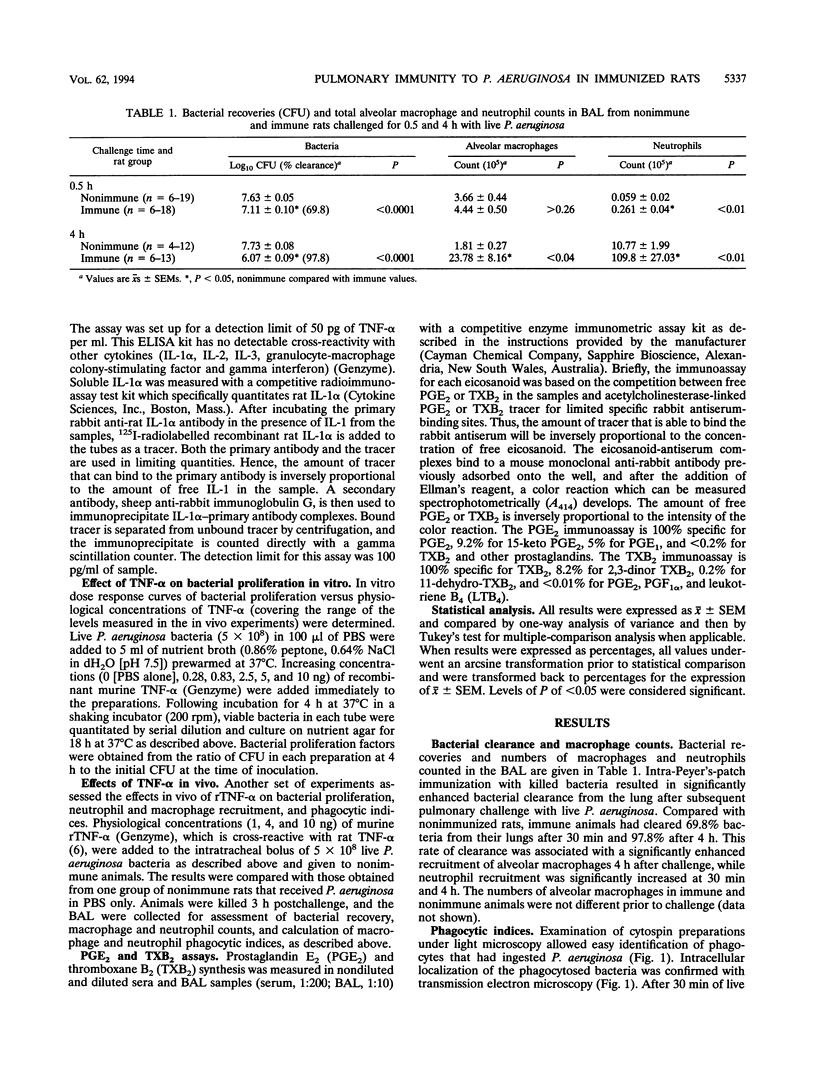
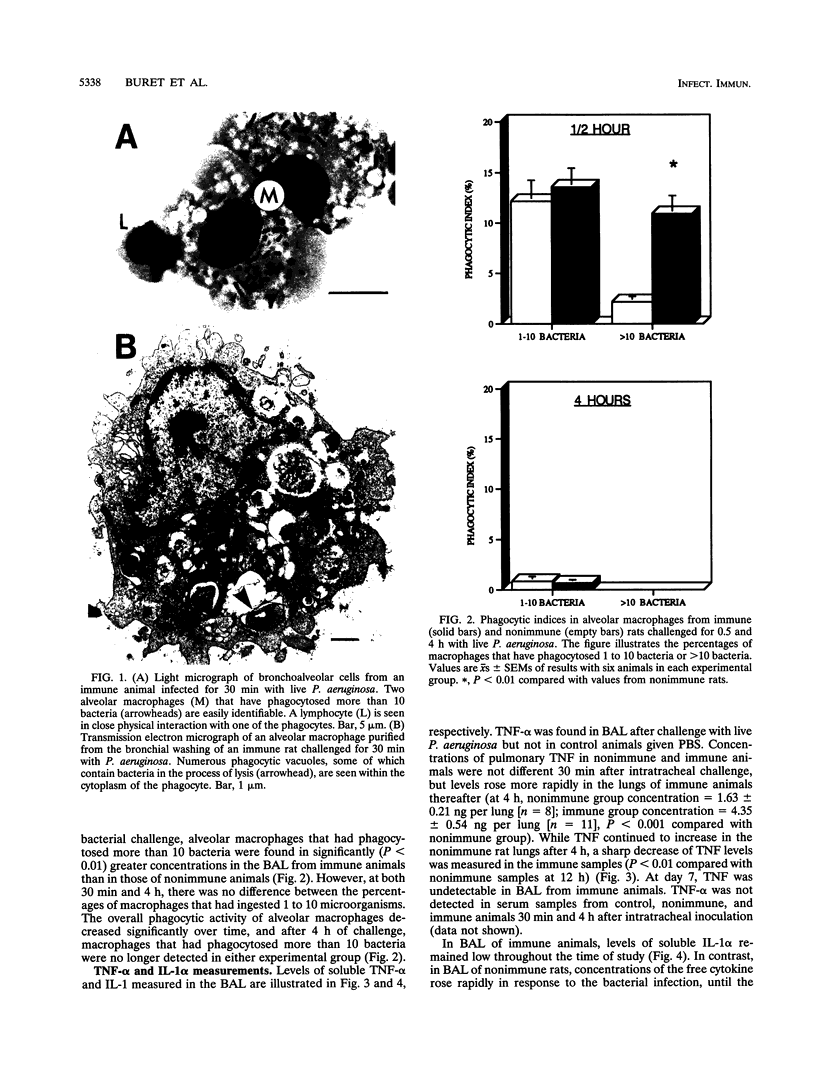
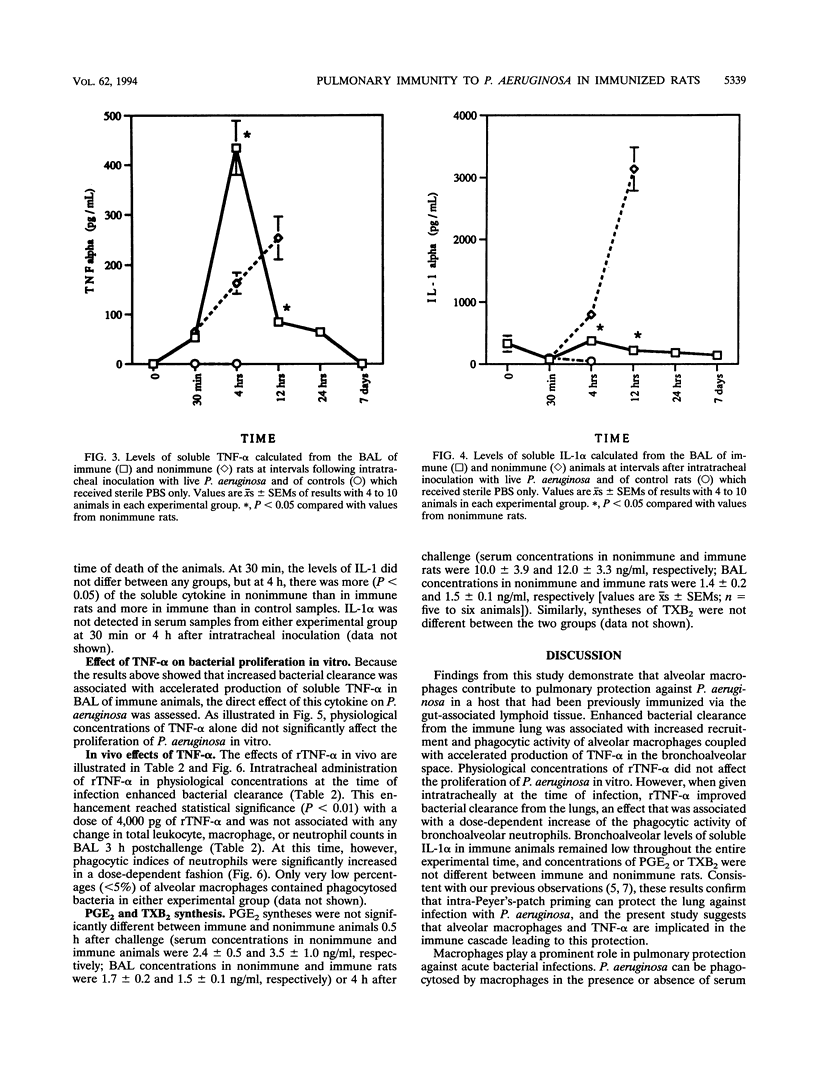
The aims of this study were to assess the role played by alveolar macrophages, tumor necrosis factor alpha (TNF-alpha), and interleukin-1 alpha (IL-1 alpha) in pulmonary immunity against Pseudomonas aeruginosa in animals that have been immunized via the gut-associated lymphoid tissue. Following intra-Peyer's patch immunization and subsequent intratracheal challenge with live bacteria, significantly enhanced bacterial clearance from the lungs correlated with an increase in bronchoalveolar neutrophils, increased recruitment and phagocytic activity of alveolar macrophages, and accelerated production of TNF-alpha in the bronchoalveolar space, while levels of IL-1 alpha remained low. Administration of recombinant TNF-alpha in physiological concentrations did not affect the proliferation of P. aeruginosa in vitro, but when given intratracheally to rats at the time of infection, recombinant TNF-alpha significantly increased bacterial clearance from the lungs. In these animals, phagocytic activity of bronchoalveolar neutrophils was enhanced, while the recruitment of alveolar macrophages and neutrophils remained unchanged. In acutely infected nonimmune animals, bronchoalveolar concentrations of soluble IL-1 alpha and TNF-alpha increased until the time of death. Levels of prostaglandin E2 and thromboxane B2 were similar in each experimental group. These results indicate that infection in immune animals enhanced both recruitment and phagocytic activity of alveolar macrophages as well as induced an accelerated production of TNF-alpha. In immune challenged animals, this cytokine enhanced the phagocytic activity of neutrophils and improved bacterial clearance from the lung. Levels of soluble IL-1 alpha and TNF-alpha in nonimmune rats increased consistently following infection until the time of death, thus implicating these cytokines in the pathogenesis of acute P. aeruginosa pneumonia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J. Regulation of magnitude of antibody response to bacterial polysaccharide antigens by thymus-derived lymphocytes. Infect Immun. 1990 Nov;58(11):3465–3468. doi: 10.1128/iai.58.11.3465-3468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buret A., Cripps A. W. The immunoevasive activities of Pseudomonas aeruginosa. Relevance for cystic fibrosis. Am Rev Respir Dis. 1993 Sep;148(3):793–805. doi: 10.1164/ajrccm/148.3.793. [DOI] [PubMed] [Google Scholar]
