Abstract
Observational epidemiologic studies of dietary antioxidant intake, serum antioxidant concentration, and lung outcomes suggest that lower levels of antioxidant defenses are associated with decreased lung function. Another approach to understanding the role of oxidant/antioxidant imbalance in risk of Chronic Obstructive Pulmonary Disease (COPD) is to investigate the role of genetic variation in antioxidant enzymes, and indeed family-based studies suggest a heritable component to lung disease. Many studies of the genes encoding antioxidant enzymes have considered COPD or COPD-related outcomes, and a systematic review is needed to summarise the evidence to date, and to provide insights for further research.
Genetic association studies of antioxidant enzymes and COPD/COPD-related traits, and comparative gene expression studies with disease or smoking as the exposure were systematically identified and reviewed. Antioxidant enzymes considered included enzymes involved in glutathione (GSH) metabolism, in the thioredoxin (TXN) system, superoxide dismutases (SOD), and catalase (CAT).
A total of 29 genetic association and 15 comparative gene expression studies met the inclusion criteria. The strongest and most consistent effects were in the genes GCL, GSTM1, GSTP1, and SOD3. This review also highlights the lack of studies for genes of interest, particularly GSR, GGT, and those related to TXN. There were limited opportunities to evaluate a gene’s contribution to disease risk through a synthesis of results from different study designs, as the majority of studies considered either association of sequence variants with disease or effect of disease on gene expression. Network-driven approaches that consider potential interaction between genes and amoung genes, smoke exposure, and antioxidant intake are needed to fully characterise the role of oxidant/antioxidant balance in pathogenesis.
Keywords: Chronic Obstructive Pulmonary Disease (COPD), Antioxidants, Oxidative Stress
INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD) is characterised by the development of airflow limitation that is not fully reversible. COPD is a major, and growing, public health burden.1
Smoking is the most important risk factor for COPD; however, there is considerable variation in the response to smoke exposure,2 and it has been estimated that only 15% of the variation in lung function is explained by smoking parameters.3 While not discounting the paramount importance of smoke exposure in the development of COPD, clearly other factors are significant. Genetic variation is a prime candidate, and many recent studies explore the contribution of genetic variation to inter-individual differences in the response to cigarette smoke.
This review focuses on genes related to antioxidant activity, as oxidant-rich cigarette smoke strains the antioxidant defenses of the lungs, leading to direct tissue damage and contributing to the inflammation and antiprotease inactivation seen in COPD. This hypothesis is supported by epidemiologic studies associating low dietary antioxidant intake and serum antioxidant concentration with decreased lung function4–9 and increased COPD mortality risk.10
Many genetic association studies and comparative expression studies investigate the relation between genes coding for antioxidant enzymes and either COPD or associated traits. An overview of the evidence is warranted to ascertain whether the pattern of published results suggests directions for future research, or whether there are apparent gaps that need to be addressed. Both genetic association studies and gene expression studies were included: polymorphisms can affect disease risk in ways that may or may not be mediated by changes in expression, and expression studies can provide a snapshot of the adaptive response to an exposure. Thus, we conducted a systematic review of the literature to characterise the evidence that genes coding for antioxidant enzymes contribute to the aetiology of COPD and related traits.
METHODS
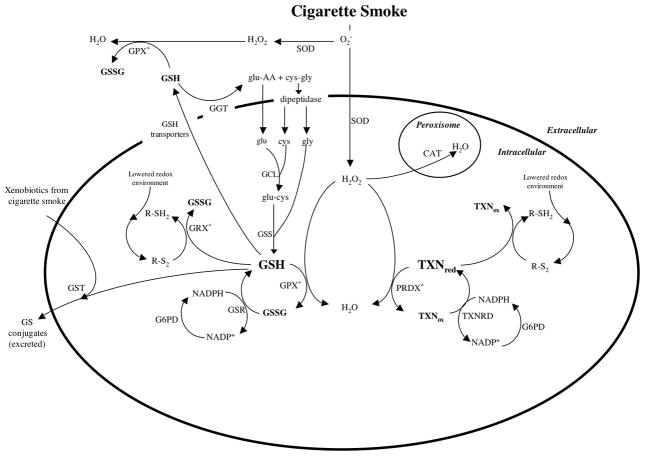
The selection of genes was based mainly on delineating important proteins and the networks of genes that may influence the amount or function of those proteins (Figure 1). As glutathione (GSH) is an antioxidant that plays a significant role in the lung, genes encoding GSH-associated enzymes were selected. Thioredoxin, which reduces oxidised glutathione and has an antioxidant function that overlaps GSH function, was included with genes encoding associated enzymes. Catalase and superoxide dismutase, two classical antioxidant enzymes of the lung, were also selected. Searches of PubMed were performed up to August 2007 (further details online). Published papers considering gene-disease association or differential gene expression in adult humans were selected. Association studies were included if the outcome was disease or lung function. Expression studies were included if the experimental exposure was disease status or smoking and if expression was measured in pulmonary tissues or cells.
Figure 1.
Interaction of antioxidant enzymes in response to oxidative stress
*: this reaction also occurs without the listed enzyme
Abbreviations are as follows:
cys: Cysteine
CAT: Catalase
G6PD: Glucose-6-phosphate dehydrogenase
GCL: Glutamate-cysteine ligase
GGT: Gamma-glutamyl transferase
glu: Glutamate
GPX: Glutathione Peroxidase
GRX: Glutaredoxin
GSH: Reduced Glutathione
GSR: Glutathione reductase
GSS: Glutathione synthetase
GSSG: Oxidised Glutathione
GST: Glutathione S-transferase
NADP+: Oxidised nicotinamide adenine dinucleotide phosphate
NADPH: Reduced nicotinamide adenine dinucleotide phosphate
PRDX: Peroxiredoxin
R-SH2: Reduced thiol
R-S2: Disulphide (could be mixed disulphides or glutathionylated proteins)
SOD: Superoxide dismutase
TXNred: Reduced thioredoxin
TXNox: Oxidised thioredoxin
TXNRD: Thioredoxin reductase
RESULTS
A total of 29 genetic association studies and 14 expression studies were identified (online supplement table 1).
