Abstract
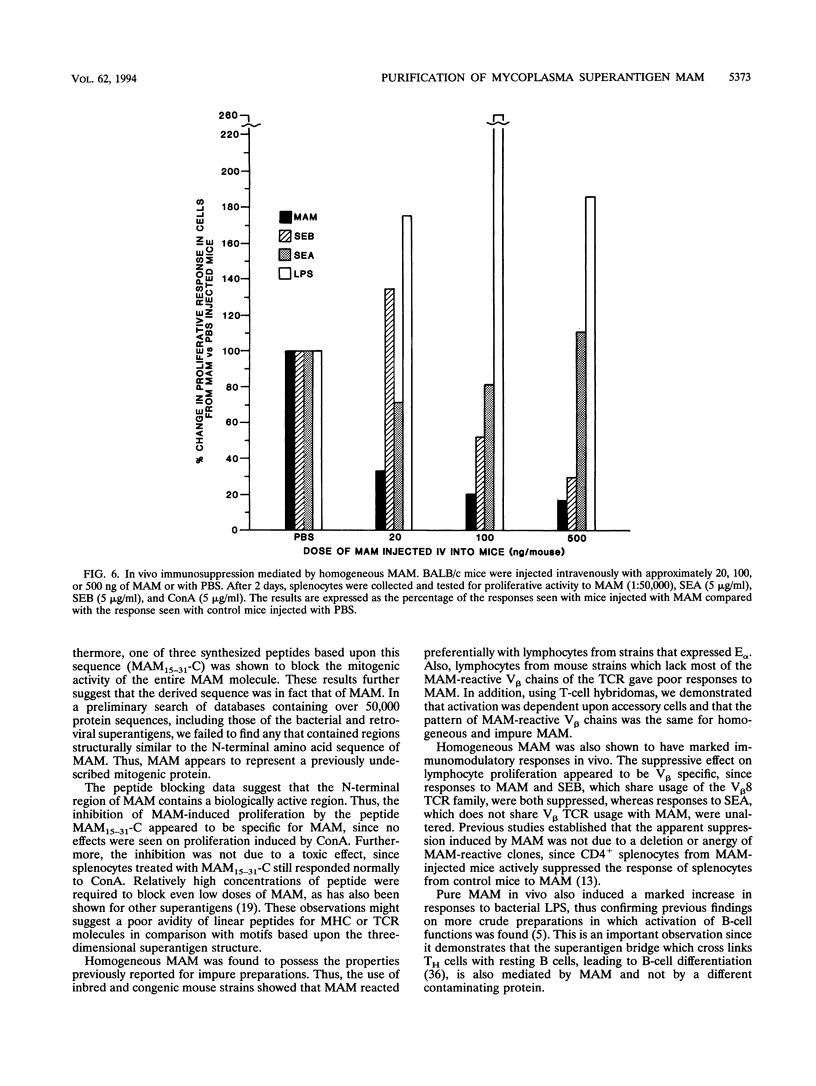
The prototypical superantigen MAM is an extracellular T-cell mitogen produced by Mycoplasma arthritidis, an organism which causes chronic proliferative arthritis of rodents. We here describe purification of MAM to homogeneity. Pure MAM exhibits all of the major properties previously described for partially purified MAM, including preference for H-2E molecules in presention to T cells, V beta T-cell receptor specificity for T-cell activation, and in vivo inhibition of T-cell functions but enhancement of B-cell activity as mediated by the superantigen bridge. Edman degradation of pure MAM gave a 54-residue partial amino-terminal sequence. The oligopeptide MAM15-31-C, synthesized according to the Edman sequence, blocked mitogenicity of MAM and supported assignment of the amino acid sequence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Palmer E. Mls--a retrovirus exploits the immune system. Immunol Today. 1991 Oct;12(10):356–361. doi: 10.1016/0167-5699(91)90066-3. [DOI] [PubMed] [Google Scholar]
- Atkin C. L., Cole B. C., Sullivan G. J., Washburn L. R., Wiley B. B. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. V. A small basic protein from culture supernatants is a potent T cell mitogen. J Immunol. 1986 Sep 1;137(5):1581–1589. [PubMed] [Google Scholar]
- Callahan J. E., Herman A., Kappler J. W., Marrack P. Stimulation of B10.BR T cells with superantigenic staphylococcal toxins. J Immunol. 1990 Apr 1;144(7):2473–2479. [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ahmed E., Araneo B. A., Shelby J., Kamerath C., Wei S., McCall S., Atkin C. L. Immunomodulation in vivo by the Mycoplasma arthritidis superantigen, MAM. Clin Infect Dis. 1993 Aug;17 (Suppl 1):S163–S169. doi: 10.1093/clinids/17.supplement_1.s163. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Araneo B. A., Sullivan G. J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. IV. Murine T hybridoma cells exhibit differential accessory cell requirements for activation by M. arthritidis T cell mitogen, concanavalin A, or hen egg-white lysozyme. J Immunol. 1986 May 15;136(10):3572–3578. [PubMed] [Google Scholar]
- Cole B. C., Atkin C. L. The Mycoplasma arthritidis T-cell mitogen, MAM: a model superantigen. Immunol Today. 1991 Aug;12(8):271–276. doi: 10.1016/0167-5699(91)90125-D. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Balderas R. A., Ahmed E. A., Kono D., Theofilopoulos A. N. Genomic composition and allelic polymorphisms influence V beta usage by the Mycoplasma arthritidis superantigen. J Immunol. 1993 Apr 15;150(8 Pt 1):3291–3299. [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. I. Transformation is associated with an H-2-linked gene that maps to the I-E/I-C subregion. J Immunol. 1981 Nov;127(5):1931–1936. [PubMed] [Google Scholar]