- Buret A., Dunkley M., Clancy R. L., Cripps A. W. Effector mechanisms of intestinally induced immunity to Pseudomonas aeruginosa in the rat lung: role of neutrophils and leukotriene B4. Infect Immun. 1993 Feb;61(2):671–679. doi: 10.1128/iai.61.2.671-679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin Y. H., Cai J. P., Johnson K. Lymphocyte adhesion to cultured Peyer's patch high endothelial venule cells is mediated by organ-specific homing receptors and can be regulated by cytokines. J Immunol. 1990 Dec 1;145(11):3669–3677. [PubMed] [Google Scholar]
- Cripps A. W., Dunkley M. L., Clancy R. L. Mucosal and systemic immunizations with killed Pseudomonas aeruginosa protect against acute respiratory infection in rats. Infect Immun. 1994 Apr;62(4):1427–1436. doi: 10.1128/iai.62.4.1427-1436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Modalities for reducing interleukin 1 activity in disease. Immunol Today. 1993 Jun;14(6):260–264. doi: 10.1016/0167-5699(93)90042-J. [DOI] [PubMed] [Google Scholar]
- Dunkley M. L., Husband A. J. Routes of priming and challenge for IgA antibody-containing cell responses in the intestine. Immunol Lett. 1990 Nov;26(2):165–170. doi: 10.1016/0165-2478(90)90140-l. [DOI] [PubMed] [Google Scholar]
- Fan S., Fehr H. G., Adams D. Activation of macrophages for ADCC in vitro: effects of IL-4, TNF, interferons-alpha/beta, interferon-gamma, and GM-CSF. Cell Immunol. 1991 Jun;135(1):78–87. doi: 10.1016/0008-8749(91)90255-a. [DOI] [PubMed] [Google Scholar]
- Galve-de Rochemonteix B., Nicod L. P., Chicheportiche R., Lacraz S., Baumberger C., Dayer J. M. Regulation of interleukin-1ra, interleukin-1 alpha, and interleukin-1 beta production by human alveolar macrophages with phorbol myristate acetate, lipopolysaccharide, and interleukin-4. Am J Respir Cell Mol Biol. 1993 Feb;8(2):160–168. doi: 10.1165/ajrcmb/8.2.160. [DOI] [PubMed] [Google Scholar]
