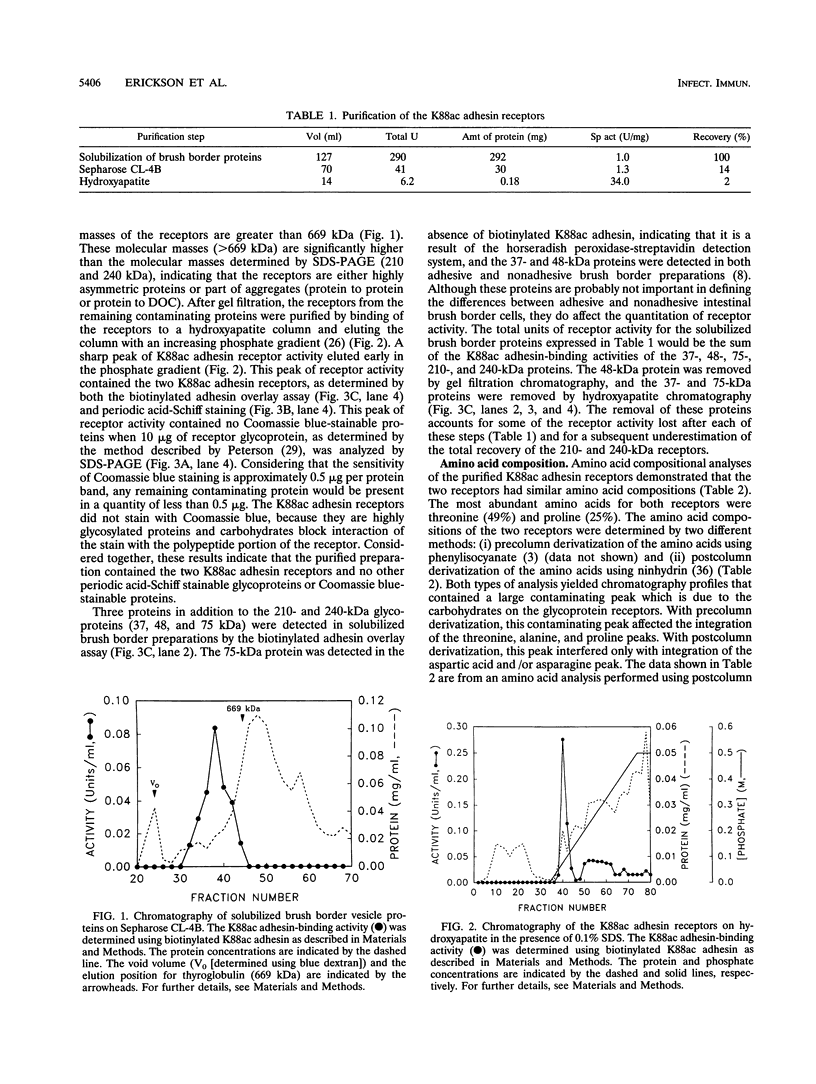
Abstract
We have previously identified two K88ac adhesion receptors (210 and 240 kDa) which are present in membrane preparations from adhesive but not nonadhesive porcine intestinal brush border cells; these adhesin receptors are postulated to be important determinants of the susceptibility of pigs to K88ac+ enterotoxigenic Escherichia coli infections (A.K. Erickson, J.A. Willgohs, S.Y. McFarland, D.A. Benfield, and D.F. Francis, Infect. Immun. 60:983-988, 1992). We now describe a procedure for the purification of these two receptors. Receptors were solubilized from adhesive intestinal brush border vesicles using deoxycholate and were purified by gel filtration chromatography on Sepharose CL-4B and then by hydroxyapatite chromatography. Amino acid compositional analyses indicated that the two receptors have similar amino acid compositions. The most distinguishing characteristic of both receptors is a high percentage of threonine and proline residues. Neuraminidase treatment caused the K88ac adhesin receptors to migrate with a slower mobility on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, indicating that these receptors are sialoglycoproteins. Results from lectin-binding studies indicated that the receptors contain O-linked oligosaccharides composed of galactosyl (beta-1,3)N-acetylgalactosamine, alpha-linked fucose, galactosyl(beta-1,4)N-acetylglucosamine, sialic acid, galactose, and N-acetylgalactosamine. Collectively, these characteristics indicate that the K88ac adhesin receptors are mucin-type sialoglycoproteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Whitehead J. S., Kim Y. S. Interaction of Escherichia coli K88 antigen with porcine intestinal brush border membranes. Infect Immun. 1980 Sep;29(3):897–901. doi: 10.1128/iai.29.3.897-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. M., Mackow E. R., Greenberg H. B. Identification and partial characterization of a rhesus rotavirus binding glycoprotein on murine enterocytes. Virology. 1991 Aug;183(2):602–610. doi: 10.1016/0042-6822(91)90989-o. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bijlsma I. G., de Nijs A., van der Meer C., Frik J. F. Different pig phenotypes affect adherence of Escherichia coli to jejunal brush borders by K88ab, K88ac, or K88ad antigen. Infect Immun. 1982 Sep;37(3):891–894. doi: 10.1128/iai.37.3.891-894.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg L., Krivan H. C., Cohen P. S., Conway P. L. Piglet ileal mucus contains protein and glycolipid (galactosylceramide) receptors specific for Escherichia coli K88 fimbriae. Infect Immun. 1993 Jun;61(6):2526–2531. doi: 10.1128/iai.61.6.2526-2531.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway K. L., Hull S. R. Cell surface mucin-type glycoproteins and mucin-like domains. Glycobiology. 1991 Mar;1(2):131–138. doi: 10.1093/glycob/1.2.131. [DOI] [PubMed] [Google Scholar]
