Abstract
Decision-making is a complex process that requires the orchestration of multiple neural systems. For example, decision-making is believed to involve areas of the brain involved in emotion (e.g., amygdala, ventromedial prefrontal cortex) and memory (e.g., hippocampus, dorsolateral prefrontal cortex). In this article, we will present findings related to the amygdala’s role in decision-making, and differentiate the contributions of the amygdala from those of other structurally and functionally connected neural regions. Decades of research have shown that the amygdala is involved in associating a stimulus with its emotional value. This tradition has been extended in newer work, which has shown that the amygdala is especially important for decision-making, by triggering autonomic responses to emotional stimuli, including monetary reward and punishment. Patients with amygdala damage lack these autonomic responses to reward and punishment, and consequently, cannot utilize “somatic marker” type cues to guide future decision-making. Studies using laboratory decision-making tests have found deficient decision-making in patients with bilateral amygdala damage, which resembles their real-world difficulties with decision-making. Additionally, we have found evidence for an interaction between sex and laterality of amygdala functioning, such that unilateral damage to the right amygdala results in greater deficits in decision-making and social behavior in men, while left amygdala damage seems to be more detrimental for women. We have posited that the amygdala is part of an “impulsive,” habit type system that triggers emotional responses to immediate outcomes.
Keywords: amygdala, decision-making, emotion, ventromedial prefrontal cortex, hippocampus
Introduction
Traditionally, the function of the amygdala has long been described as involving emotion and especially fear-related processes. Classic studies from animal and human lesion research have identified the amygdala as a critical structure for the expression and perception of fear and the development of fear conditioning (e.g., Adolphs, Tranel, Damasio, & Damasio, 1994; Bechara et al., 1995; Kluver & Bucy, 1939; LeDoux, 1993a, 1993b). However, much of later research has demonstrated a role for the amygdala in appetitive processes as well (e.g., Baxter & Murray, 2002; Everitt, Cardinal, Parkinson, & Robbins, 2003; Everitt & Robbins, 2005). Recent research in humans has explored the amygdala’s contributions to more complex processes, such as social interaction (Gupta, Duff, & Tranel, in press; Kennedy, Glascher, Tyszka, & Adolphs, 2009; Spezio, Huang, Castelli, & Adolphs, 2007; Tranel & Hyman, 1990), social judgments (e.g., trustworthiness, stereotyping) (Adolphs, Tranel, & Damasio, 1998; Phelps et al., 2000; Winston, Strange, O'Doherty, & Dolan, 2002), and decision-making (Bechara, Damasio, Damasio, & Lee, 1999; Brand, Grabenhorst, Starcke, Vandekerckhove, & Markowitsch, 2007; De Martino, Kumaran, Seymour, & Dolan, 2006; Weller, Levin, Shiv, & Bechara, 2007). Here, we review work elucidating the role of the amygdala in decision-making in humans.
A frequently used tool to study decision-making is the Iowa Gambling Task (IGT), which was designed to simulate real-life decisions in terms of uncertainty of outcomes and variable reward and punishment (Bechara, Damasio, Damasio, & Anderson, 1994). The task has been described in detail elsewhere (Bechara, Tranel, & Damasio, 2000). Briefly, across trials, participants select from decks of cards, which are associated with monetary rewards and punishments. In order to gain the largest amount of money, participants must learn over trials that certain decks (decks C and D) are more rewarding overall as they are associated with small rewards but have small punishments. By contrast, the other decks (decks A and B) are disadvantageous overall because despite having larger immediate gains, they also have larger long-term punishments (for additional details regarding the task see Bechara et al., 2000). This task has been used to investigate the decision-making abilities of numerous populations, including participants with amygdala damage (Bechara et al., 1999; Brand et al., 2007), ventromedial prefrontal cortex damage (Bechara et al., 1994; Clark, Manes, Antoun, Sahakian, & Robbins, 2003; Fellows & Farah, 2005), schizophrenia (Sevy et al., 2007), Huntington’s Disease (Stout, Rodawalt, & Siemers, 2001), and substance abuse (Martin & Bechara, 2003; van der Plas, Crone, van den Wildenberg, Tranel, & Bechara, 2009; Woicik et al., 2009), among others.
Decision-making involves the orchestration of multiple neural structures and cognitive systems. Research has shown that areas such as the ventromedial prefrontal cortex (VMPC), amygdala, insula, somatosensory cortex, dorsolateral prefrontal cortex and hippocampus are all involved in various aspects of decision-making (Bechara & Damasio, 2005; Bechara, Damasio, & Damasio, 2003; Bechara, Tranel, & Damasio, 2000; Clark, et al., 2008; Clark & Manes, 2004; Dunn, Dalgleish, & Lawrence, 2006; Naqvi, Shiv, & Bechara, 2006; Gupta et al., 2009; Manes, et al., 2002). Here, we review some of the pertinent findings related to the role of the amygdala in decision-making, and differentiate its role from the roles of other structures functionally and anatomically connected to the amygdala, such as the VMPC and hippocampus.