- Gilligan P. H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991 Jan;4(1):35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Kolls J. K., Beck J. M., Nelson S., Summer W. R., Shellito J. Alveolar macrophage release of tumor necrosis factor during murine Pneumocystis carinii pneumonia. Am J Respir Cell Mol Biol. 1993 Apr;8(4):370–376. doi: 10.1165/ajrcmb/8.4.370. [DOI] [PubMed] [Google Scholar]
- Luo G., Niesel D. W., Shaban R. A., Grimm E. A., Klimpel G. R. Tumor necrosis factor alpha binding to bacteria: evidence for a high-affinity receptor and alteration of bacterial virulence properties. Infect Immun. 1993 Mar;61(3):830–835. doi: 10.1128/iai.61.3.830-835.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham R. B., Pier G. B., Goellner J. J., Mizel S. B. In vitro T cell-mediated killing of Pseudomonas aeruginosa. II. The role of macrophages and T cell subsets in T cell killing. J Immunol. 1985 Jun;134(6):4112–4117. [PubMed] [Google Scholar]
- Mork T., Hancock R. E. Mechanisms of nonopsonic phagocytosis of Pseudomonas aeruginosa. Infect Immun. 1993 Aug;61(8):3287–3293. doi: 10.1128/iai.61.8.3287-3293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster A. M., Leary A. G. Cell-mediated immune responses to Pseudomonas aeruginosa. Am J Surg. 1977 Jun;133(6):710–712. doi: 10.1016/0002-9610(77)90160-x. [DOI] [PubMed] [Google Scholar]
- Ogle J. D., Noel J. G., Sramkoski R. M., Ogle C. K., Alexander J. W. Effects of combination of tumor necrosis factor alpha and chemotactic peptide, f-Met-Leu-Phe, on phagocytosis of opsonized microspheres by human neutrophils. Inflammation. 1992 Feb;16(1):57–68. doi: 10.1007/BF00917515. [DOI] [PubMed] [Google Scholar]
- Ozaki Y., Ohashi T., Minami A., Nakamura S. Enhanced resistance of mice to bacterial infection induced by recombinant human interleukin-1a. Infect Immun. 1987 Jun;55(6):1436–1440. doi: 10.1128/iai.55.6.1436-1440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmely M., Gale A., Clabaugh M., Horvat R., Zhou W. W. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect Immun. 1990 Sep;58(9):3009–3014. doi: 10.1128/iai.58.9.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesanti E. L. Interaction of cytokines and alveolar cells with Pneumocystis carinii in vitro. J Infect Dis. 1991 Mar;163(3):611–616. doi: 10.1093/infdis/163.3.611. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Markham R. B. Induction in mice of cell-mediated immunity to Pseudomonas aeruginosa by high molecular weight polysaccharide and vinblastine. J Immunol. 1982 May;128(5):2121–2125. [PubMed] [Google Scholar]
