Abstract
Africans have elevated T cell activation compared to residents of Europe or the USA. Levels of T cell activation also correlate with disease progression in HIV-infected individuals. We sought to determine if treatment with antiretroviral therapy (ART) would reduce levels of T cell activation (CD38 and HLADR co-expression) in HIV-infected Ugandan children. The median CD8+ T cell activation level among 199 ART-treated children (30%) was lower than in 57 ART-naïve children (45%, p < 0.001), but remained higher than in 30 HIV-uninfected children (18%, p < 0.001). Among ART-treated children, CD4% was inversely correlated with both CD8− (ρ = −0.61, p < 0.001) and CD8+ (ρ = −0.38, p < 0.001) T cell activation. Prospectively, CD4 recovery correlated with post-treatment CD8+ T cell activation level (p = 0.008). Our data suggest that significant decreases in T cell activation accompany CD4 recovery in ART-treated HIV-infected African children, to levels that approach but do not reach those of uninfected children.
Keywords: T cell activation, Children, Antiretroviral treatment, Uganda
Introduction
In addition to CD4+ T cell lymphopenia, HIV infection leads to other significant immunologic derangements including depletion of naïve T cell pools and increased expression of markers of activation including CD38 and HLADR [1,2]. Levels of T cell activation have proven to be important markers of disease progression; elevated levels are found in the HIV-infected children that have rapidly progressive HIV disease [3,4] and are predictive of CD4+ T cell decline in adults independent of plasma HIV RNA [5]. Conversely, there is evidence that recovery of CD4+ T cell counts following antiretroviral therapy (ART) is associated with reduction of activation levels in both adults [6] and children [7]. Residents of Africa, both HIV-infected and uninfected, appear to have elevated T cell activation compared to residents of Europe and the USA, potentially related to increased stimulation from other endemic infectious diseases [8]. ART has been shown to reduce levels of activation in HIV-infected Ugandan adults [9,10], but there has been, to date, no similar study in African children. To characterize changes in T cell activation following the initiation of ART in African children, we evaluated CD38 and HLA-DR expression on T cells in the Children with HIV and Malaria Project (CHAMP), an observational cohort of HIV-infected Ugandan children [11]. In cross sectional analysis, we compared levels of T cell activation in HIV-infected children that had received ART for at least 24 weeks to levels in ART-naïve HIV-infected and HIV-uninfected children and investigated the relationship between activation and CD4+ T cell status and HIV RNA level. Additionally, the change in T cell activation following the initiation of ART was assessed prospectively in a subset of HIV-infected children.
Materials and methods
Subjects
The CHAMP enrolled 300 HIV-infected children aged 1 to 10 years from a pediatric HIV clinic at Mulago Hospital, Kampala, Uganda, as described elsewhere [11,12]; levels of T cell activation were determined in 291 participants. Results from children who were either ART-naïve or had received ART for at least 24 weeks (“ART-treated”) at the time of testing were included; results from those with an acute illness on the day of testing were excluded. ART was initiated per WHO guidelines in children who experienced WHO Stage 3 or 4 clinical events, or who developed severe immunodeficiency as defined by having a % or absolute count of CD4+ T cells count below age-specific cutoffs [13]. For 8 children that initiated ART during follow up, levels of T cell activation were available at 2 time points: before and 10–14 weeks after initiation of ART. A total of 30 HIV-uninfected children were selected by convenience sampling from a local, parallel cohort of 601 HIV-uninfected children for comparison; no child had malaria or other significant illness at the time of sampling [14]. Informed consent was obtained from the parents or guardians of all children. The research was approved by the Uganda National Council of Science and Technology, the Makerere University Research and Ethics Committee, and the University of California, San Francisco Committee on Human Research and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Laboratory techniques