The amygdala and VMPC are critical for decision-making as measured by the IGT
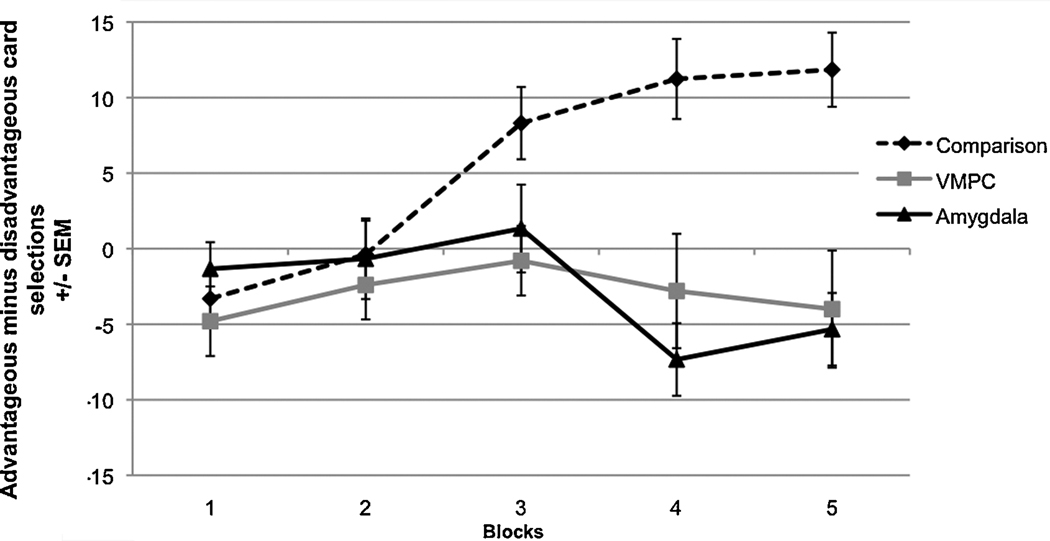
In the IGT, healthy normal participants learn over trials to avoid the decks that are disadvantageous overall (A and B), as they yield overall monetary loss, and prefer the advantageous decks (C and D) which yield overall monetary gain. However, participants with bilateral amygdala damage as well as participants with bilateral ventromedial prefrontal cortex (VMPC) damage do not learn to avoid (i.e., they continue to prefer) the disadvantageous decks (A and B) (see Fig. 1) (Bechara, Damasio, & Damasio, 2003; Bechara et al., 1999). This decision-making behavior results in monetary losses overall. This was one of the first findings from a laboratory test in which the impaired decision-making performance of both of these participant populations resembled their real-life inability to make advantageous decisions (Bechara et al., 1994; Bechara et al., 1999). Using skin conductance recording, it was found that normal participants generate skin conductance responses (SCRs) prior to the selection of any cards, i.e., during the time when they were pondering from which deck to choose. The SCRs generated before picking a card from the risky decks A and B were more pronounced compared to the SCRs generated prior to picking from the advantageous decks. However, participants with VMPC or amygdala damage failed to generate this anticipatory SCR before selecting a card (Bechara et al., 1999). Additionally, healthy participants generate a skin conductance response (SCR) after selecting a card and receiving a monetary reward or punishment. VMPC participants generated these reward and punishment SCRs normally; however, participants with amygdala damage failed to generate these responses after winning or losing money.
Figure 1.
Participants with bilateral amygdala damage and participants with bilateral ventromedial prefrontal cortex damage have impaired performance on the IGT. (Data used with permission from Bechara et al., 2003)
These findings have been interpreted within the framework of the somatic marker hypothesis (Damasio, 1994) (while acknowledging that other frameworks may also provide reasonable explanations; e.g., see Dunn, Dalgleish, & Lawrence, 2006, for review). Dunn and colleagues noted that the somatic marker theory enjoys strong scientific support in terms of anatomical circuitry. The weakest link in the theory, according to Dunn and colleagues, is the role of the peripheral signals (body signals) in influencing decision-making, and this is an accurate appraisal that we ourselves accept. However, the somatic marker theory does not hinge upon this periheral link, since the as-if-body loop of the theory operates entirely in the brain. As such, the somatic marker has robust support for its proposed anatomical circuit, especially the central nervous system components, i.e., the amygdala, VMPC, and insula (Dunn et al., 2006), and we maintain that this theory remians the most parsimonous theory which can account for the different roles of the various neural structures involved in decision-making. The somatic marker hypothesis states that somatic signals tied to stimuli or events will be reactivated in future encounters with those stimuli or events and will bias behavior related to the stimuli (see Figure 2 for a schematic model). As seen in the IGT, decision-making is believed to be guided by emotional signaling (or the reactivation of somatic states) that are generated in anticipation of future events based on past experience. Behaviorally, VMPC patients and amygdala patients perform similarly on the IGT; both participant groups select more from the disadvantageous decks than from the advantageous decks. However, as suggested by the differences in SCR responses during the IGT in amygdala and VMPC participants, these two structures are believed to play distinct roles in decision-making. Where the VMPC appears necessary for reactivating previously acquired information regarding the value of stimuli or events (as revealed by a lack of anticipatory SCRs), the amygdala appears to be necessary for acquiring and/or associating information on the value of stimuli or events (as revealed by the lack of SCRs to reward or punishment). The amygdala is involved in inducing these somatic states from primary inducers, or stimuli/entities that are innate or highly learned to be pleasurable or aversive (e.g., snakes; monetary reward or punishment). The VMPC, by contrast, is involved in inducing somatic states from secondary inducers, or entities generated by the recall of a personal or hypothesized emotional event. These are “thoughts” or “memories” of a primary inducer, that when brought into memory, elicit a somatic state. For example, the memory of losing or winning money, or simply imagining winning or losing money will also elicit a somatic response.