- Doucet J. P., Trifaró J. M. A discontinuous and highly porous sodium dodecyl sulfate-polyacrylamide slab gel system of high resolution. Anal Biochem. 1988 Feb 1;168(2):265–271. doi: 10.1016/0003-2697(88)90317-x. [DOI] [PubMed] [Google Scholar]
- Erickson A. K., Willgohs J. A., McFarland S. Y., Benfield D. A., Francis D. H. Identification of two porcine brush border glycoproteins that bind the K88ac adhesin of Escherichia coli and correlation of these glycoproteins with the adhesive phenotype. Infect Immun. 1992 Mar;60(3):983–988. doi: 10.1128/iai.60.3.983-988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D. H., Ryan C. J., Fritzemeier J. D. Effect of sodium acetate on expression of K99 pili by Escherichia coli. Infect Immun. 1983 Sep;41(3):1368–1369. doi: 10.1128/iai.41.3.1368-1369.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Carlsson S. R. Leukosialin, a major sialoglycoprotein on human leukocytes as differentiation antigens. Med Biol. 1986;64(6):335–343. [PubMed] [Google Scholar]
- Gelberg H., Whiteley H., Ballard G., Scott J., Kuhlenschmidt M. Temporal lectin histochemical characterization of porcine small intestine. Am J Vet Res. 1992 Oct;53(10):1873–1880. [PubMed] [Google Scholar]
- Gibbons R. A., Jones G. W., Sellwood R. An attempt to identify the intestinal receptor for the K88 adhesin by means of a haemagglutination inhibition test using glycoproteins and fractions from sow colostrum. J Gen Microbiol. 1975 Feb;86(2):228–240. doi: 10.1099/00221287-86-2-228. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H. Behavior of Escherichia coli K antigens K88ab, K88ac, and K88ad in immunoelectrophoresis, double diffusion, and hemagglutination. Infect Immun. 1979 Mar;23(3):700–705. doi: 10.1128/iai.23.3.700-705.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Dowbenko D., Imai Y., Henzel W. J., Grimley C., Fennie C., Gillett N., Watson S. R., Rosen S. D. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992 Jun 12;69(6):927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- Laux D. C., McSweegan E. F., Williams T. J., Wadolkowski E. A., Cohen P. S. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12(K88ab). Infect Immun. 1986 Apr;52(1):18–25. doi: 10.1128/iai.52.1.18-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligtenberg M. J., Vos H. L., Gennissen A. M., Hilkens J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem. 1990 Apr 5;265(10):5573–5578. [PubMed] [Google Scholar]