Levels of T cell activation were measured in peripheral blood mononuclear cells using the following antibodies: CD3 APC, CD8 PerCp-Cy5.5, HLA-DR FITC and CD38 PE. Fresh samples were prepared within 4 h and analyzed within 24 h of collection. Isolation of peripheral blood mononuclear cells (PBMC) was performed by Ficol-Paque Plus (GE Healthcare, Piscataway, NJ) density centrifugation. A minimum of 30,000 CD3+ cells per sample were acquired using a 4-color FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA). T cells were defined by expression of CD3. Levels of activation were defined by the % expressing both CD38 and HLADR in both CD8− and CD8+ T cell subpopulations. Preset gating for CD38 and HLA-DR expression on T cells was based on a natural break seen in HIV-uninfected Ugandans and utilized for all samples, as described previously [15]. In addition, identical gates were validated in the United States to ensure that they provided activation values consistent with what has been previously reported for US samples. Analysis was performed using the FLOWJO software (TreeStar, San Carlos, CA). There is high correlation between PBMC and whole blood staining using this technique; several PBMC and whole blood specimens from study subjects were processed in parallel and yielded similar results. Percentage of cells expressing both CD38 and HLADR is a validated measure of T cell activation that has been shown to be equivalent to mean fluorescence intensity in predicting clinical outcomes in HIV infected [16]. For HIV-infected children, plasma HIV RNA levels were determined using the Roche Amplicor kit (Version 1.5, level of detection 400 copies/ml). Absolute CD4+ T cell count and CD4% were determined from whole blood samples obtained from the same blood draw as the PBMC samples, using a 4-color FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA); CD4% was calculated using CD45 as the anchor marker.
Statistics
Levels of T cell activation were compared in cross section among ART-naïve, ART-treated and HIV-uninfected children and stratified based on CD4% and HIV RNA level using the non-parametric Wilcoxon two-sample test (normal approximation, 1 sided). Correlation of CD8− and CD8+ T cell activation with absolute CD4+ T cell count, CD4+ T cell %, and HIV plasma RNA levels were tested using Spearman’s rank correlation testing. For matched comparisons, the Wilcoxon signed rank test was used on difference values. Values are listed as median (IQR: 25%’ile–75%’ile unless otherwise stated).
Results
Levels of T cell activation from 57 ART-treated children were compared to 199 ART-naïve and 30 HIV-uninfected children (Table 1). The median age of ART-treated children was 5.1 years (IQR: 3.2–7.0), ART-naïve children 6.4 years (IQR: 4.4–8.3) and HIV-uninfected children, 8.2 years (IQR: 6.0–10.1). ART-treated children had a median CD4 count of 887 cells/μl (IQR: 638–1245), CD4% of 25 (IQR: 18–30). Forty-five of the 57 ART-treated children had undetectable plasma HIV RNA (< 400 copies/ml) on the day of activation testing. Of the 12 children with detectable HIV RNA, the median value was 4.7 log10 copies/ml (IQR: 4.4–4.8). ART-naïve children had a median CD4+ T cell count of 731 cells/μl (500–1074), CD4% of 23 (IQR: 18–29) and HIV plasma RNA of 5.0 log10 (copies/ml) (4.5–5.5). ART regimens included either efavirenz or nevirapine and two nucleoside reverse transcriptase inhibitors – lamivudine with either stavudine or zidovudine – for a median duration of 38 weeks (IQR: 32–62 weeks).
Table 1.
CD38 and HLADR Expression on T cells in HIV-infected and uninfected Ugandan children.
