Abstract
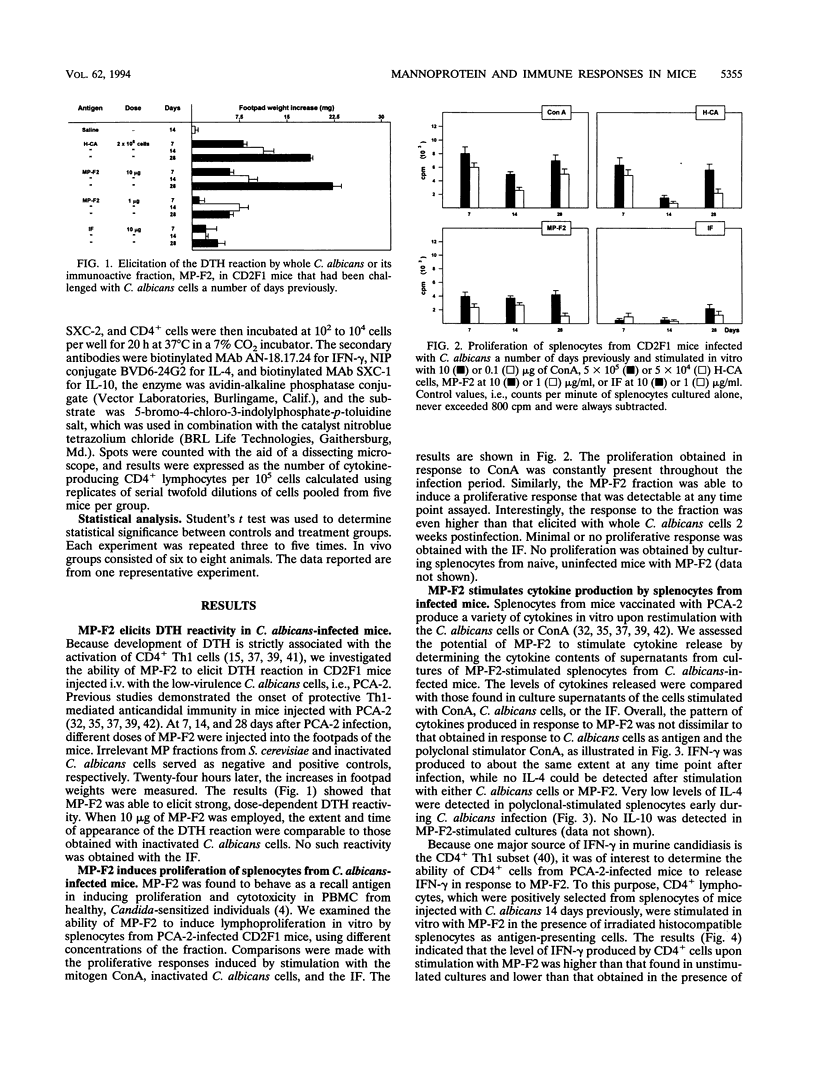
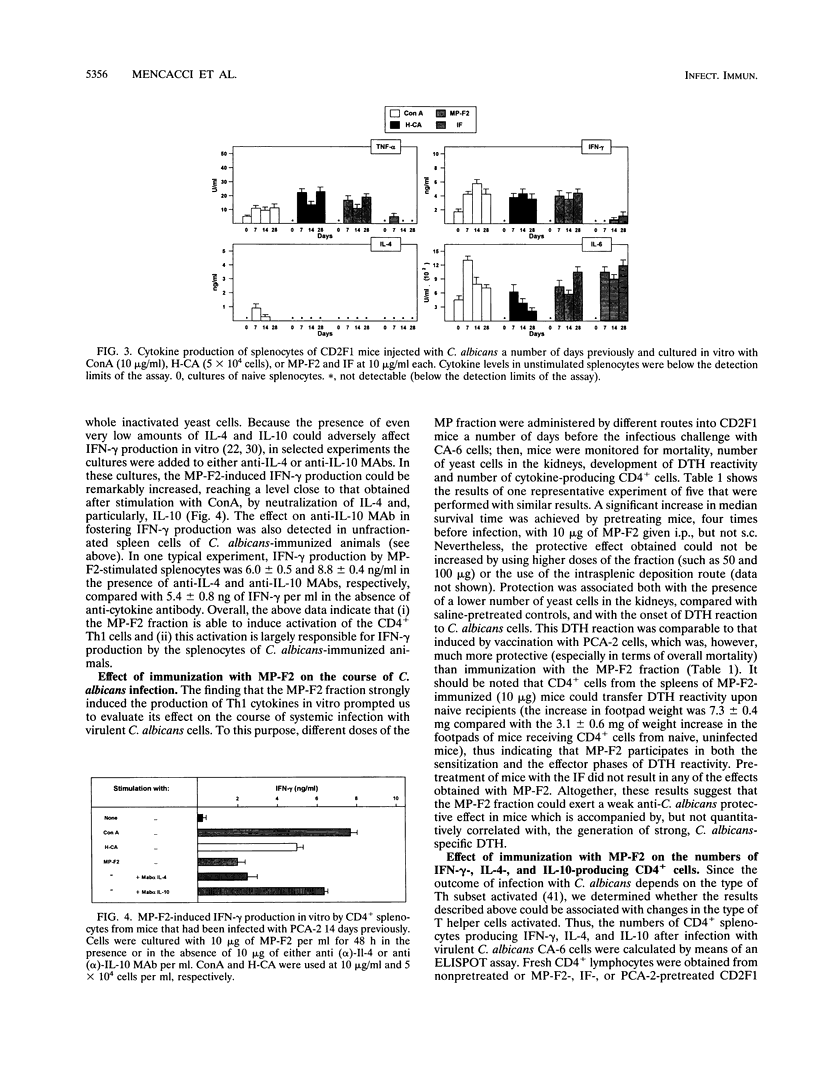
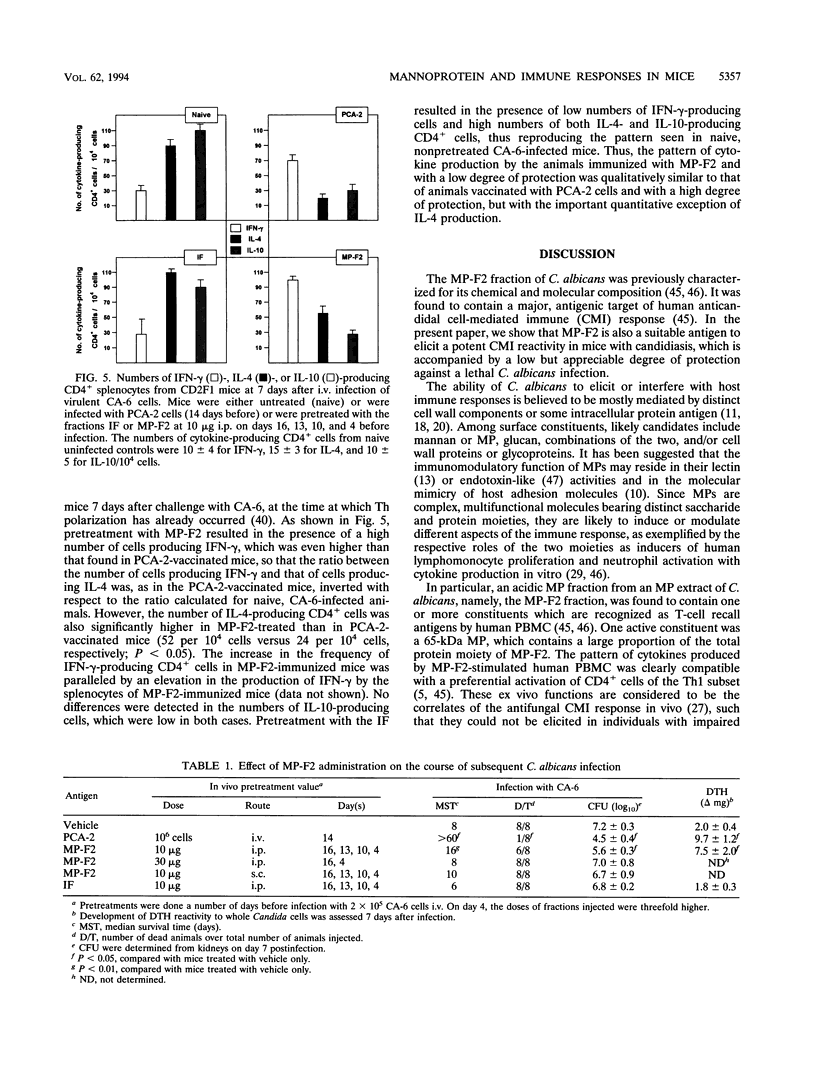
To identify major immunogenic constituents of Candida albicans, the effect of a mannoprotein fraction (MP-F2) on the elicitation of a delayed-type hypersensitivity (DTH) reaction, cytokine production, and protection from a virulent Candida challenge in a mouse candidiasis model was studied. In mice immunized with whole cells of a low-virulence strain of C. albicans and thus protected against a challenge with a highly virulent strain of this fungus, MP-F2 was able to elicit a strong DTH response that was accompanied by splenocyte proliferation in vitro in the presence of Candida antigen. The supernatants of MP-F2-stimulated splenocyte cultures contained gamma interferon (IFN-gamma, a typical CD4+ T helper-1 (Th1) cytokine, but no interleukin-4, (IL-4), a typical CD4+ Th2 cytokine. IFN-gamma was produced by CD4+ cells, and its level could be greatly increased by the addition of anti-IL-4 or, mostly, anti-IL-10 antibodies to the CD4+ cell cultures. Upon a suitable schedule of immunization, MP-F2 was also able to induce a vigorous DTH response in Candida-uninfected mice, a response that could be efficiently transferred into naive recipients by CD4+ cells from the spleens of MP-F2-immunized mice. The immunization described above also conferred to mice a low degree of protection against a virulent Candida challenge, both in terms of median survival time and in the number of Candida cells in the kidney. However, while DTH induction by MP-F2 was as strong as that induced by whole cells, MP-F2-induced protection was significantly weaker than that conferred by Candida whole-cell immunization. Mice immunized with either MP-F2 or Candida whole cells had an inverted ratio between the number of CD4+ splenocytes producing IFN-gamma and that of cells producing IL-4, compared with nonimmunized animals. However, the number of IL-4-producing CD4+ cells was significantly higher in MP-F2-vaccinated, weakly protected mice than in Candida whole-cell-vaccinated, highly protected animals. Overall, our data suggest that the MP-F2 fraction contains one or more major immunogens of C. albicans which are capable of interfering with the balance of CD4+ Th1 and Th2 responses that is so critical in the outcome of host-Candida relationship and are thus potentially relevant in the mechanisms of Candida-specific DTH regulation and protection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. B. Mouse candidiasis. II. Host responses are T-cell dependent and regulated by genes in the major histocompatibility complex. Immunogenetics. 1987;25(3):200–203. doi: 10.1007/BF00344035. [DOI] [PubMed] [Google Scholar]