- Cole B. C., Griffiths M. M. Triggering and exacerbation of autoimmune arthritis by the Mycoplasma arthritidis superantigen MAM. Arthritis Rheum. 1993 Jul;36(7):994–1002. doi: 10.1002/art.1780360717. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Kartchner D. R., Wells D. J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. VII. Responsiveness is associated with expression of a product(s) of the V beta 8 gene family present on the T cell receptor alpha/beta for antigen. J Immunol. 1989 Jun 15;142(12):4131–4137. [PubMed] [Google Scholar]
- Cole B. C., Tuller J. W., Sullivan G. J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. VI. Detection of a non-MHC gene(s) in the E alpha-bearing RIIIS mouse strain that is associated with a specific lack of T cell responses to the M. arthritidis soluble mitogen. J Immunol. 1987 Aug 1;139(3):927–935. [PubMed] [Google Scholar]
- Cole B. C., Wells D. J. Immunosuppressive properties of the Mycoplasma arthritidis T-cell mitogen in vivo: inhibition of proliferative responses to T-cell mitogens. Infect Immun. 1990 Jan;58(1):228–236. doi: 10.1128/iai.58.1.228-236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. K., Zagon G., Chu Z., Ravina B., Tumang J. R., Cole B. C., Friedman S. M. Human B cell differentiation induced by microbial superantigens: unselected peripheral blood lymphocytes secrete polyclonal immunoglobulin in response to Mycoplasma arthritidis mitogen. Autoimmunity. 1992;14(1):23–32. doi: 10.3109/08916939309077353. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Gerardy-Schahn R., Metzroth B., Carrel S., Gerlach D., Köhler W. An evolutionary conserved mechanism of T cell activation by microbial toxins. Evidence for different affinities of T cell receptor-toxin interaction. J Immunol. 1991 Jan 1;146(1):11–17. [PubMed] [Google Scholar]
- Friedman S. M., Posnett D. N., Tumang J. R., Cole B. C., Crow M. K. A potential role for microbial superantigens in the pathogenesis of systemic autoimmune disease. Arthritis Rheum. 1991 Apr;34(4):468–480. doi: 10.1002/art.1780340412. [DOI] [PubMed] [Google Scholar]
- Griggs N. D., Pontzer C. H., Jarpe M. A., Johnson H. M. Mapping of multiple binding domains of the superantigen staphylococcal enterotoxin A for HLA. J Immunol. 1992 Apr 15;148(8):2516–2521. [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Homfeld J., Homfeld A., Nicklas W., Rink L., Weyland A., Kirchner H. Induction of interleukin-6 in murine bone marrow-derived macrophages stimulated by the Mycoplasma arthritidis mitogen MAS. Autoimmunity. 1990;7(4):317–327. doi: 10.3109/08916939009087591. [DOI] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügin A. W., Vacchio M. S., Morse H. C., 3rd A virus-encoded "superantigen" in a retrovirus-induced immunodeficiency syndrome of mice. Science. 1991 Apr 19;252(5004):424–427. doi: 10.1126/science.1850169. [DOI] [PubMed] [Google Scholar]