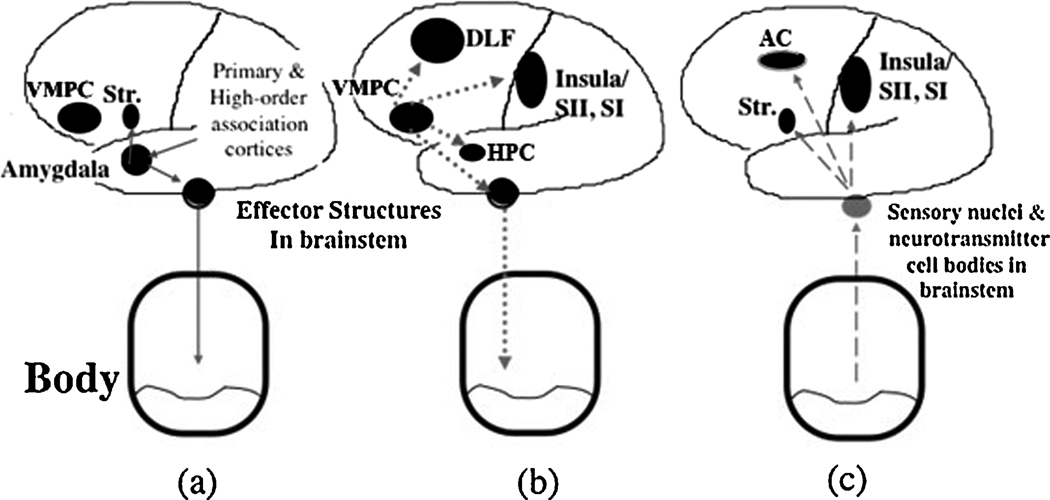
Figure 2.
A schematic model of somatic state activation and decision-making. (a) The amygdala triggers emotional (somatic) states from primary inducers. It does so by coupling the features of primary inducers, received via early sensory and high-order association cortices, with effector structures (e.g., hypothalamus) that trigger the emotional/somatic response. (b) The ventromedial prefrontal cortex (VMPC) is a trigger structure for emotional/somatic states from secondary inducers. It couples systems involved in memory (including doroslateral prefrontal cortex (DLF) and hippocampus (HPC) which bind the context of the stimulus to its somatic and emotional outcome. The VMPC also couples to effector structures that induce the somatic responses, and to structures holding representations of previous feeling states (e.g., Insula and Somatosensory I (SI) and Somatosensory II (SII) cortices). During the pondering of a decision, somatic states are triggered by primary or secondary inducers. Once induced, their ascending feedback signals (c) provide a substrate for feeling the emotional state, through the Insula/SII, SI as well as bias decisions through motor effector structures such as the striatum (Str.) and anterior cingulate cortex (AC).
A. The role of the amygdala
Decades of animal and human research have shown that the amygdala is involved in conditioned and unconditioned responses to stimuli (Amorapanth, LeDoux, & Nader, 2000; Bechara et al., 1995; Davis, 1992a, 1992b; LaBar, LeDoux, Spencer, & Phelps, 1995; LeDoux, 1993a, 1993b; Malkova, Mishkin, Suomi, & Bachevalier, 1997). It is believed that the amygdala is involved in coupling a stimulus which evokes an emotional response (i.e., a primary inducer, such as a snake) with its affective value. The evidence for this comes not only from fear conditioning work, but from the classic work of Kluver and Bucy (1939) who showed that monkeys with mesial temporal lesions that included the amygdala have an increased tendency to approach emotionally salient stimuli, e.g., snakes (Aggleton, 1992; Emery et al., 2001; Zola-Morgan, Squire, Alvarez-Royo, & Clower, 1991), suggesting that the stimuli no longer evoke fear. In humans, amygdala lesions reduce, but do not block, autonomic response (e.g., SCR) to an aversive loud sound (Bechara et al., 1999), and block the conditioned autonomic response to the same aversive loud sound (Bechara et al., 1995; LaBar et al., 1995). Amygdala damage in humans reduces autonomic responses to a variety of stressful or emotionally salient stimuli (Feinstein & Tranel, 2009; Lee et al., 1988; Lee et al., 1998; Tranel & Hyman, 1990). Functional neuroimaging studies have supported these findings, for example activation of the amygdala has been found in classical conditioning experiments (LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; Schiller, Levy, Niv, LeDoux, & Phelps, 2008), and a meta-analysis has shown that across 114 studies, the amygdala reliably responds to both positive and aversive stimuli (Ball et al., 2009). For example, amygdala activation has been found in response to emotionally salient pictures and emotional facial expressions (Breiter et al., 1996; Graham, Devinsky, & LaBar, 2007; Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002; Whalen et al., 1998).
The amygdala has been considered part of an “impulsive system” involved in decision-making, which triggers emotional responses to immediate outcomes (Bechara, 2005). Especially important for human decision-making, amygdala lesions impair the emotional response to learned, complex, cognitive information which through learning has acquired properties that automatically and obligatorily elicit emotional responses. Examples of this type of cognitive information are learned concepts such as “winning” or “losing.” The previously described findings from the IGT support this idea, as amygdala patients have reduced skin conductance response to winning or losing of various amounts of money (Bechara et al., 1999). In line with findings from the IGT, recent research has shown that participants with amygdala damage have reduced aversion to monetary loss (De Martino, Camerer, & Adolphs, 2010). Participants with amygdala lesions display impairments in decision-making on other tasks, including decision-making under risk, as measured by tasks such as the Game of Dice Task (Brand et al., 2007) and the Cups Task (Weller et al., 2007). Functional neuroimaging studies have also supported the notion that the amygdala is involved in reward/loss and value. Increased amygdala activation has been found in reaction to winning and losing money (Zalla et al., 2000). The amygdala has also been found to be active when subjects choose options associated with large reward magnitudes (Smith et al., 2009), when they make choices that reflect regret avoidance (Coricelli et al., 2005), or when evaluating risk in contexts of both certain gain and certain loss (De Martino et al., 2006). Also, recent research in patients with unilateral amygdala damage, utilizing a version of the Trust Game, reveals that such patients have abnormal responses to defections or betrayals of trust, whereby negative outcomes are not treated in kind; rather, they are treated with increased and maladaptive generosity (Koscik & Tranel, this issue).