- Ausiello C. M., Palma C., Maleci A., Spagnoli G. C., Amici C., Antonelli G., Casciani C. U., Cassone A. Cell mediated cytotoxicity and cytokine production in peripheral blood mononuclear cells of glioma patients. Eur J Cancer. 1991;27(5):646–650. doi: 10.1016/0277-5379(91)90235-6. [DOI] [PubMed] [Google Scholar]
- Ausiello C. M., Palma C., Spagnoli G. C., Piazza A., Casciani C. U., Cassone A. Cytotoxic effectors in human peripheral blood mononuclear cells induced by a mannoprotein complex of Candida albicans: a comparison with interleukin 2-activated killer cells. Cell Immunol. 1989 Jul;121(2):349–359. doi: 10.1016/0008-8749(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Ausiello C. M., Spagnoli G. C., Boccanera M., Casalinuovo I., Malavasi F., Casciani C. U., Cassone A. Proliferation of human peripheral blood mononuclear cells induced by Candida albicans and its cell wall fractions. J Med Microbiol. 1986 Nov;22(3):195–202. doi: 10.1099/00222615-22-3-195. [DOI] [PubMed] [Google Scholar]
- Ausiello C. M., Urbani F., Gessani S., Spagnoli G. C., Gomez M. J., Cassone A. Cytokine gene expression in human peripheral blood mononuclear cells stimulated by mannoprotein constituents from Candida albicans. Infect Immun. 1993 Oct;61(10):4105–4111. doi: 10.1128/iai.61.10.4105-4111.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini M., Bistoni F., Puccetti P., Garaci E. Natural cell-mediated cytotoxicity against Candida albicans induced by cyclophosphamide: nature of the in vitro cytotoxic effector. Infect Immun. 1983 Oct;42(1):1–9. doi: 10.1128/iai.42.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Cenci E., Mencacci A., Schiaffella E., Mosci P., Puccetti P., Romani L. Mucosal and systemic T helper cell function after intragastric colonization of adult mice with Candida albicans. J Infect Dis. 1993 Dec;168(6):1449–1457. doi: 10.1093/infdis/168.6.1449. [DOI] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Puccetti P., Marconi P., Cassone A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun. 1986 Feb;51(2):668–674. doi: 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomly K., Janeway C. A., Jr Antigen presentation by B cells. Nature. 1989 Jan 5;337(6202):24–24. doi: 10.1038/337024a0. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A. Cell wall of Candida albicans: its functions and its impact on the host. Curr Top Med Mycol. 1989;3:248–314. doi: 10.1007/978-1-4612-3624-5_10. [DOI] [PubMed] [Google Scholar]