Glutathione Synthesis
Three enzymes that relate to glutathione synthesis were considered: gamma-glutamyl transpeptidase (GGT, no studies found), glutamate-cysteine ligase (GCL), and glutathione synthetase (GSS). A substitution in the promoter region of GCLM (GCL’s modulatory subunit), leading to decreased glutathione levels,11 was associated with a 3-fold increased risk of COPD in Chinese smokers.12 In the single study of a substitution in GCL’s catalytic subunit (GCLC) that results in decreased expression,13 an increased prevalence was observed in patients vs. healthy controls (OR 1.83, 95% CI[1.00, 3.36]).14
Expression studies of glutathione synthesis compared expression in COPD patients with asymptomatic smokers and/or nonsmokers. Eight of the 9 comparisons of GCLC expression in lung epithelium found upregulation with disease.15–18 Two comparisons of GCLM expression in lung epithelium of lung tumor patients with and without COPD showed decreased GCLM expression with disease,17 while the single study of COPD patients without lung tumor found upregulation.18
There were 4 comparisons of GCL expression in lung epithelium of asymptomatic smokers and nonsmokers: expression of both subunits was increased in smokers who were healthy volunteers18, 19 and unchanged or decreased in smokers with lung tumor.17
Expression of the GCL subunits in alveolar macrophages/inflammatory cells showed a more consistent pattern. Both GCLC16, 17, 20 and GCLM20 were increased in COPD patients vs. smokers, and smokers had lower expression of both subunits compared to nonsmokers.17, 20 A 27% upregulation of the mRNA of GSS, the final step of GSH synthesis, was reported in smokers vs. nonsmokers (p=0.08),19 but was not replicated in comparisons of nonsmokers with either asymptomatic smokers or COPD patients.18, 19
Antioxidant Activity of GSH and Recycling
Glutathione peroxidase (GPX), glutaredoxin (GLRX), glutathione reductase (GSR), and glucose-6-phosphate dehydrogenase (G6PD) were considered for their role in the antioxidant activity of GSH and in GSH recycling. There were no association studies of variants in these genes.
Two studies evaluated expression in COPD patients versus healthy controls. In the single study of GPX2, it was strongly upregulated in COPD patients at all stages compared to nonsmokers (and modestly upregulated compared to smokers).18 GPX3 was upregulated in COPD patients compared to nonsmokers, though this difference was not seen in comparison with asymptomatic smokers.18, 21 There was little evidence of differential regulation of GPX4, GPX5, or GPX7 by disease status.18
GPX2 showed a 3 to 5-fold upregulation in epithelial cells of smokers compared to nonsmokers.18, 19 Each of the 4 studies of GPX3 expression in epithelial cells reported upregulation in smokers: two studies found a 2-fold difference,19, 22 and a study of alveolar macrophages reported similar differences.22
Two expression studies evaluated regulation of GLRX. A statistically significant downregulation was observed in the tissue homogenate of COPD patients compared to smokers (patients had either resection for lung tumor or lung transplantation for severe COPD),23 but similar results were not observed in an analysis of bronchial epithelial cells.18 A statistically significant upregulation of GLRX in the sputum of COPD patients in exacerbation was reported vs. nonsmokers.23
The sole comparison of GSR expression in epithelial cells by disease groups showed upregulation in COPD patients.18 In the 2 comparisons by smoking status, upregulation was observed among smokers.18, 19 Very similar results were found in the 3 studies of G6PD expression: 2-fold upregulation in epithelial cells 18, 19 and in alveolar macrophages.24
In agreement with biological networks, the 4 expression studies that considered GPX (GPX1, GPX2, and GPX3), GSR, and G6PD all showed upregulation of each of these genes with smoking.
GSH Conjugation and Export
There were 24 association and 4 expression studies of Glutathione S-Transferases (GSTs), which play a role in GSH conjugation and export.
A homozygous deletion of GSTM1, resulting in a complete lack of activity,25 was associated with increased COPD risk in 3 of 7 association studies (range in OR: 2.2 to 8.0).26–28 The prevalence of the deletion was increased in emphysema patients compared to non-diseased participants,29, 30 though no association was observed with emphysematous changes in heavy smokers.31 Both studies of chronic bronchitis reported a 3-fold increased risk associated with the null genotype.32, 33
Five studies investigated the association of the GSTM1 deletion with COPD-related quantitative traits. Conflicting evidence was reported: 1 of 2 studies of rate of FEV1 decline reported an association in men only34 and 1 of 3 studies of FEV1%predicted reported lower lung function with the null genotype.35 In a single study of FVC %predicted, the null genotype was significantly associated with decreased lung function.35 However, it was not associated with increased rate of FVC decline.34 Null genotype was associated with a steeper rate of FEF25–75 decline (among men) in one study.34
In GSTP1, the Ile105Val substitution, which causes altered affinity for specific substrates,36 was associated with COPD-related outcomes. A protective effect of the heterozygous genotype was reported in 7 of 11 studies of COPD patients vs. asymptomatic participants; the magnitude of the effect varied and was statistically significant in 2 studies.37, 38 A study of smokers with emphysematous changes (vs. normal smokers) reported a protective effect of heterozygosity.31 However, 7 of 10 studies of the homozygous variant genotype in relation to COPD risk reported an increased risk: the difference was statistically significant in one study39 and 4 estimates were based on small numbers.