Thus, as participants with amygdala damage have impaired emotional responses to primary inducers, such as winning or losing money, this emotional information cannot guide their future decisions. Therefore, in the IGT, because they do not mount an autonomic response to reward or punishment, these somatic states cannot be tied back to the associated stimuli (good or bad decks), and reconstituted by the VMPC when deliberating the consequences of a future decision, since they do not exist in the first place. Previous work has indicated that the development of the amygdala system may be a necessary step for the intact functioning of the VMPC system to trigger somatic states from secondary inducers. Evidence for this comes from a patient with focal bilateral amygdala damage who had intact skin conductance responses to the recall of emotional memories which occurred before brain damage, but not emotional memories which occurred after amygdala damage (Bechara et al., 2003). This suggests that the VMPC can only reconstitute somatic states for which the amygdala was intact and functioning when the primary inducer occurred. Therefore, it seems likely that the age of amygdala lesion onset might affect decision-making ability as well, such that earlier lesions might be more detrimental, similar to the pattern of findings with participants with early-onset damage to the VMPC (Anderson, Bechara, Damasio, Tranel, & Damasio, 1999). However, while age of amygdala lesion onset has been examined for abilities such as theory of mind (Shaw et al., 2004) and emotional facial expression recognition (Meletti et al., 2003), both showing that earlier damage is more detrimental, to our knowledge, this has not been systematically examined for decision-making.
B. The role of the VMPC
While damage to the amygdala impairs the somatic response to reward and punishment, thus hindering future decision-making, VMPC participants do have intact somatic responses to reward and punishment. It has been hypothesized that the VMPC is a “reflective system” which is involved in integrating information, including autonomic responses generated by the “impulsive” amygdala-driven system, and controls these impulses to allow flexible pursuit of long-term goals and to use this information advantageously in the future. (Bechara, 2005) The VMPC is believed to link memory systems (including both working memory and declarative memory) and emotional systems (especially involving the amygdala) in order to analyze the decision and re-evoke the associated somatic states (Bechara, 2005; Bechara & Van Der Linden, 2005). Thus in the case of the IGT, participants with VMPC damage are unable to properly re-evoke the somatic state that is associated with reward and punishment after selecting from a deck; this information (as represented by an anticipatory SCR) cannot be used to guide future decision-making and card selections.
Hemispheric and sex-related asymmetry and decision-making
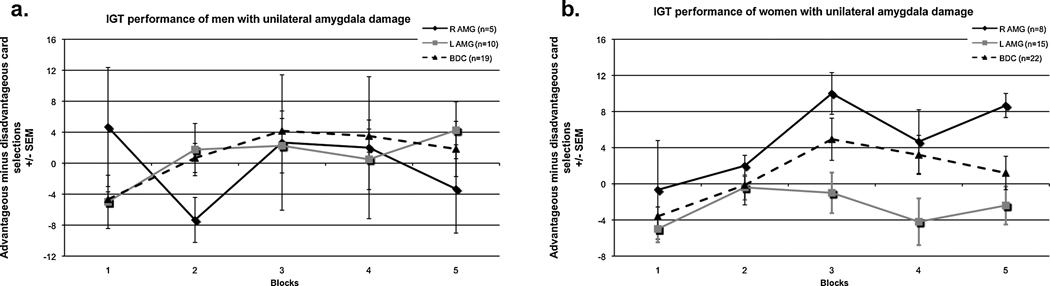
Most of the previous research on decision-making in VMPC and amygdala participants has focused on patients with bilateral damage to these structures. However, research has suggested that there may be functional differences in VMPC and amygdala that are driven by laterality (Cahill et al., 2001; Cahill, Uncapher, Kilpatrick, Alkire, & Turner, 2004). In fact, in our examinations of participants with VMPC or amygdala damage, we have found interesting sex-related functional asymmetries regarding social functioning and decision-making. Using a matched case study approach, same-sex pairs with comparable unilateral lesions in opposite hemispheres were compared on a number of variables including social conduct (as measured by ratings from neuropsychologists and family members), emotional functioning and personality (as measured by the Iowa Scales of Personality Change), and decision-making (as measured by the IGT). We found that right (but not left) VMPC damage in men is more likely to cause deficits in social conduct, emotional functioning and decision-making, while left (but not right) VMPC damage in women is more likely to lead to impairments (Tranel, Damasio, Denburg, & Bechara, 2005). A similar pattern has emerged from preliminary investigations in participants with unilateral amygdala damage. Men with unilateral right (but not left) amygdala damage tend to have greater disturbances in social and emotional functioning and decision-making, while left (but not right) amygdala damage in women is more likely to impair social conduct and decision-making (Tranel & Bechara, 2009). Recently, IGT data in an expanded sample of men and women participants with unilateral amygdala damage have provided additional support for our original conclusions regarding the sex-related asymmetry of amygdala function (see Fig. 2). To summarize, men patients with right amygdala damage had the poorest overall performance on the IGT, whilst men patients with left amygdala damage performed similarly to sex-matched brain-damaged comparison participants (Fig. 2a). The reverse outcome obtained in women: the women with left amygdala damage performed the worst on the IGT, whilst women with right amygdala damage performed similarly to sex-matched brain-damaged comparison participants (Fig. 2b). As our hypothesis suggests that these decision-making deficits arise from impairments in the autonomic response to emotionally salient stimuli (e.g., reward and punishment), it would be interesting to investigate if patients with unilateral amygdala damage who have impaired IGT performance also show a similar pattern of impairment in basic autonomic responses to emotional stimuli. Previous research has found that unilateral amygdala damage is sufficient to impair autonomic responses to conditioned stimuli (e.g., LaBar et al., 1995; Peper, Karcher, Wohlfarth, Reinshagen, & LeDoux, 2001; Weike, et al., 2005), but to our knowledge, these previous studies have not examined or report the relationship between sex and laterality.