- Cassone A., Mattia E., Boldrini L. Agglutination of blastospores of Candida albicans by concanavalin A and its relationship with the distribution of mannan polymers and the ultrastructure of the cell wall. J Gen Microbiol. 1978 Apr;105(2):263–273. doi: 10.1099/00221287-105-2-263. [DOI] [PubMed] [Google Scholar]
- Cassone A., Palma C., Djeu J. Y., Aiuti F., Quinti I. Anticandidal activity and interleukin-1 beta and interleukin-6 production by polymorphonuclear leukocytes are preserved in subjects with AIDS. J Clin Microbiol. 1993 May;31(5):1354–1357. doi: 10.1128/jcm.31.5.1354-1357.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Romani L., Vecchiarelli A., Puccetti P., Bistoni F. Role of L3T4+ lymphocytes in protective immunity to systemic Candida albicans infection in mice. Infect Immun. 1989 Nov;57(11):3581–3587. doi: 10.1128/iai.57.11.3581-3587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Domer J. E. Candida cell wall mannan: a polysaccharide with diverse immunologic properties. Crit Rev Microbiol. 1989;17(1):33–51. doi: 10.3109/10408418909105721. [DOI] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Le Deist F., Drouhet E., Griscelli C. Mannan-specific and mannan-induced T-cell suppressive activity in patients with chronic mucocutaneous candidiasis. J Clin Immunol. 1987 Sep;7(5):400–409. doi: 10.1007/BF00917018. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Differential activation of murine TH1 and TH2 clones. Res Immunol. 1991 Jan;142(1):19–23. doi: 10.1016/0923-2494(91)90005-4. [DOI] [PubMed] [Google Scholar]
- Garner R. E., Domer J. E. Lack of effect of Candida albicans mannan on development of protective immune responses in experimental murine candidiasis. Infect Immun. 1994 Feb;62(2):738–741. doi: 10.1128/iai.62.2.738-741.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., O'Garra A., Murphy K. M. Pathogen-induced Th1 phenotype development in CD4+ alpha beta-TCR transgenic T cells is macrophage dependent. Int Immunol. 1993 Apr;5(4):371–382. doi: 10.1093/intimm/5.4.371. [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Furotani M., Matsuura N., Kakudo K. The role of antigen-presenting cells in the regulation of delayed-type hypersensitivity. II. Epidermal Langerhans' cells and peritoneal exudate macrophages. Cell Immunol. 1993 Nov;152(1):200–210. doi: 10.1006/cimm.1993.1279. [DOI] [PubMed] [Google Scholar]