The Ile105Val genotype had little or no relation to the rate of decline in FEV1, although the direction of effect was consistent with the hypothesis: risk was increased in those homozygous for the variant allele.34 Greater effect sizes were found for risk of being in the tails of the FEV1%predicted distribution,40 but there was little or no continuous relation with FEV1%predicted.41
A GSTP1 polymorphism with unknown biological effect (Ala114Val) was investigated in 3 studies. One study of Indian smokers observed a statistically significant graded increase in prevalence of COPD with the variant allele,39 but a similar association with emphysema risk was not observed in an American population.30 In 3 comparisons of lung function within disease groups, statistically significantly lower lung function was observed with the variant allele in COPD patients,39 but not in emphysema patients41 or asymptomatic smokers.39
There was little or no association of a homozygous deletion of GSTT1 with COPD risk; 3 of 4 studies reported a slightly decreased risk of disease with the null genotype, but the interval estimates of the effect were wide. There was no association of GSTT1 null and risk of emphysematous changes in smokers.31 Three of 4 studies of lung function reported an association: null genotype was associated with a steeper decline in FEV1 in a general population,42 with steeper decline in FEV1, FVC, and FEF25–75 among men,34 and with an increased risk of being in the lowest compared to the highest group of %predicted FEV1.40 The only study of mGST1 found no association between 4 markers and FEV1 %predicted.41
There were 3 studies of gene expression differences by disease group. Upregulation of GSTM3 and mGST1 expression was observed in COPD patients.18 GSTO1 was significantly downregulated in the single study of lung tissue and sputum from COPD patients with lung tumor,43 but was upregulated in the epithelial cells of patients with COPD only (less severe stages).18 Four studies investigated the expression of GSTs by smoking status. Both studies of GSTA1 expression showed upregulation among smokers in lung tissue.18, 21 Statistically significant upregulation was associated with smoking in a single study of GSTA2.19 GSTM3 expression was increased among smokers to the same extent (approximately 50%) in both studies of epithelial cells.18, 19 There was some evidence of upregulation of mGST1 among smokers in 2 studies.18, 19 In 6 of 7 comparisons of GSTO1 expression in various lung tissues, expression was unrelated to smoking.
Thioredoxin Metabolism
Thioredoxin metabolism was evaluated by considering the enzymes thioredoxin (TXN), thioredoxin reductase (TXNRD), and peroxiredoxin (PRDX). No association studies and 2 expression studies were found. A single study considering epithelial cell expression in COPD vs. non-diseased reported upregulation of TXN, TXNRD1, and PRDX1 with disease, and downregulation of PRDX5 with disease.18 Both studies of expression by smoking status reported increased expression of TXN and TXNRD1 with smoking.18, 19 In the study that also evaluated peroxiredoxins, PRDX1 was upregulated and PRDX3 and PRDX5 were both downregulated in smokers.18
Other Enzymes
Two classic antioxidants, superoxide dismutase (SOD) and catalase (CAT), were considered. Three association studies (evaluating 5 variants) and 6 expression studies were identified.
There was no association between an intronic SNP in SOD1 and COPD. SOD2 Val16Ala was associated with disease in a Chinese population,44 but not in persons of European descent.45 The association between SOD3 Arg213Gly and COPD was studied in 2 large populations.45, 46 Heterozygosity was associated with a strong, statistically significant decreased risk of disease (~40–75% reduction) 45, 46 and a 70% reduction in risk of COPD hospitalization or death during follow-up.46 Genotype was not associated with lung function in a general population, but FEV1/FVC ratio was higher among smokers with the heterozygous genotype (p=0.04).46 There were no homozygous variants among diseased individuals in either study, precluding odds ratio calculation.
Three studies of SOD expression compared COPD patients to healthy controls. SOD activity was increased in the bronchial lavage fluid of nonsmokers with COPD compared to smokers with COPD and healthy controls.47 No evidence for differential expression by disease status was seen in 3 studies of SOD1.18, 21, 48 In the 6 comparisons of SOD2 in lung tumor patients with COPD versus controls, COPD was associated with increased SOD2 concentration.21, 48 An increase in expression was not observed in the single study of COPD patients without lung tumor.18 Neither of the studies of SOD3 expression in disease groups provided strong evidence for differential regulation by disease.18, 21
Little or no evidence of differential expression of SOD1 and SOD3 by smoking status was observed. SOD2 was upregulated in smokers vs. nonsmokers in 3 comparisons of epithelial cells (with one showing strong, significant upregulation) and downregulated in 3 other comparisons. SOD2 was also upregulated in lung tissue homogenate and alveolar macrophages, but downregulated in the pulmonary blood vessels of smokers vs. nonsmokers.
Two studies evaluated polymorphisms in CAT, but provided no strong evidence for an association of two promoter region SNPs with disease risk.44, 45 Two studies compared CAT expression in disease groups, with little evidence for differential regulation by COPD status, though statistically significant downregulation was observed in lung tumor patients with COPD in 1 study.21 Three studies compared CAT expression by smoking status, with inconsistent results.