| Surface marker expression* | HIV(+) ART-naïve (n = 199) | HIV(+) ART ≥ 24 weeks§ (n = 57) | HIV (−) (n = 30) | pa | pb | pc |
|---|---|---|---|---|---|---|
| CD8− | ||||||
| CD3+CD8− CD38+ HLADR+ | 13 (10–17) | 10 (6–14) | 5 (4–6) | 0.005 | < 0.001 | < 0.001 |
| CD3+CD8− CD38+ HLADR− | 76 (68–81) | 76 (68–80) | 78 (72–80) | 0.80 | 0.32 | 0.25 |
| CD3+CD8− CD38− HLADR+ | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.25 | 0.25 | 0.94 |
| CD8 | ||||||
| CD3+CD8+ CD38+ HLADR+ | 45 (34–54) | 30 (21–40) | 18 (13–21) | < 0.001 | < 0.001 | < 0.001 |
| CD3+CD8+ CD38+ HLADR− | 50 (40–58) | 62 (47–66) | 66 (62–72) | < 0.001 | < 0.001 | 0.004 |
| CD3+CD8+ CD38− HLADR+ | 1 (1–3) | 2 (1–4) | 3 (2–6) | 0.003 | < 0.001 | 0.08 |
Percent of cells expressing, median (25th–75th%’ile);
median: 37.9 week (32.0–60.1).
p: p-value for Wilcoxon two-sample test;
HIV(+) ART-naïve vs. ART ≥ 24 weeks;
HIV(+) ART-naïve vs. HIV (−);
HIV(+) ART ≥ 24 weeks vs. HIV (−).
The median level of activation in CD8− and CD8+ T cell populations of ART-treated children was lower compared to ART-naïve children (p < 0.001) but remained higher compared to HIV-uninfected children (p < 0.001) (Table 1). To further investigate the relationship between T cell activation, CD4 status and plasma HIV RNA, subgroup analyses were performed in “ART-suppressed children” (ART-treated with undetectable HIV Plasma RNA), ART-naïve and HIV-uninfected children, stratifying by CD4+ T cell % into groups of normal (≥25%), moderate immunosuppression (15–25%) and severe immunosuppression (< 15%) (Figs. 1a and b). Among ART-suppressed children, those with normal CD4+ T cell % had lower median levels of CD8− T cell activation (6.8%) than those with moderate (10.9%, p = 0.002) or severe immunosuppression (24.4%, p = 0.001). Similarly, the level of CD8+ T cell activation ART-suppressed children with normal CD4% (25.1%) was lower compared to levels observed in moderate (29.3%, p = 0.064) or severe immunosuppression subgroups (46.4%, p = 0.029), although the former did not reach statistical significance. T cell activation, both CD8− and CD8+, in ART-suppressed children with normal CD4+ T cell status remained significantly higher compared to levels seen in HIV negative children (4.6%, p = 0.004 and 17.7%, p = 0.001, respectively). CD4+ T cell % was negatively associated with CD8− T cell activation in both ART-suppressed (ρ = −0.65, p < 0.001) or ART-naïve children (ρ = −0.61, p < 0.001). Similar correlation was observed for CD8+ T cell activation in ART-suppressed as well as ART-naïve (ρ = −0.047, p = 0.001 and ρ = −0.38, p < 0.001, respectively).
Figure 1.
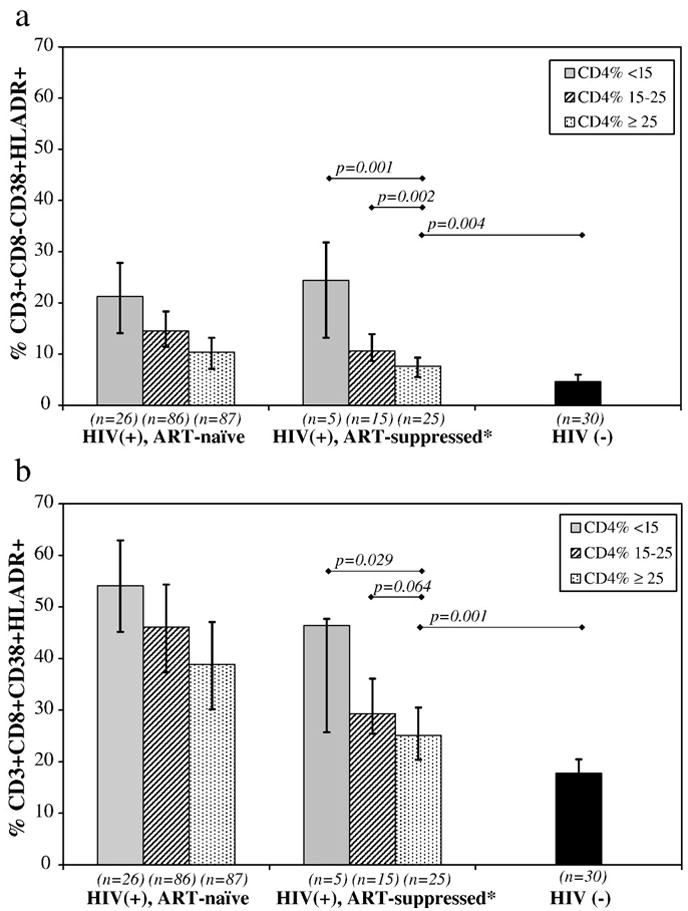
Levels of T cell activation in T cell populations stratified by CD4% in Ugandan Children. The percentage of CD8− (a) and CD8+ (b) lymphocytes co-expressing CD38 and HLADR were determined by 4-color FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA) using fresh peripheral blood mononuclear cells. Bar heights represent median values. Vertical lines represent interquartile ranges (25%–75%’ile). Horizontal lines indicate comparison by Wilcoxon Rank Sum, with applicable p values. *ART-treated ≥ 24 weeks with undetectable HIV plasma RNA.