- Morris L., Troutt A. B., McLeod K. S., Kelso A., Handman E., Aebischer T. Interleukin-4 but not gamma interferon production correlates with the severity of murine cutaneous leishmaniasis. Infect Immun. 1993 Aug;61(8):3459–3465. doi: 10.1128/iai.61.8.3459-3465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma C., Serbousek D., Torosantucci A., Cassone A., Djeu J. Y. Identification of a mannoprotein fraction from Candida albicans that enhances human polymorphonuclear leukocyte (PMNL) functions and stimulates lactoferrin in PMNL inhibition of candidal growth. J Infect Dis. 1992 Nov;166(5):1103–1112. doi: 10.1093/infdis/166.5.1103. [DOI] [PubMed] [Google Scholar]
- Pitzurra L., Blasi E., Puliti M., Bistoni F. Toxic effects of tetanus toxin on GG2EE macrophages: prevention of gamma interferon-mediated upregulation of lysozyme-specific mRNA levels. Infect Immun. 1993 Sep;61(9):3605–3610. doi: 10.1128/iai.61.9.3605-3610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Leach M. W., Mauze S., Caddle L. B., Coffman R. L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993 Nov;5(11):1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- Powrie F., Menon S., Coffman R. L. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993 Sep;23(9):2223–2229. doi: 10.1002/eji.1830230926. [DOI] [PubMed] [Google Scholar]
- Quinti I., Palma C., Guerra E. C., Gomez M. J., Mezzaroma I., Aiuti F., Cassone A. Proliferative and cytotoxic responses to mannoproteins of Candida albicans by peripheral blood lymphocytes of HIV-infected subjects. Clin Exp Immunol. 1991 Sep;85(3):485–492. doi: 10.1111/j.1365-2249.1991.tb05754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Romani L., Cenci E., Mencacci A., Spaccapelo R., Grohmann U., Puccetti P., Bistoni F. Gamma interferon modifies CD4+ subset expression in murine candidiasis. Infect Immun. 1992 Nov;60(11):4950–4952. doi: 10.1128/iai.60.11.4950-4952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Mencacci A., Cenci E., Mosci P., Vitellozzi G., Grohmann U., Puccetti P., Bistoni F. Course of primary candidiasis in T cell-depleted mice infected with attenuated variant cells. J Infect Dis. 1992 Dec;166(6):1384–1392. doi: 10.1093/infdis/166.6.1384. [DOI] [PubMed] [Google Scholar]
- Romani L., Mencacci A., Cenci E., Spaccapelo R., Mosci P., Puccetti P., Bistoni F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993 Feb 1;150(3):925–931. [PubMed] [Google Scholar]