- Pierangeli S. S., Sonnenfeld G. Treatment of murine macrophages with murine interferon-gamma and tumour necrosis factor-alpha enhances uptake and intracellular killing of Pseudomonas aeruginosa. Clin Exp Immunol. 1993 Aug;93(2):165–171. doi: 10.1111/j.1365-2249.1993.tb07960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y. Phagocytic defense in the lung. Antibiot Chemother (1971) 1985;36:74–87. [PubMed] [Google Scholar]
- Rola-Pleszczynski M., Lemaire I. Leukotrienes augment interleukin 1 production by human monocytes. J Immunol. 1985 Dec;135(6):3958–3961. [PubMed] [Google Scholar]
- Sauder D. N., Mounessa N. L., Katz S. I., Dinarello C. A., Gallin J. I. Chemotactic cytokines: the role of leukocytic pyrogen and epidermal cell thymocyte-activating factor in neutrophil chemotaxis. J Immunol. 1984 Feb;132(2):828–832. [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Speert D. P., Loh B. A., Cabral D. A., Salit I. E. Nonopsonic phagocytosis of nonmucoid Pseudomonas aeruginosa by human neutrophils and monocyte-derived macrophages is correlated with bacterial piliation and hydrophobicity. Infect Immun. 1986 Jul;53(1):207–212. doi: 10.1128/iai.53.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck M. J., Roth J. A. Neutrophil activation by recombinant cytokines. Rev Infect Dis. 1989 Jul-Aug;11(4):549–568. doi: 10.1093/clinids/11.4.549. [DOI] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Roux-Lombard P., Girardin E., Grau G., Dayer J. M. Relation between tumor necrosis factor-alpha and granulocyte elastase-alpha 1-proteinase inhibitor complexes in the plasma of patients with cystic fibrosis. Am Rev Respir Dis. 1989 Dec;140(6):1640–1644. doi: 10.1164/ajrccm/140.6.1640. [DOI] [PubMed] [Google Scholar]