Gene-Gene Interaction
Increased risk of COPD, and decreased FEV1 %predicted among COPD patients, was reported for various genotype combinations that included either GSTP1 105Val or 114Val vs. wildtype for both polymorphisms (OR 1.99 for COPD risk; 95% CI 1.28, 3.09).39 In an analysis of GSTM1 null, GSTT1 null, and GSTP1 Ile105Val, most combinations of the “higher risk” genotypes were associated with an increased risk of disease, with the strongest associations observed with the GSTP1 105Ile allele and the null genotype for either GSTM1 (OR 11.3; 95% CI 1.3, 98.6) or GSTT1 (OR 12.1; 95% CI 1.3, 116.96).37 Although the combination of GSTM1 null, GSTT1 null, and GSTP1 105Ile was not associated with COPD risk in another study,49 it was associated with steeper lung function decline (p=0.026)40 and risk of rapid decline in lung function (OR 2.83; 95% CI 1.1, 7.2).42 Men with the null genotype for both GSTM1 and GSTT1 had 8.3 ml/year greater decline in FEV1 vs. those with at least one copy of both (p<0.001), with similar results reported for both FVC and FEF25–75.34 The National Emphysema Treatment Trial reported little or no association between combinations of GSTM1 null and GSTP1 105Ile and disease.30 There was little or no association of GSTM1 and GSTT1 null genotypes and emphysematous changes in Japanese heavy smokers.31 The combination of GSTM1 null, GSTP1 105 Ile/Ile, and at least one slow allele for microsomal epoxide hydrolase increased risk of COPD (OR 6.8; 95% CI 1.6, 17.2).26 The combination of GSTM1 null and a matrix metalloproteinase 9 polymorphism increased COPD risk by about 8-fold (OR 7.7; 95% CI 1.1, 53.3).27
DISCUSSION
Observational epidemiologic evidence suggests a role for nutrients contributing to antioxidant function in the prevention of lung disease.4–9 Whether these findings reflect underlying biological mechanisms or methodologic bias (e.g., confounding) is unclear. The consideration of genetic variants that affect antioxidant/oxidant balance and that may be sensitive to dietary intake of antioxidants can help address this question. The study of genetic variants affecting antioxidant capacity allows an unbiased approach, in comparison to observational studies of diet, based in part on the principles of Mendelian randomisation.50 Thus, this review was designed to evaluate the evidence that antioxidant enzyme function and/or regulation is related to COPD risk.
This systematic review included studies that addressed gene-disease associations as well as those that evaluated differences in gene expression. There were limited opportunities to synthesise results from both approaches as genes were often considered either in association studies or in expression studies, yet such synthesis may reveal complementary data.51 For example, a variant allele that leads to decreased glutathione was associated with an increased risk of COPD among smokers,12 and an expression study of GCLM reported upregulation in smokers.19 The combined results support the hypothesis that increasing available glutathione in persons with a high oxidant load may protect against lung damage. A lack of agreement between association and gene expression studies may also be informative. While association studies suggest a protective effect of heterozygosity for the GSTP1 Ile105Val polymorphism in COPD, no differences in expression of GSTP1 were reported in smokers compared to nonsmokers, suggesting that the effect of the genotype is not mediated through mRNA quantity.
Comparisons with animal studies provide an additional context in which to interpret the findings from human studies, but caution is warranted. Mice and adult rats (in contrast to humans) synthesise ascorbate,52 which protects GSH from oxidation and reduces it from its disulphide form.53 The interaction between ascorbate and other antioxidants suggests that animal studies of genetic manipulation or oxidant insult may not be predictive of results in humans. While two human studies of GSR expression reported significantly increased mRNA expression in the airway epithelial cells of asymptomatic smokers,18, 19 findings in smoke-exposed rats were mainly negative.54–56 Reduction of GSSG by endogenously formed ascorbate may blunt the rat’s need for GSR to perform the same function.
This review reveals very few instances where the evidence base contains enough information to make a strong statement of effect, but a few examples deserve mention. The GSTM1 null genotype (no enzyme activity) was consistently associated with increased COPD risk.26–29, 32–35, 37, 57 A substitution in another GST, GSTP1 Ile105Val, which affects catalytic activity and binding affinity for particular substrates, was consistently inversely associated with disease.31, 34, 37, 38, 40, 58–60 A rare substitution in SOD3, which increases plasma SOD levels, was also associated with a significantly decreased COPD risk,45, 46 a result supported by an animal study: transgenic mice overexpressing human SOD3 had attenuated lung damage and inflammatory response in hyperoxic conditions.61 In addition, there was simultaneous upregulation of GSR, GPX, and G6PD in the airway epithelial cells of smokers,19 highlighting the importance of a network of genes in the lung’s response to oxidative stress.
Several elements of the selected interrelated pathways have received minimal or no attention in human studies to date. For example, targeted disruption of the TXN gene produced early embryo lethality in mice,62, 63 and transgenic mice over-expressing human TXN1 had increased survival and decreased hydroxyl radical production during exposure to diesel exhaust particles.64 The two studies of TXN-related enzymes in humans reported upregulation of TXNRD1 and TXN in the airway epithelial cells of smokers and those with COPD18, 19: further investigation is warranted. Other understudied genes of interest include GGT, PRDX6, and GLRX. GGT is the key enzyme in one pathway for the intracellular supply of cysteine for GSH synthesis: GGT-deficient mice show a reduced ability for de novo synthesis65 and decreased intracellular GSH concentration.66 Furthermore, pulmonary GGT activity was increased during hyperoxia in rats,67 and GGT-deficient mice had worse survival in hyperoxic conditions.65, 66 PRDX6 is a peroxiredoxin that uses GSH as a cofactor. PRDX6 null mice had more severe lung injury and significantly decreased survival in conditions of hyperoxia vs. wildtype.68 Transgenic mice over-expressing PRDX6 had greater survival and attenuated lung damage in hyperoxia.69 Finally, GLRX comprises part of a major thiol-disulphide redox buffer in the cell. The activity of this enzyme suggests a possible role in relation to the oxidation of GSH and the redox status of the cell, recommending it for further study.