Convergent evidence from other approaches supports the notion of sex-related functional asymmetry in the brain, particularly the female-left and male-right pattern that we have observed in lesion patients. For example, fMRI studies of brain responses to social and emotional stimuli display a consistent pattern of amygdala activation, including sex-related differences (for review see Hamann, 2005). In a study examining amygdala responses to happy and fearful faces, amygdala activation was more strongly lateralized for men than women, and right-sided activations were greater than left for men but not women, although both men and women displayed greater activation of the left amygdala for fearful faces (Killgore & Yurgelun-Todd, 2001). Memory for emotionally negative films was found to relate to right amygdala activity in men and left amygdala activity in women (Cahill, Uncapher, Kilpatrick, Alkire, & Turner, 2004). A recent meta-analysis reported lateralization of periamygdalar regions consistent with the women-left and men-right pattern (Wager, Phan, Liberzon, & Taylor, 2003). In a PET study, bilateral frontal activation was observed in women during recognition of facial emotions whereas unilateral right activation was observed in men (Hall, Witelson, Szechtman, & Nahmias, 2004). An ERP study examining responses to neutral and emotional faces revealed a similar pattern, whereby a strong right hemispheric dominance was observed in men and women showed a lack of asymmetry (Proverbio, Brignone, Matarazzo, Del Zotto, & Zani, 2006). Finally, in a functional connectivity study, increased connectivity has been observed in regard to the right amygdala of men and the left amygdala of women. There are some exceptions to the basic male-right, female-left pattern. On a task where participants either focused on their own emotion or evaluated the emotion of another, women tended to show activation in right hemisphere regions including frontal cortex, whereas men tended to have greater activations in the left temporoparietal junction (Schulte-Rüther, Markowitsch, Shah, Fink, & Piefke, 2008). An ERP study examining responses to emotional pictures found a similar effect, whereby women displayed reduced frontal latency preferentially in the right hemisphere, and men did not show this effect (Kemp, Silberstein, Armstrong, & Nathan, 2004).
These sex-related hemispheric asymmetries may reflect the unique social roles and goals of men and women (Koscik, Bechara, & Tranel, 2010). Given that men and women have distinct roles in human groups and societies, the most obvious of which is the fact that women bear children and men do not, there is ample reason to suspect that men and women have different emotional goals such that the same information may be more or less relevant to either sex or interpreted in line with the distinct goals (but likely complementary) of the sex in question. These different information processing goals undoubtedly require appropriate neural machinery capable of processing similar information in different ways. It may be that the genetic and developmental systems that determine sexual differentiation (e.g., the sex-related hormones) have been exapted to influence and at least partially determine the structure and functioning of neural systems that are needed to meet the differential goals of each sex. Given that sex differences exist in regard to the biological realities associated with sexual reproduction, natural selection is likely to proceed via a “path of least resistance” in finding solutions to ecological problems. Moreover, the cognitive power of a group can be increased by complementary specialization of individuals within the group. From these premises, we can make several predictions concerning sex differences in the brain. First, signaling mechanisms (e.g., sex hormones) that create sex differences in reproductive biology will have been exapted to create sex differences in neural substrates that support specialized cognition. Second, cognitive specializations are likely to be manifest as differences in hemispheric specializations as the hemispheres present easily differentiable targets to signaling mechanisms and unilateral alterations represent no-cost solutions to cognitive adaptation (Gazzaniga, 2000). Third, sex differences are most likely to be observed for brain regions that are unique, highly developed, or expanded in humans compared to our non-human relatives, and this likelihood will increase as phylogenetic distance from the last common ancestor increases. And fourth, where dichotomous specialization is insufficient, perhaps because specialization for one cognitive type interferes with more than one other important cognitive process, other signaling mechanisms may be exapted or sex hormone signaling may be exapted in other ways to create other complementarily specialized phenotypes. In short, sex-related functional asymmetry is not an evolutionary fluke, but rather, may be an adaptive solution to increase brain power without increasing individual brain size.
Differential contributions of hippocampus and amygdala in decision-making
Previous research has suggested that the dorsolateral prefrontal cortex and working memory are important for intact decision-making (e.g., Bechara, Damasio, Tranel, & Anderson, 1998; Manes et al., 2002). Research with participants with bilateral hippocampal damage has shown that the hippocampus and declarative memory also play a critical role in intact decision-making (Gupta et al., 2009; Gutbrod et al., 2006). However, participants with hippocampal damage display a distinct pattern of performance on the Iowa Gambling Task, different from the performance patterns of participants with amygdala or VMPC damage. While participants with VMPC or amygdala damage tend to select more from the disadvantageous decks than the advantageous decks, participants with bilateral hippocampal damage tend to choose equally from the advantageous and disadvantageous decks, resulting in IGT performances scores around zero throughout the task (Gupta et al., 2009). Additionally, unlike participants with amygdala damage, participants with bilateral hippocampal damage have normal SCRs in response to punishment or reward after selecting a card (Gutbrod et al., 2006). They also respond to punishment behaviorally, as they tend to always shift away from the most recent deck which has yielded punishment (Gupta et al., 2009). In the IGT however, this is not the most favorable strategy, and normal, healthy participants realize that the decks that are advantageous overall (C and D) are also associated with smaller, more frequent punishments than the disadvantageous decks (A and B). Thus, as the participants with hippocampal damage are unable to build these representations of the decks over trials, they respond only to the most immediate punishment. We suggest that declarative memory is critical for building choice-outcome representations for each deck of cards, as these relations must be built up flexibly across time.