Because CD38 and HLA-DR expressions have been shown to vary by age in healthy children that live in the USA [17], we repeated the analyses stratifying by age and CD4+ T cell %. The findings remained the same, with the lowest levels of both CD8− and CD8+ T cell activation observed in children with normal CD4+ T cell % in both age groups regardless of ART status. Additionally, no correlation was found with age and CD8− or CD8+ T cell activation in either ART-naïve or ART-treated children (data not shown).
T cell activation was measured before and 10–14 weeks after ART initiation in 8 children. The median plasma HIV plasma RNA level at baseline was 5.3 log10(copies/ml); 6 children achieved full viral suppression (< 400 copies/ml) following ART initiation. The median level of CD8− and CD8+ T cell activation prior to ART initiation was 22.8% (IQR: 16.4%–38.9%) and 55.1% (IQR: 46.3%–60.7%), respectively. Following viral suppression, CD8− T cell activation decreased in every child by a median 8.7% points (p = 0.01) and CD8+ T cell activation decreased in every child by a median 17.2% points (p = 0.01). The median CD4% recovery was 6.5% points. CD4+ T cell % recovery was significantly associated with lower post-treatment CD8 activation level (ρ = 0.73, p = 0.008), but not with pre-treatment CD8− or CD8+ T cell activation or post treatment CD8− T cell activation level.
Discussion
While it is clear that the initiation of ART leads to viral suppression and recovery of the immune functions in most HIV-infected children, the understanding of factors influencing immune reconstitution and the role of T cell activation in this population remains limited. This is the first study, to our knowledge, to investigate the dynamics of T cell activation in HIV-infected African children following the initiation of ART. We found high levels of T cell activation at baseline in our cohort of HIV-infected Ugandan children. In this context, HIV-infected Ugandan children who have received ART for at least 24 weeks experienced significantly reduced levels of activation compared to ART-naïve HIV-infected children, with rapid reductions in the first 10–14 weeks of therapy. This is similar to what has been shown in ART-treated HIV-infected children in the USA (PACTG 338 [18] and 377 [19]).
Additionally, we found that among ART-treated children with undetectable plasma HIV RNA, those with high CD4+ T cell counts had the least T cell activation with levels that approached but did not reach those of HIV-uninfected children. Conversely, ART-treated children with severe immunosuppression despite undetectable plasma HIV RNA maintained high levels of CD8− and CD8+ T cell activation. A recent study of 143 ART-treated HIV-infected children in Spain showed similarly that CD4 status correlated closely with levels of T cell activation independent of HIV plasma RNA level [7]. We further show that the degree of CD4+ T cell recovery strongly correlated with post-treatment CD8+ T cell activation.