- Imanishi K., Igarashi H., Uchiyama T. Activation of murine T cells by streptococcal pyrogenic exotoxin type A. Requirement for MHC class II molecules on accessory cells and identification of V beta elements in T cell receptor of toxin-reactive T cells. J Immunol. 1990 Nov 15;145(10):3170–3176. [PubMed] [Google Scholar]
- Kirchner H., Brehm G., Nicklas W., Beck R., Herbst F. Biochemical characterization of the T-cell mitogen derived from Mycoplasma arthritidis. Scand J Immunol. 1986 Sep;24(3):245–249. doi: 10.1111/j.1365-3083.1986.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum D. M. Molar absorptivity and A 1 1cm values for proteins at selected wavelengths of the ultraviolet and visible region. II. Int J Protein Res. 1971;3(3):157–164. doi: 10.1111/j.1399-3011.1971.tb01706.x. [DOI] [PubMed] [Google Scholar]
- Lambin P., Rochu D., Fine J. M. A new method for determination of molecular weights of proteins by electrophoresis across a sodium dodecyl sulfate (SDS)-polyacrylamide gradient gel. Anal Biochem. 1976 Aug;74(2):567–575. doi: 10.1016/0003-2697(76)90239-6. [DOI] [PubMed] [Google Scholar]
- Legaard P. K., LeGrand R. D., Misfeldt M. L. Lymphoproliferative activity of Pseudomonas exotoxin A is dependent on intracellular processing and is associated with the carboxyl-terminal portion. Infect Immun. 1992 Apr;60(4):1273–1278. doi: 10.1128/iai.60.4.1273-1278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Posnett D. N. Do superantigens play a role in autoimmunity? Semin Immunol. 1993 Feb;5(1):65–72. doi: 10.1006/smim.1993.1009. [DOI] [PubMed] [Google Scholar]
- RAZIN S., ARGAMAN M. Lysis of Mycoplasma, bacterial protoplasts, spheroplasts and L-forms by various agents. J Gen Microbiol. 1963 Jan;30:155–172. doi: 10.1099/00221287-30-1-155. [DOI] [PubMed] [Google Scholar]
- Stuart P. M., Woodward J. G. Yersinia enterocolitica produces superantigenic activity. J Immunol. 1992 Jan 1;148(1):225–233. [PubMed] [Google Scholar]
- Tomai M. A., Aelion J. A., Dockter M. E., Majumdar G., Spinella D. G., Kotb M. T cell receptor V gene usage by human T cells stimulated with the superantigen streptococcal M protein. J Exp Med. 1991 Jul 1;174(1):285–288. doi: 10.1084/jem.174.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumang J. R., Posnett D. N., Cole B. C., Crow M. K., Friedman S. M. Helper T cell-dependent human B cell differentiation mediated by a mycoplasmal superantigen bridge. J Exp Med. 1990 Jun 1;171(6):2153–2158. doi: 10.1084/jem.171.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]