Several methodological considerations deserve mention. COPD aetiology is expected to include gene-environment interactions, given the clear role of smoking in this disease and the inter-individual differences in response to cigarette smoke. Thus, comparison groups must be carefully selected with regard to smoke exposure. In association studies in which the non-diseased group is comprised of non-smokers and the diseased group of smokers, for instance, the estimate of a true effect may be diluted. Similarly, in expression studies, a comparison between individuals with equivalent exposures, but whose disease outcome differed may be most informative. Studies published in other languages were included to avoid the “Tower of Babel error”.70 A comprehensive set of enzymes based on biological networks were the starting point for the review, however our selections may have led to inadvertent omission of relevant enzymes. Disturbances in a broad range of redox-related enzymes are likely to affect disease risk, suggesting that complex interactions cannot be ignored. A broader network approach may ultimately lead to more robust epidemiologic findings.
In conclusion, the evidence summarised in this review supports the continued investigation of the hypothesis that variation in genes that code for enzymes that can alter the redox environment of the lungs may contribute to COPD risk. Future directions suggested by this summary are: more network-driven approaches that include broader consideration of enzymes whose related, redundant and linked activities might alter disease risk, further integration of association and expression studies to determine the nature of the biological relationships that may lead to disease, and careful consideration, in both study design and analysis, of environmental exposures (e.g., smoking and nutritional status) that are likely to modify the gene-disease associations.
Supplementary Material
Acknowledgments
Funding: Supported by USDA (CSREES) Subproject on grant number 2003-34324-13135 and by NIH R01 HL 0701022; ARB supported in part by NIH training grant T32 DK007158-31.
Abbreviations used
- ABCC1
ATP-binding cassette C1
- CAT
catalase
- COPD
chronic obstructive pulmonary disease
- G6PD
glucose-6-phosphate dehydrogenase
- GCL
glutamate-cysteine ligase
- GCLC
GCL catalytic subunit
- GCLM
GCL modulatory subunit
- GGT
gamma-glutamyl transpeptidase
- GPX
glutathione peroxidase
- GLRX
glutaredoxin
- GSH
glutathione
- GSR
glutathione reductase
- GSS
glutathione synthetase
- GSSG
oxidised glutathione
- GST
glutathione S-transferase
- H202
hydrogen peroxide
- PRDX
peroxiredoxin
- SOD
superoxide dismutase
- TXN
thioredoxin
- TXNRD
thioredoxin reductase
Footnotes
No reprints available
Competing Interests: No conflict of interest exists.
Publisher's Disclaimer: Licence for Publication: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article to be published in Thorax editions and any other BMJPG Ltd products to exploit all subsidiary rights, as set out in our licence http://thorax.bmj.com/ifora/licence.pdf.
References
- 1.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4:1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CM. The Natural History of Chronic Bronchitis and Emphysema : An Eight-Year Study of Early Chronic Obstructive Lung Disease in Working Men in London. Oxford ; New York: Oxford University Press; 1976. p. xix.p. 272. [Google Scholar]
- 3.Beck GJ, Doyle CA, Schachter EN. Smoking and lung function. Am Rev Respir Dis. 1981;123:149–155. doi: 10.1164/arrd.1981.123.2.149. [DOI] [PubMed] [Google Scholar]
- 4.Butland BK, Fehily AM, Elwood PC. Diet, lung function, and lung function decline in a cohort of 2512 middle aged men. Thorax. 2000;55:102–108. doi: 10.1136/thorax.55.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Browne RW, et al. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur J Clin Nutr. 2006 doi: 10.1038/sj.ejcn.1602410. [DOI] [PubMed] [Google Scholar]
- 6.Grievink L, Smit HA, Ocke MC, van 't Veer P, Kromhout D. Dietary intake of antioxidant (pro)-vitamins, respiratory symptoms and pulmonary function: the MORGEN study. Thorax. 1998;53:166–171. doi: 10.1136/thx.53.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu G, Zhang X, Chen J, Peto R, Campbell TC, Cassano PA. Dietary vitamin C intake and lung function in rural China. Am J Epidemiol. 1998;148:594–599. doi: 10.1093/oxfordjournals.aje.a009685. [DOI] [PubMed] [Google Scholar]
- 8.Tabak C, Smit HA, Heederik D, Ocke MC, Kromhout D. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study) Clin Exp Allergy. 2001;31:747–755. doi: 10.1046/j.1365-2222.2001.01064.x. [DOI] [PubMed] [Google Scholar]
- 9.McKeever TM, Scrivener S, Broadfield E, Jones Z, Britton J, Lewis SA. Prospective study of diet and decline in lung function in a general population. Am J Respir Crit Care Med. 2002;165:1299–1303. doi: 10.1164/rccm.2109030. [DOI] [PubMed] [Google Scholar]