The levels of activation among CD3+CD8− cells measured in this study are likely to approximate those of CD4+ cells. In additional study of 50 children, we found that 79–87% of CD3+ CD8− T cells express CD4 (unpublished). That said, activation levels among CD3+CD8−CD4− T cells may also be important in this population; further study is indicated.
Taken together, these data support the current model in which the reduction of T cell activation is a critical factor in CD4+ T cell recovery [6]. Mechanisms of CD4+ T cell depletion in HIV-infection in children are not fully understood, but bystander killing of activated, uninfected cells and ineffective thymic production seem to be important factors. Optimal CD4+ T cell recovery likely requires restoration of thymic function and a decrease in the T cell activation that arises from HIV infection as well as other stimuli.
The majority of the world’s HIV-infected children live in sub-Saharan Africa and there is mounting evidence that factors in pathogenesis and CD4+ T cell recovery may differ for this population. Several studies have now documented that residents of sub-Saharan Africa have higher levels of T cell activation compared to residents of the US and Europe [15,20], potentially related to the burden of chronic parasitic infections [21]. But whether the high levels of T cell activation seen in HIV-infected African children lead to faster CD4 immune depletion or incomplete immune recovery remains to be determined. Further study identifying the determinants of activation such as HIV subtype, host genetic factors, or non-HIV infectious burden will be important and may lead to novel treatment strategies for this population.
Acknowledgments
We would like to thank the staff and families of the Children with HIV and Malaria Project.
This research was funded by grants from the NIAID (AI062677-01, AI51982, and MH083573) and supported by the University of California, San Francisco (UCSF) – Gladstone Institute of Virology and Immunology (GIVI) – Center for Center for AIDS Research (CFAR).
The Children with HIV and Malaria Project is also supported by the President’s Emergency Plan for AIDS Relief (Centers for Disease Control, Cooperative Agreement number 1U2GPS000942-01). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
References
- 1.Plaeger-Marshall S, Isacescu V, O’Rourke S, Bertolli J, Bryson YJ, Stiehm ER. T cell activation in pediatric AIDS pathogenesis: three-color immunophenotyping. Clin Immunol Immunopathol. 1994;71:19–26. doi: 10.1006/clin.1994.1046. [DOI] [PubMed] [Google Scholar]
- 2.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 3.Paul ME, Shearer WT, Kozinetz CA, Lewis DE. Comparison of CD8+ T-cell subsets in HIV-infected rapid progressor children versus non-rapid progressor children. J Allergy Clin Immunol. 2001;108:258–264. doi: 10.1067/mai.2001.117179. [DOI] [PubMed] [Google Scholar]
- 4.Paul ME, Mao C, Charurat M, Serchuck L, Foca M, Hayani K, Handelsman EL, Diaz C, McIntosh K, Shearer WT. Predictors of immunologic long-term nonprogression in HIV-infected children: implications for initiating therapy. J Allergy Clin Immunol. 2005;115:848–855. doi: 10.1016/j.jaci.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 5.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 6.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 7.Resino SE, Gutierrez MD, Leon JA, Munoz Fernandez MA. CD4+ T-cell immunodeficiency is more dependent on immune activation than viral load in HIV-infected children on highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;42:269–276. doi: 10.1097/01.qai.0000222287.90201.d7. [DOI] [PubMed] [Google Scholar]