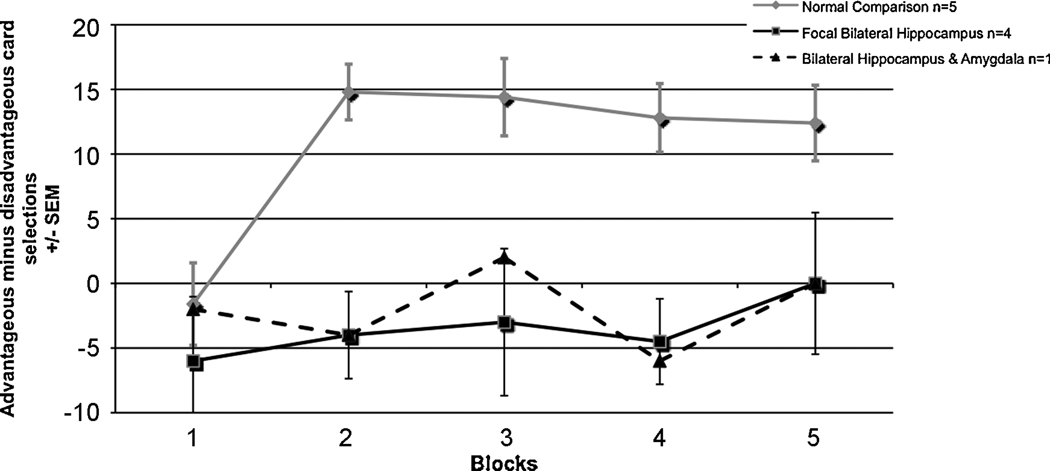
In order to further understand the relationship between contributions of the amygdala and hippocampus to decision-making, data were collected from a participant with bilateral damage to both the hippocampus and amygdala (Gupta et al., 2009). We found that this participant performed more similarly to the other participants with bilateral hippocampal damage, rather than focal bilateral amygdala damaged participants (Fig. 3). This suggests that advantageous decision-making requires the contributions multiple cognitive systems involved in at least two separate but related processes. Specifically, one of these processes seems to be related to the triggering and representation of the emotional “tag” or marker related to an outcome value, mediated by the VMPC and amygdala; this is not simply valence but a non-linear combination of valence and magnitude. However, the flexible formation and maintenance of a choice-outcome value seems to rely on declarative memory. Since the participant with bilateral damage to both the hippocampus and amygdala performs more like participants with bilateral hippocampal damage, this suggests that the contribution of declarative memory to decision-making may be necessary for the formation of an emotional marker to complex choice-outcome values which must be continually updated over time. In line with this finding is research that shows that patients with mild dementia of the Alzheimer’s type show a pattern similar to that of patients with hippocampal damage, as the Alzheimer’s patients a preference for advantageous or disadvantageous cards, and choose equally from both types across trials (Sinz, Zamarian, Benke, Wenning, & Delazer, 2008). This provides further evidence for the importance of declarative memory for decision-making.
Figure 3.
(a) Unilateral right amygdala damage, more so than damage on the left, seems to impair IGT performance in men, (b) while in women, the left amygdala, but not the right, seems to be critical for intact IGT performance. Note: BDC=brain-damaged comparison; R AMG=right amygdala; L AMG=left amygdala.
Conclusions
Overall, we have seen that the amygdala plays a distinct role in decision-making, separate from and complementary to the roles played by the VMPC and hippocampus. It is worth adding here that the decision-making deficit in amygdala participants, which has been demonstrated in the laboratory using the Iowa Gambling Task, is reflective of their real-world behavior (Bechara et al., 1999). For example, a patient with focal bilateral amygdala damage displays defective real-world decision-making as seen by inappropriate social behavior (e.g., flirtatiousness with strangers), inability to maintain employment, and inability to maintain stable interpersonal relationships (Adolphs, Tranel, Damasio, & Damasio, 1995; Tranel & Hyman, 1990). These decision-making deficits are in the same social realm as the real-world deficits seen in VMPC patients, but it is noteworthy that amygdala patients, unlike VMPC patients, may engage in actions that might lead to physical harm of themselves or others, whereas VMPC patients’ defective decisions typically do not lead to physical harm (Bechara et al., 1999). Potentially compounding their decision-making deficit is the lack of insight that patients with amygdala or VMPC damage often have regarding their faulty decision-making, thus hindering their ability to call upon compensatory strategies. This is especially notable in real-world situations where the patients seem to lack awareness that they are making bad decisions, even though in laboratory tasks they may realize what is right and what is wrong, but do not act according to that knowledge (Barrash, Tranel & Anderson, 2000; Tranel et al., 2005; Tranel & Bechara, 2009).
In sum, during decision-making, an initial choice is made, and the outcome of this choice (e.g., reward or punishment) is associated with an emotional, somatic response, which is mediated by the amygdala. Over time, the choice-outcome representation must be flexibly created such that even a choice that is not always associated with the same outcome has an overall positive or negative somatic response associated with it. This process of creating a choice-outcome representation flexibly across time is dependent on the hippocampus. When the choice is encountered in the future, the VMPC evaluates options and re-evokes the associated somatic states, which are used to guide decision-making (Bechara et al., 1999; Bechara et al., 2000; Weller et al., 2007). Future research is needed to better understand the effect of age of onset of amygdala dysfunction on decision-making, and the relationship between decision-making and other social abilities in which the amygdala is believed to be involved, such as theory of mind and perspective taking (Fine, Lumsden, & Blair, 2001; Gupta et al., in press; Shaw, et al., 2004; Stone, Baron-Cohen, Calder, Keane, & Young, 2003), in order to better understand the neural network invovled in these processes. Additionally, research is ongoing to better understand the contribution of these neural systems to impairments in decision-making in addiction and substance abuse (e.g., Bechara, 2005; Clark & Robbins, 2002). Future work should be mindful of potential differences in the functional laterality of these structures, as well as sex-related differences, as our recent work suggests that there are interesting interactions of sex and laterality of functioning in the amygdala and VMPC possibly reflecting sex differences in social roles.