- 10.Walda IC, Tabak C, Smit HA, Rasanen L, Fidanza F, Menotti A, et al. Diet and 20-year chronic obstructive pulmonary disease mortality in middle-aged men from three European countries. Eur J Clin Nutr. 2002;56:638–643. doi: 10.1038/sj.ejcn.1601370. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura S, Kugiyama K, Sugiyama S, Miyamoto S, Koide S, Fukushima H, et al. Polymorphism in the 5'-flanking region of human glutamate-cysteine ligase modifier subunit gene is associated with myocardial infarction. Circulation. 2002;105:2968–2973. doi: 10.1161/01.cir.0000019739.66514.1e. [DOI] [PubMed] [Google Scholar]
- 12.Hu RC, Tan SX, Dai AG. The relationship between the polymorphism of glutamate cysteine ligase modulatory subunit gene and the susceptibility to chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. 2006;29:100–103. [PubMed] [Google Scholar]
- 13.Koide S, Kugiyama K, Sugiyama S, Nakamura S, Fukushima H, Honda O, et al. Association of polymorphism in glutamate-cysteine ligase catalytic subunit gene with coronary vasomotor dysfunction and myocardial infarction. J Am Coll Cardiol. 2003;41:539–545. doi: 10.1016/s0735-1097(02)02866-8. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Li B, Zhou Y, Zhong N, Ran P. Genetic analysis of CC16, OGG1 and GCLC polymorphisms and susceptibility to COPD. Respirology. 2007;12:29–33. doi: 10.1111/j.1440-1843.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 15.Rahman I, van Schadewijk AA, Hiemstra PS, Stolk J, van Krieken JH, MacNee W, et al. Localization of gamma-glutamylcysteine synthetase messenger rna expression in lungs of smokers and patients with chronic obstructive pulmonary disease. Free Radic Biol Med. 2000;28:920–925. doi: 10.1016/s0891-5849(00)00179-9. [DOI] [PubMed] [Google Scholar]
- 16.Lin SD, Dai AG, Xu P. Changes of the activity and expression of gamma-glutamylcysteine synthetase in patients with chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. 2005;28:97–101. [PubMed] [Google Scholar]
- 17.Harju T, Kaarteenaho-Wiik R, Soini Y, Sormunen R, Kinnula VL. Diminished immunoreactivity of gamma-glutamylcysteine synthetase in the airways of smokers' lung. Am J Respir Crit Care Med. 2002;166:754–759. doi: 10.1164/rccm.2112014. [DOI] [PubMed] [Google Scholar]
- 18.Pierrou S, Broberg P, O'Donnell RA, Pawlowski K, Virtala R, Lindqvist E, et al. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:577–586. doi: 10.1164/rccm.200607-931OC. [DOI] [PubMed] [Google Scholar]
- 19.Hackett NR, Heguy A, Harvey BG, O'Connor TP, Luettich K, Flieder DB, et al. Variability of antioxidant-related gene expression in the airway epithelium of cigarette smokers. Am J Respir Cell Mol Biol. 2003;29:331–343. doi: 10.1165/rcmb.2002-0321OC. [DOI] [PubMed] [Google Scholar]
- 20.Neurohr C, Lenz AG, Ding I, Leuchte H, Kolbe T, Behr J. Glutamate-cysteine ligase modulatory subunit in BAL alveolar macrophages of healthy smokers. Eur Respir J. 2003;22:82–87. doi: 10.1183/09031936.03.00080403. [DOI] [PubMed] [Google Scholar]
- 21.Tomaki M, Sugiura H, Koarai A, Komaki Y, Akita T, Matsumoto T, et al. Decreased expression of antioxidant enzymes and increased expression of chemokines in COPD lung. Pulm Pharmacol Ther. 2007;20:596–605. doi: 10.1016/j.pupt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Comhair SA, Thomassen MJ, Erzurum SC. Differential induction of extracellular glutathione peroxidase and nitric oxide synthase 2 in airways of healthy individuals exposed to 100% O(2) or cigarette smoke. Am J Respir Cell Mol Biol. 2000;23:350–354. doi: 10.1165/ajrcmb.23.3.4076. [DOI] [PubMed] [Google Scholar]
- 23.Peltoniemi MJ, Rytila PH, Harju TH, Soini YM, Salmenkivi KM, Ruddock LW, et al. Modulation of glutaredoxin in the lung and sputum of cigarette smokers and chronic obstructive pulmonary disease. Respir Res. 2006;7:133. doi: 10.1186/1465-9921-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heguy A, O'connor TP, Luettich K, Worgall S, Cieciuch A, Harvey BG, et al. Gene expression profiling of human alveolar macrophages of phenotypically normal smokers and nonsmokers reveals a previously unrecognized subset of genes modulated by cigarette smoking. J Mol Med. 2006;84:318–328. doi: 10.1007/s00109-005-0008-2. [DOI] [PubMed] [Google Scholar]
- 25.Xu S, Wang Y, Roe B, Pearson WR. Characterization of the human class Mu glutathione S-transferase gene cluster and the GSTM1 deletion. J Biol Chem. 1998;273:3517–3527. doi: 10.1074/jbc.273.6.3517. [DOI] [PubMed] [Google Scholar]
- 26.Cheng SL, Yu CJ, Chen CJ, Yang PC. Genetic polymorphism of epoxide hydrolase and glutathione S-transferase in COPD. Eur Respir J. 2004;23:818–824. doi: 10.1183/09031936.04.00104904. [DOI] [PubMed] [Google Scholar]
- 27.Yanchina ED, Ivchik TV, Shvarts EI, Kokosov AN, Khodzhayantz NE. Gene-gene interactions between glutathione-s transferase M1 and matrix metalloproteinase 9 in the formation of hereditary predisposition to chronic obstructive pulmonary disease. Bull Exp Biol Med. 2004;137:64–66. doi: 10.1023/b:bebm.0000024389.16247.0a. [DOI] [PubMed] [Google Scholar]
- 28.Dialyna IA, Miyakis S, Georgatou N, Spandidos DA. Genetic polymorphisms of CYP1A1, GSTM1 and GSTT1 genes and lung cancer risk. Oncol Rep. 2003;10:1829–1835. [PubMed] [Google Scholar]