- 8.Rizzardini G, Trabattoni D, Saresella M, Piconi S, Lukwiya M, Declich S, Fabiani M, Ferrante P, Clerici M. Immune activation in HIV-infected African individuals. Italian-Ugandan AIDS cooperation program. AIDS. 1998;12:2387–2396. doi: 10.1097/00002030-199818000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Baker CA, Emenyonu N, Ssewanyana I, Jones NG, Elrefaei M, Nghania F, Nakiwala J, Andia I, Clark R, Martin J, Bangsberg DR, Cao H. Profile of immunologic recovery in HIV-infected Ugandan adults after antiretroviral therapy. AIDS Res Hum Retrovir. 2007;23:900–905. doi: 10.1089/aid.2006.0309. [DOI] [PubMed] [Google Scholar]
- 10.Ondoa P, Koblavi-Deme S, Borget MY, Nolan ML, Nkengasong JN, Kestens L. Assessment of CD8 T cell immune activation markers to monitor response to antiretroviral therapy among HIV-1 infected patients in Cote d’Ivoire. Clin Exp Immunol. 2005;140:138–148. doi: 10.1111/j.1365-2249.2005.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamya MR, Gasasira AF, Achan J, Mebrahtu T, Ruel T, Kekitiinwa A, Charlebois ED, Rosenthal PJ, Havlir D, Dorsey G. Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. AIDS. 2007;21:2059–2066. doi: 10.1097/QAD.0b013e3282ef6da1. [DOI] [PubMed] [Google Scholar]
- 12.Ssewanyana I, Elrefaei M, Dorsey G, Ruel T, Jones NG, Gasasira A, Kamya M, Nakiwala J, Achan J, Charlebois E, Havlir D, Cao H. Profile of T cell immune responses in HIV-infected children from Uganda. J Infect Dis. 2007;196:1667–1670. doi: 10.1086/522013. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. 2006:158. www.who.int/hiv/pub/guidelines/paediatric020907.pdf.
- 14.Dorsey G, Staedke S, Clark TD, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Dokomajilar C, Kamya MR, Rosenthal PJ. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA. 2007;297:2210–2219. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- 15.Eggena MP, Barugahare B, Okello M, Mutyala S, Jones N, Ma Y, Kityo C, Mugyenyi P, Cao H. T cell activation in HIV-seropositive Ugandans: differential associations with viral load, CD4+ T cell depletion, and coinfection. J Infect Dis. 2005;191:694–701. doi: 10.1086/427516. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Hultin LE, Cumberland WG, Hultin P, Schmid I, Matud JL, Detels R, Giorgi JV. Elevated relative fluorescence intensity of CD38 antigen expression on CD8+ T cells is a marker of poor prognosis in HIV infection: results of 6 years of follow-up. Cytometry. 1996;26:1–7. doi: 10.1002/(SICI)1097-0320(19960315)26:1<1::AID-CYTO1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, Wara DW, Douglas SD, Luzuriaga K, McFarland EJ. Lymphocyte subsets in healthy children from birth through 18 years of age: the pediatric AIDS clinical trials group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Borkowsky W, Stanley K, Douglas S, Lee S, Wiznia A, Pelton S, Yogev R, McIntosh K, Nachman S. Immunologic response to combination nucleoside analogue plus protease inhibitor therapy in stable antiretroviral therapy-experienced human immunodeficiency virus-infected children. J Infect Dis. 2000;182:96–103. doi: 10.1086/315672. [DOI] [PubMed] [Google Scholar]
- 19.Rosenblatt HM, Stanley KE, Song LY, Johnson GM, Wiznia AA, Nachman SA, Krogstad PA. Immunological response to highly active antiretroviral therapy in children with clinically stable HIV-1 infection. J Infect Dis. 2005;192:445–455. doi: 10.1086/431597. [DOI] [PubMed] [Google Scholar]
- 20.Clerici BS, Lukwiya M, Saresella M, Declich S, Trabattoni D, Pastori C, Piconi S, Fracasso C, Fabiani M, Ferrante P, Rizzardini G, Lopalco L. Immune activation in Africa is environmentally-driven and is associated with upregulation of CCR5. AIDS. 2000;14:2083–2092. doi: 10.1097/00002030-200009290-00003. [DOI] [PubMed] [Google Scholar]
- 21.Kalinkovich A, Weisman Z, Greenberg Z, Nahmias J, Eitan S, Stein M, Bentwich Z. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin Exp Immunol. 1998;114:414–421. doi: 10.1046/j.1365-2249.1998.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