- Sypek J. P., Jacobson S., Vorys A., Wyler D. J. Comparison of gamma interferon, tumor necrosis factor, and direct cell contact in activation of antimycobacterial defense in murine macrophages. Infect Immun. 1993 Sep;61(9):3901–3906. doi: 10.1128/iai.61.9.3901-3906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Vannier E., Miller L. C., Dinarello C. A. Coordinated antiinflammatory effects of interleukin 4: interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4076–4080. doi: 10.1073/pnas.89.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A., Dottorini M., Puliti M., Todisco T., Cenci E., Bistoni F. Defective candidacidal activity of alveolar macrophages and peripheral blood monocytes from patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991 May;143(5 Pt 1):1049–1054. doi: 10.1164/ajrccm/143.5_Pt_1.1049. [DOI] [PubMed] [Google Scholar]
- Wallace F. J., Clancy R. L., Cripps A. W. An animal model demonstration of enhanced clearance of nontypable Haemophilus influenzae from the respiratory tract after antigen stimulation of gut-associated lymphoid tissue. Am Rev Respir Dis. 1989 Aug;140(2):311–316. doi: 10.1164/ajrccm/140.2.311. [DOI] [PubMed] [Google Scholar]
- Watson M. L., Smith D., Bourne A. D., Thompson R. C., Westwick J. Cytokines contribute to airway dysfunction in antigen-challenged guinea pigs: inhibition of airway hyperreactivity, pulmonary eosinophil accumulation, and tumor necrosis factor generation by pretreatment with an interleukin-1 receptor antagonist. Am J Respir Cell Mol Biol. 1993 Apr;8(4):365–369. doi: 10.1165/ajrcmb/8.4.365. [DOI] [PubMed] [Google Scholar]
- Wilmott R. W., Kassab J. T., Kilian P. L., Benjamin W. R., Douglas S. D., Wood R. E. Increased levels of interleukin-1 in bronchoalveolar washings from children with bacterial pulmonary infections. Am Rev Respir Dis. 1990 Aug;142(2):365–368. doi: 10.1164/ajrccm/142.2.365. [DOI] [PubMed] [Google Scholar]
- Yasuda H., Ajiki Y., Shimozato T., Kasahara M., Kawada H., Iwata M., Shimizu K. Therapeutic efficacy of granulocyte colony-stimulating factor alone and in combination with antibiotics against Pseudomonas aeruginosa infections in mice. Infect Immun. 1990 Aug;58(8):2502–2509. doi: 10.1128/iai.58.8.2502-2509.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Doerfler M., Lee T. C., Guillemin B., Rom W. N. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosis components. J Clin Invest. 1993 May;91(5):2076–2083. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. W., Barza M., Wolff S. M., Dinarello C. A. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]