- 29.Harrison DJ, Cantlay AM, Rae F, Lamb D, Smith CA. Frequency of glutathione S-transferase M1 deletion in smokers with emphysema and lung cancer. Hum Exp Toxicol. 1997;16:356–360. doi: 10.1177/096032719701600703. [DOI] [PubMed] [Google Scholar]
- 30.Hersh CP, Demeo DL, Lange C, Litonjua AA, Reilly JJ, Kwiatkowski D, et al. Attempted replication of reported chronic obstructive pulmonary disease candidate gene associations. Am J Respir Cell Mol Biol. 2005;33:71–78. doi: 10.1165/rcmb.2005-0073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Budhi A, Hiyama K, Isobe T, Oshima Y, Hara H, Maeda H, et al. Genetic susceptibility for emphysematous changes of the lung in Japanese. Int J Mol Med. 2003;11:321–329. [PubMed] [Google Scholar]
- 32.Baranova H, Perriot J, Albuisson E, Ivaschenko T, Baranov VS, Hemery B, et al. Peculiarities of the GSTM1 0/0 genotype in French heavy smokers with various types of chronic bronchitis. Hum Genet. 1997;99:822–826. doi: 10.1007/s004390050455. [DOI] [PubMed] [Google Scholar]
- 33.Baranov VS, Ivaschenko T, Bakay B, Aseev M, Belotserkovskaya R, Baranova H, et al. Proportion of the GSTM1 0/0 genotype in some Slavic populations and its correlation with cystic fibrosis and some multifactorial diseases. Hum Genet. 1996;97:516–520. doi: 10.1007/BF02267078. [DOI] [PubMed] [Google Scholar]
- 34.Imboden M, Downs SH, Senn O, Matyas G, Brandli O, Russi EW, et al. Glutathione S-transferase genotypes modify lung function decline in the general population: SAPALDIA cohort study. Respir Res. 2007;8:2. doi: 10.1186/1465-9921-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tkacova R, Salagovic J, Ceripkova M, Tkac I, Stubna J, Kalina I. Glutathione S-transferase M1 gene polymorphism is related to COPD in patients with non-small-cell lung cancer. Wien Klin Wochenschr. 2004;116:131–134. doi: 10.1007/BF03040750. [DOI] [PubMed] [Google Scholar]
- 36.Zimniak P, Nanduri B, Pikula S, Bandorowicz-Pikula J, Singhal SS, Srivastava SK, et al. Naturally occurring human glutathione S-transferase GSTP1–1 isoforms with isoleucine and valine in position 104 differ in enzymic properties. Eur J Biochem. 1994;224:893–899. doi: 10.1111/j.1432-1033.1994.00893.x. [DOI] [PubMed] [Google Scholar]
- 37.Calikoglu M, Tamer L, Ates Aras N, Karakas S, Ercan B. The association between polymorphic genotypes of glutathione S-transferases and COPD in the Turkish population. Biochem Genet. 2006;44:307–319. doi: 10.1007/s10528-006-9031-4. [DOI] [PubMed] [Google Scholar]
- 38.Ishii T, Matsuse T, Teramoto S, Matsui H, Miyao M, Hosoi T, et al. Glutathione S-transferase P1 (GSTP1) polymorphism in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:693–696. doi: 10.1136/thx.54.8.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vibhuti A, Arif E, Deepak D, Singh B, Qadar Pasha MA. Genetic polymorphisms of GSTP1 and mEPHX correlate with oxidative stress markers and lung function in COPD. Biochem Biophys Res Commun. 2007;359:136–142. doi: 10.1016/j.bbrc.2007.05.076. [DOI] [PubMed] [Google Scholar]
- 40.He JQ, Connett JE, Anthonisen NR, Pare PD, Sandford AJ. Glutathione S-transferase variants and their interaction with smoking on lung function. Am J Respir Crit Care Med. 2004;170:388–394. doi: 10.1164/rccm.200312-1763OC. [DOI] [PubMed] [Google Scholar]
- 41.Hersh CP, Demeo DL, Lazarus R, Celedon JC, Raby BA, Benditt JO, et al. Genetic association analysis of functional impairment in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:977–984. doi: 10.1164/rccm.200509-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He JQ, Ruan J, Connett JE, Anthonisen NR, Pare PD, Sandford AJ. Antioxidant gene polymorphisms and susceptibility to a rapid decline in lung function in smokers. Am J Respir Crit Care Med. 2002;166:323–328. doi: 10.1164/rccm.2111059. [DOI] [PubMed] [Google Scholar]
- 43.Harju TH, Peltoniemi MJ, Rytila PH, Soini Y, Salmenkivi KM, Board PG, et al. Glutathione S-transferase omega in the lung and sputum supernatants of COPD patients. Respir Res. 2007;8:48. doi: 10.1186/1465-9921-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mak JC, Ho SP, Yu WC, Choo KL, Chu CM, Yew WW, et al. Polymorphisms and functional activity in SOD and catalase genes in smokers with COPD. Eur Respir J. 2007 doi: 10.1183/09031936.00015207. [DOI] [PubMed] [Google Scholar]
- 45.Young RP, Hopkins R, Black PN, Eddy C, Wu L, Gamble GD, et al. Functional variants of antioxidant genes in smokers with COPD and in those with normal lung function. Thorax. 2006;61:394–399. doi: 10.1136/thx.2005.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juul K, Tybjaerg-Hansen A, Marklund S, Lange P, Nordestgaard BG. Genetically Increased Antioxidative Protection and Decreased Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200509-1387OC. [DOI] [PubMed] [Google Scholar]