Figure 4.
A participant with bilateral damage to the hippocampus and amygdala performs similarly on the IGT to other participants with bilateral hippocampal damage, where scores remain close to zero throughout the task.
Acknowledgements
Supported by NIDA R01 DA022549, R01 DA023051, and NINDS P50 NS19632.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372(6507):669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15(9):5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP. The functional effects of amygdala lesions in humans: a comparison with findings from monkeys. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss; 1992. pp. 485–504. [Google Scholar]
- Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3(1):74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2(11):1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Ball T, Derix J, Wentlandt J, Wieckhorst B, Speck O, Schulze-Bonhage A, et al. Anatomical specificity of functional amygdala imaging of responses to stimuli with positive and negative emotional valence. J Neurosci Methods. 2009;180(1):57–70. doi: 10.1016/j.jneumeth.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52(2):336–372. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann N Y Acad Sci. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19(13):5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation Of working memory from decision making within the human prefrontal cortex. J Neurosci. 1998;18(1):428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269(5227):1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18(6):734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Brand M, Grabenhorst F, Starcke K, Vandekerckhove MM, Markowitsch HJ. Role of the amygdala in decisions under ambiguity and decisions under risk: evidence from patients with Urbach-Wiethe disease. Neuropsychologia. 2007;45(6):1305–1317. doi: 10.1016/j.neuropsychologia.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, et al. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem. 2001;75(1):1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learn Mem. 2004;11(3):261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131(Pt 5):1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Manes F. Social and emotional decision-making following frontal lobe injury. Neurocase. 2004;10(5):398–403. doi: 10.1080/13554790490882799. [DOI] [PubMed] [Google Scholar]
- Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia. 2003;41(11):1474–1483. doi: 10.1016/s0028-3932(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Clark L, Robbins T. Decision-making deficits in drug addiction. Trends Cogn Sci. 2002;6(9):361. doi: 10.1016/s1364-6613(02)01960-5. [DOI] [PubMed] [Google Scholar]
- Coricelli G, Critchley HD, Joffily M, O'Doherty JP, Sirigu A, Dolan RJ. Regret and its avoidance: a neuroimaging study of choice behavior. Nat Neurosci. 2005;8(9):1255–1262. doi: 10.1038/nn1514. [DOI] [PubMed] [Google Scholar]
- Damasio A. Descartes' error: Emotion, reason, and the human brain. New York: Putnam; 1994. [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992a;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. 1992b;13(1):35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313(5787):684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Camerer CF, Adolphs R. Amygdala damage eliminates monetary loss aversion. Proc Natl Acad Sci U S A. 2010;107(8):3788–3792. doi: 10.1073/pnas.0910230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: a critical evaluation. Neurosci Biobehav Rev. 2006;30(2):239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Emery N, Capitanio J, Mason W, Machado C, Mendoza S, Amaral D. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115(3):515–544. [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Feinstein J, Tranel D. Probing the experience of fear in patient SM. Society for Neuroscience Abstracts. 2009 91.12. [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex. 2005;15(1):58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fine C, Lumsden J, Blair RJ. Dissociation between 'theory of mind' and executive functions in a patient with early left amygdala damage. Brain. 2001;124(Pt 2):287–298. doi: 10.1093/brain/124.2.287. [DOI] [PubMed] [Google Scholar]
- Gazzaniga M. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain. 2000;123(7):1293. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Graham R, Devinsky O, LaBar KS. Quantifying deficits in the perception of fear and anger in morphed facial expressions after bilateral amygdala damage. Neuropsychologia. 2007;45(1):42–54. doi: 10.1016/j.neuropsychologia.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Denburg NL, Cohen NJ, Bechara A, Tranel D. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47(7):1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Tranel D. Bilateral amygdala damage impairs the acquisition and use of common ground in social interaction. Neuropsychology. doi: 10.1037/a0021123. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutbrod K, Krouzel C, Hofer H, Muri R, Perrig W, Ptak R. Decision-making in amnesia: do advantageous decisions require conscious knowledge of previous behavioural choices? Neuropsychologia. 2006;44(8):1315–1324. doi: 10.1016/j.neuropsychologia.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hall G, Witelson S, Szechtman H, Nahmias C. Sex differences in functional activation patterns revealed by increased emotion processing demands. NeuroReport. 2004;15(2):219. doi: 10.1097/00001756-200402090-00001. [DOI] [PubMed] [Google Scholar]
- Hamann S. Sex differences in the responses of the human amygdala. Neuroscientist. 2005;11:288–293. doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- Kemp A, Silberstein R, Armstrong S, Nathan P. Gender differences in the cortical electrophysiological processing of visual emotional stimuli. Neuroimage. 2004;21(2):632–646. doi: 10.1016/j.neuroimage.2003.09.055. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Glascher J, Tyszka JM, Adolphs R. Personal space regulation by the human amygdala. Nat Neurosci. 2009 doi: 10.1038/nn.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W, Yurgelun-Todd D. Sex differences in amygdala activation during the perception of facial affect. NeuroReport. 2001;12(11):2543. doi: 10.1097/00001756-200108080-00050. [DOI] [PubMed] [Google Scholar]
- Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42(6):979–1000. [Google Scholar]
- Koscik T, Bechara A, Tranel D. Sex-related functional asymmetry in the limbic brain. Neuropsychopharmacology. 2010;35(1):340–341. doi: 10.1038/npp.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci. 1995;15(10):6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993a;58(1–2):69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory: in search of systems and synapses. Ann N Y Acad Sci. 1993b;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- Lee GP, Arena JG, Meador KJ, Smith JR, Loring DW, Flanigin HF. Changes in autonomic responsiveness following bilateral amygdalotomy in humans. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:119–129. [Google Scholar]
- Lee GP, Bechara A, Adolphs R, Arena J, Meador KJ, Loring DW, et al. Clinical and physiological effects of stereotaxic bilateral amygdalotomy for intractable aggression. J Neuropsychiatry Clin Neurosci. 1998;10(4):413–420. doi: 10.1176/jnp.10.4.413. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M, Suomi SJ, Bachevalier J. Socioemotional behavior in adult rhesus monkeys after early versus late lesions of the medial temporal lobe. Ann N Y Acad Sci. 1997;807:538–540. doi: 10.1111/j.1749-6632.1997.tb51961.x. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125(Pt 3):624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Martin EM, Bechara A. Decision-making and drug of choice in substance-dependent individuals: A preliminary report. Biological Psychiatry. 2003;53(8):97S–97S. [Google Scholar]
- Meletti S, Benuzzi F, Rubboli G, Cantalupo G, Stanzani Maserati M, Nichelli P, et al. Impaired facial emotion recognition in early-onset right mesial temporal lobe epilepsy. Neurology. 2003;60(3):426–431. doi: 10.1212/wnl.60.3.426. [DOI] [PubMed] [Google Scholar]
- Naqvi N, Shiv B, Bechara A. The role of emotion in decision making: A cognitive neuroscience perspective. Current Directions in Psychological Science. 2006;15(5):260–264. [Google Scholar]
- Peper M, Karcher S, Wohlfarth R, Reinshagen G, LeDoux JE. Aversive learning in patients with unilateral lesions of the amygdala and hippocampus. Biol Psychol. 2001;58(1):1–23. doi: 10.1016/s0301-0511(01)00098-9. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, et al. Performance on indirect measures of race evaluation predicts amygdala activation. J Cogn Neurosci. 2000;12(5):729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Proverbio A, Brignone V, Matarazzo S, Del Zotto M, Zani A. Gender differences in hemispheric asymmetry for face processing. BMC neuroscience. 2006;7(1):44. doi: 10.1186/1471-2202-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. J Neurosci. 2008;28(45):11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Rüther M, Markowitsch H, Shah N, Fink G, Piefke M. Gender differences in brain networks supporting empathy. Neuroimage. 2008;42(1):393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, et al. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr Res. 2007;92(1–3):74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lawrence EJ, Radbourne C, Bramham J, Polkey CE, David AS. The impact of early and late damage to the human amygdala on 'theory of mind' reasoning. Brain. 2004;127:1535–1548. doi: 10.1093/brain/awh168. [DOI] [PubMed] [Google Scholar]
- Sinz H, Zamarian L, Benke T, Wenning GK, Delazer M. Impact of ambiguity and risk on decision making in mild Alzheimer's disease. Neuropsychologia. 2008;46(7):2043–2055. doi: 10.1016/j.neuropsychologia.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Smith BW, Mitchell DG, Hardin MG, Jazbec S, Fridberg D, Blair RJ, et al. Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. Neuroimage. 2009;44(2):600–609. doi: 10.1016/j.neuroimage.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spezio ML, Huang PYS, Castelli F, Adolphs R. Amygdala damage impairs eye contact during conversations with real people. Journal of Neuroscience. 2007;27(15):3994–3997. doi: 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Calder AJ, Keane J, Young A. Acquired theory of mind impairments in individuals with bilateral amygdala lesions. Neuropsychologia. 2003;41(2):209–220. doi: 10.1016/s0028-3932(02)00151-3. [DOI] [PubMed] [Google Scholar]
- Stout JC, Rodawalt WC, Siemers ER. Risky decision making in Huntington's disease. J Int Neuropsychol Soc. 2001;7(1):92–101. doi: 10.1017/s1355617701711095. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A. Sex-related functional asymmetry of the amygdala: preliminary evidence using a case-matched lesion approach. Neurocase. 2009;15(3):217–234. doi: 10.1080/13554790902775492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128(Pt 12):2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Tranel D, Hyman BT. Neuropsychological correlates of bilateral amygdala damage. Archives of Neurology. 1990;47(3):349–355. doi: 10.1001/archneur.1990.00530030131029. [DOI] [PubMed] [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2009;31(6):706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T, Phan K, Liberzon I, Taylor S. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19(3):513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Weike AI, Hamm AO, Schupp HT, Runge U, Schroeder HW, Kessler C. Fear conditioning following unilateral temporal lobectomy: dissociation of conditioned startle potentiation and autonomic learning. J Neurosci. 2005;25(48):11117–11124. doi: 10.1523/JNEUROSCI.2032-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JA, Levin IP, Shiv B, Bechara A. Neural correlates of adaptive decision making for risky gains and losses. Psychol Sci. 2007;18(11):958–964. doi: 10.1111/j.1467-9280.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18(1):411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5(3):277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, et al. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34(5):1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalla T, Koechlin E, Pietrini P, Basso G, Aquino P, Sirigu A, et al. Differential amygdala responses to winning and losing: a functional magnetic resonance imaging study in humans. Eur J Neurosci. 2000;12(5):1764–1770. doi: 10.1046/j.1460-9568.2000.00064.x. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Alvarez-Royo P, Clower RP. Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1(2):207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]