- Romani L., Mencacci A., Grohmann U., Mocci S., Mosci P., Puccetti P., Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992 Jul 1;176(1):19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Mencacci A., Tonnetti L., Spaccapelo R., Cenci E., Wolf S., Puccetti P., Bistoni F. Interleukin-12 but not interferon-gamma production correlates with induction of T helper type-1 phenotype in murine candidiasis. Eur J Immunol. 1994 Apr;24(4):909–915. doi: 10.1002/eji.1830240419. [DOI] [PubMed] [Google Scholar]
- Romani L., Mocci S., Bietta C., Lanfaloni L., Puccetti P., Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991 Dec;59(12):4647–4654. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaringi L., Marconi P., Boccanera M., Tissi L., Bistoni F., Cassone A. Cell wall components of Candida albicans as immunomodulators: induction of natural killer and macrophage-mediated peritoneal cell cytotoxicity in mice by mannoprotein and glucan fractions. J Gen Microbiol. 1988 May;134(5):1265–1274. doi: 10.1099/00221287-134-5-1265. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A. Accessory cells control induction of CD4+ T cells with specific effector function. Res Immunol. 1991 Jan;142(1):50–54. doi: 10.1016/0923-2494(91)90012-8. [DOI] [PubMed] [Google Scholar]
- Torosantucci A., Bromuro C., Gomez M. J., Ausiello C. M., Urbani F., Cassone A. Identification of a 65-kDa mannoprotein as a main target of human cell-mediated immune response to Candida albicans. J Infect Dis. 1993 Aug;168(2):427–435. doi: 10.1093/infdis/168.2.427. [DOI] [PubMed] [Google Scholar]
- Torosantucci A., Palma C., Boccanera M., Ausiello C. M., Spagnoli G. C., Cassone A. Lymphoproliferative and cytotoxic responses of human peripheral blood mononuclear cells to mannoprotein constituents of Candida albicans. J Gen Microbiol. 1990 Nov;136(11):2155–2163. doi: 10.1099/00221287-136-11-2155. [DOI] [PubMed] [Google Scholar]
- Trinel P. A., Borg-von-Zepelin M., Lepage G., Jouault T., Mackenzie D., Poulain D. Isolation and preliminary characterization of the 14- to 18-kilodalton Candida albicans antigen as a phospholipomannan containing beta-1,2-linked oligomannosides. Infect Immun. 1993 Oct;61(10):4398–4405. doi: 10.1128/iai.61.10.4398-4405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada H., Kawamura I., Arakawa M., Nomoto K., Mitsuyama M. Dissociated development of T cells mediating delayed-type hypersensitivity and protective T cells against Listeria monocytogenes and their functional difference in lymphokine production. Infect Immun. 1991 Oct;59(10):3589–3595. doi: 10.1128/iai.59.10.3589-3595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