- Metcalfe J. W., Krogfelt K. A., Krivan H. C., Cohen P. S., Laux D. C. Characterization and identification of a porcine small intestine mucus receptor for the K88ab fimbrial adhesin. Infect Immun. 1991 Jan;59(1):91–96. doi: 10.1128/iai.59.1.91-96.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Bunn T. O. Vaccines for preventing enterotoxigenic Escherichia coli infections in farm animals. Vaccine. 1993;11(2):213–200. doi: 10.1016/0264-410X(93)90020-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Hydroxylapatite chromatography of protein-sodium dodecyl sulfate complexes. A new method for the separation of polypeptide subunits. J Biol Chem. 1972 Aug 25;247(16):5194–5198. [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F., SOJKA W. J., WITTIG W. K ANTIGENS K88AB(L) AND K88AC(L) IN E. COLI. A NEW O ANTIGEN: 0147 AND A NEW K ANTIGEN: K89(B). Acta Pathol Microbiol Scand. 1964;62:439–447. doi: 10.1111/apm.1964.62.3.439. [DOI] [PubMed] [Google Scholar]
- Payne D., O'Reilly M., Williamson D. The K88 fimbrial adhesin of enterotoxigenic Escherichia coli binds to beta 1-linked galactosyl residues in glycosphingolipids. Infect Immun. 1993 Sep;61(9):3673–3677. doi: 10.1128/iai.61.9.3673-3677.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Rafiee P., Leffler H., Byrd J. C., Cassels F. J., Boedeker E. C., Kim Y. S. A sialoglycoprotein complex linked to the microvillus cytoskeleton acts as a receptor for pilus (AF/R1) mediated adhesion of enteropathogenic Escherichia coli (RDEC-1) in rabbit small intestine. J Cell Biol. 1991 Nov;115(4):1021–1029. doi: 10.1083/jcb.115.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J. M., Burrows M. R., Sellwood R., Gibbons R. A. A genetic basis for resistance to enteric disease caused by E. coli. Nature. 1975 Sep 11;257(5522):135–136. doi: 10.1038/257135a0. [DOI] [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- Sellwood R. The interaction of the K88 antigen with porcine intestinal epithelial cell brush borders. Biochim Biophys Acta. 1980 Oct 1;632(2):326–335. doi: 10.1016/0304-4165(80)90090-2. [DOI] [PubMed] [Google Scholar]
- Strömqvist M., Gruffman H. Periodic acid/Schiff staining of glycoproteins immobilized on a blotting matrix. Biotechniques. 1992 Nov;13(5):744-6, 749. [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman R. B., Mills K. W., Phillips R. M., Fortner G. W., Greenwood J. M. Predominance of the ac variant in K88-positive Escherichia coli isolates from swine. J Clin Microbiol. 1988 Jan;26(1):149–150. doi: 10.1128/jcm.26.1.149-150.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen P. T., de Graaf F. K. Age and serotype dependent binding of K88 fimbriae to porcine intestinal receptors. Microb Pathog. 1992 May;12(5):367–375. doi: 10.1016/0882-4010(92)90099-a. [DOI] [PubMed] [Google Scholar]
- Willoughby R. E. Rotaviruses preferentially bind O-linked sialylglycoconjugates and sialomucins. Glycobiology. 1993 Oct;3(5):437–445. doi: 10.1093/glycob/3.5.437. [DOI] [PubMed] [Google Scholar]
- Wilson I. B., Staley T. E., Bush L. J., Gilliland S. E. Recovery of intestinal membrane binding sites for K88 E. coli from pig mucosal organ cultures. Mol Cell Biochem. 1984 Apr;62(1):57–65. doi: 10.1007/BF00230078. [DOI] [PubMed] [Google Scholar]