- 47.Yigla M, Berkovich Y, Nagler RM. Oxidative stress indices in COPD--Broncho-alveolar lavage and salivary analysis. Arch Oral Biol. 2007;52:36–43. doi: 10.1016/j.archoralbio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Harju T, Kaarteenaho-Wiik R, Sirvio R, Paakko P, Crapo JD, Oury TD, et al. Manganese superoxide dismutase is increased in the airways of smokers' lungs. Eur Respir J. 2004;24:765–771. doi: 10.1183/09031936.04.00121203. [DOI] [PubMed] [Google Scholar]
- 49.Chan-Yeung M, Ho SP, Cheung AH, So LK, Wong PC, Chan KK, et al. Polymorphisms of glutathione S-transferase genes and functional activity in smokers with or without COPD. Int J Tuberc Lung Dis. 2007;11:508–514. [PubMed] [Google Scholar]
- 50.Davey Smith G, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Burmeister M. Genetical genomics: combining genetics with gene expression analysis. Hum Mol Genet. 2005;14(Spec No 2):R163–9. doi: 10.1093/hmg/ddi267. [DOI] [PubMed] [Google Scholar]
- 52.Banhegyi G, Braun L, Csala M, Puskas F, Mandl J. Ascorbate metabolism and its regulation in animals. Free Radic Biol Med. 1997;23:793–803. doi: 10.1016/s0891-5849(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 53.Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994;269:9397–9400. [PubMed] [Google Scholar]
- 54.York GK, Peirce TH, Schwartz LW, Cross CE. Stimulation by cigarette smoke of glutathione peroxidase system enzyme activities in rat lung. Arch Environ Health. 1976;31:286–290. doi: 10.1080/00039896.1976.10667237. [DOI] [PubMed] [Google Scholar]
- 55.Joshi UM, Kodavanti PR, Mehendale HM. Glutathione metabolism and utilization of external thiols by cigarette smoke-challenged, isolated rat and rabbit lungs. Toxicol Appl Pharmacol. 1988;96:324–335. doi: 10.1016/0041-008x(88)90091-9. [DOI] [PubMed] [Google Scholar]
- 56.Gupta MP, Khanduja KL, Sharma RR. Effect of cigarette smoke inhalation on antioxidant enzymes and lipid peroxidation in the rat. Toxicol Lett. 1988;41:107–114. doi: 10.1016/0378-4274(88)90084-7. [DOI] [PubMed] [Google Scholar]
- 57.Korytina GF, Iaibaeva DG, Viktorova TV. Polymorphism of glutathione-S-transferase M1 and P1 genes in patients with cystic fibrosis and chronic respiratory tract diseases. Genetika. 2004;40:401–408. [PubMed] [Google Scholar]
- 58.Lu B, He Q. Correlation between exon5 polymorphism of glutathione S-transferase P1 gene and susceptibility to chronic obstructive pulmonary disease in northern Chinese population of Han nationality living in Beijing, China. Zhonghua Nei Ke Za Zhi. 2002;41:678–681. [PubMed] [Google Scholar]
- 59.Rodriguez F, de la Roza C, Jardi R, Schaper M, Vidal R, Miravitlles M. Glutathione S-transferase P1 and lung function in patients with alpha1-antitrypsin deficiency and COPD. Chest. 2005;127:1537–1543. doi: 10.1378/chest.127.5.1537. [DOI] [PubMed] [Google Scholar]
- 60.Yim JJ, Yoo CG, Lee CT, Kim YW, Han SK, Shim YS. Lack of association between glutathione S-transferase P1 polymorphism and COPD in Koreans. Lung. 2002;180:119–125. doi: 10.1007/s004080000086. [DOI] [PubMed] [Google Scholar]
- 61.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999;103:1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, et al. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol. 1996;178:179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 63.Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaimul Ahsan M, Nakamura H, Tanito M, Yamada K, Utsumi H, Yodoi J. Thioredoxin-1 suppresses lung injury and apoptosis induced by diesel exhaust particles (DEP) by scavenging reactive oxygen species and by inhibiting DEP-induced downregulation of Akt. Free Radic Biol Med. 2005;39:1549–1559. doi: 10.1016/j.freeradbiomed.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 65.Barrios R, Shi ZZ, Kala SV, Wiseman AL, Welty SE, Kala G, et al. Oxygen-induced pulmonary injury in gamma-glutamyl transpeptidase-deficient mice. Lung. 2001;179:319–330. doi: 10.1007/s004080000071. [DOI] [PubMed] [Google Scholar]
- 66.Jean JC, Liu Y, Brown LA, Marc RE, Klings E, Joyce-Brady M. Gamma-glutamyl transferase deficiency results in lung oxidant stress in normoxia. Am J Physiol Lung Cell Mol Physiol. 2002;283:L766–76. doi: 10.1152/ajplung.00250.2000. [DOI] [PubMed] [Google Scholar]
- 67.Van Klaveren RJ, Dinsdale D, Pype JL, Demedts M, Nemery B. Changes in gamma-glutamyltransferase activity in rat lung tissue, BAL, and type II cells after hyperoxia. Am J Physiol. 1997;273:L537–47. doi: 10.1152/ajplung.1997.273.3.L537. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Lung injury and mortality with hyperoxia are increased in peroxiredoxin 6 gene-targeted mice. Free Radic Biol Med. 2004;37:1736–1743. doi: 10.1016/j.freeradbiomed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Phelan SA, Manevich Y, Feinstein SI, Fisher AB. Transgenic Mice Overexpressing Peroxiredoxin 6 Show Increased Resistance to Lung Injury in Hyperoxia. Am J Respir Cell Mol Biol. 2006 doi: 10.1165/rcmb.2005-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gregoire G, Derderian F, Le Lorier J. Selecting the language of the publications included in a meta-analysis: is there a Tower of Babel bias? J Clin Epidemiol. 1995;48:159–163. doi: 10.1016/0895-4356(94)00098-b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.