Abstract
A large body of evidence has shown that stromal cells play a significant role in determining the fate of neighboring tumor cells through the secretion of various cytokines. How cytokine secretion by stromal cells is regulated in this context is poorly understood. In this study, we used a bioengineered human tissue model of skin squamous cell carcinoma progression to reveal that RalA function in dermal fibroblasts is required for tumor progression of neighboring neoplastic keratinocytes. This conclusion is based on the observations that suppression of RalA expression in dermal fibroblasts blocked tumorigenic keratinocytes from invading into the dermal compartment of engineered tissues, and suppressed more advanced tumor progression after these tissues were transplanted onto the dorsum of mice. RalA executes this tumor-promoting function of dermal fibroblasts, at least in part, by mediating HGF secretion through its effector proteins, the Sec5 and Exo84 subunits of the exocyst complex. These findings reveal a new level of HGF regulation and highlight the RalA signaling cascade in dermal fibroblasts as a potential anti-cancer target.
INTRODUCTION
Solid tumors consist of oncogenically transformed cells embedded in a tissue microenvironment containing a multitude of additional cell types including fibroblasts, immune cells and endothelial cells. Recent studies have established that these stromal cells surrounding cancer cells are key mediators in the process of tumor progression (for review see (1)). Furthermore, as the tumor progresses the surrounding tissue evolves as well in ways that support tumor progression (2). For example, immune cells are recruited to the growing tumor mass and “cancer associated fibroblasts” with novel properties appear. These stromal constituents secrete factors that act either directly on tumor cells or indirectly such as by promoting angiogenesis.
Hepatocyte growth factor (scatter factor, HGF) is a multifunctional cytokine that is secreted by fibroblasts to promote the maintenance of neighboring epithelial cells (3). HGF acts on epithelial cells through the c-Met receptor, to upregulate genes involved in the epithelial-to-mesenchymal transition, a process important during development and tissue repair. When deregulated, it can also contribute to early steps in tumorigenesis (4). There is growing evidence that tumor cells can activate stromal cells to stimulate the secretion of HGF, promoting their own tumorigenicity (5). Understanding how stromal cells regulate the secretion of tumorigenic factors such as HGF may reveal new strategies to suppress progression of tumor cells to malignant states by targeting stromal cells.
Ral GTPases, RalA and RalB, are best known for their roles as downstream targets of Ras GTPases (6). Ras binds to and contributes to the activation of Ral-specific nucleotide exchange factors (Ral-GEFs), which subsequently activate RalA and/or RalB. The resulting active GTP-bound Ral proteins have the capacity to regulate many cellular functions by binding to and altering the activities of a set of effector proteins, including the Sec5 and Exo84 subunits of the exocyst complex (7-10), the CDC42 GTPase activating protein RalBP-1 (11-13), and the transcription factor Zonab (14).
Although RalA and RalB are quite similar (>85% identity) and have the potential to activate the same effectors, they actually play remarkably distinct roles in cells, most likely because of distinct subcellular localizations (15, 16) and differences in effector binding efficiency (15). For example, RalA, but not RalB, promotes the delivery of E-cadherin to the basal membrane of polarized epithelial cells through the exocyst subunit Exo84 (15). RalA, but not RalB, also uses the exocyst to promote early steps in cytokinesis (17) as well as cell polarity in neurons (18). Endocytosis of AMPA receptors in neurons that induces LTD, an important form of synaptic plasticity, is regulated specifically by RalA through RalBP1 (19). Finally, RalA promotes insulin exocytosis from islet beta-cells through the exocyst (20). RalB also has distinct functions, such as the ability to activate the TBK1 kinase through the exocyst subunit Sec5 to mount an innate immune response (21). It also differs from RalA in its ability to promote the completion of cytokinesis (17).
As downstream effectors of Ras, Ral proteins have been intensively investigated in cancer cells for their contributions to Ras-induced tumorigenesis (22). As expected from their differing normal functions described above, RalA and RalB can also play distinct roles in mediating carcinogenesis. For example, RalB activation of TBK-1 through the Sec5 subunit of the exocyst is important for tumor cells to avoid apoptosis (21), while RalA function through the exocyst is involved in promoting anchorage independent growth (22) via integrin-dependent exocytosis of lipid rafts (23). Moreover, RalB appears to be more critical than RalA for metastasis in tail vein injection assays, although the effector involved has not been revealed (24). Also, knock-down of RalA, but not RalB, blocked RalGEF-induced tumorigenesis in primary epithelial cells (16). Moreover, enhanced RalA and RalB activity correlates with tumorigenicity of pancreatic cells better than enhanced Erk activity (24). Finally, the tumor-suppressor PP2A inhibits RalA activation by dephosphorylating its C-terminus (25), implying that a super-active RalA may participate in cancers associated with the loss of PP2A such as those of the lung, breast and colon.
Interestingly, Ral proteins can also play inhibitory roles in tumorigenesis. For example, knock-down of RalB actually increases RalGEF-induced tumorigenesis in primary epithelial cells (16). Moreover, RalA through RalBP-1 suppresses tumor progression via inhibition of translation of the antiapoptotic protein FLIP(s) (26). Finally, we recently reported that RalA through Exo84, inhibits tumor progression in oncogenic Ras-expressing keratinocytes by promoting delivery of the suppressor of cell invasion, E-cadherin, to the plasma membrane (27). These examples highlight the fact that Ral GTPases can play distinct functions in different cell types and even a single cell type depending upon which effectors are activated.
In this study, we used a bioengineered human tissue model of Ras-induced skin squamous cell carcinoma to find a new tumor-promoting function for RalA in stromal fibroblasts. RalA, through the exocyst, promotes fibroblast secretion of HGF that is required for tumor progression.
METHODS AND MATERIALS
Cell Lines
HaCaT-II-4, HaCaT-II-4-H-2Kd-E-cad, HaCaT-II-4-sh-RalA, MSCC-1-Inv-1, and human foreskin fibroblast (HFF) cells were maintained as previously described (27). Cell lines were tested and authenticated by their ability to generate a 3-D skin tissues in vitro.
Three-dimensional cell culture
Bioengineered human skin tissues were prepared as previously described (28). Briefly, normal or manipulated human foreskin fibroblasts were mixed with Bovine Type I collagen (2.5×104 cells/ml) and the gels were allowed to contract for seven days in deep-well polycarbonate tissue culture inserts (Organogenesis, Canton, MA). 5×105 epithelial cells of the different keratinocyte cell lines mentioned above were seeded on top of the contracted collagen gels and grown submerged for three days in low-calcium epidermal growth medium. Cultures were then maintained for an additional two days in normal-calcium epidermal growth medium and subsequently raised to grow at an air-liquid interface for seven days.
Tissue transplantation
All animal experiments were performed according to a protocol approved by the Tufts University Institutional Animal Care and Use Committee. Briefly, for each cell line tested a 1.3cm dorsal skin section was removed from at least 5 athymic nude mice (Taconic Labs, Hudson, NY). Bioengineered tissues were grown as described above and transplanted onto fascia at the site of skin excision. Bandages were removed after 2 weeks and mice were sacrificed four weeks after transplantation. Skin tumors were excised, fixed in formalin, and processed according to common methods.
Quantification of cell invasion
For each tissue generated, epithelial cells that had invaded into the underlying matrix were counted and averaged from approximately 150 microscope fields (≥5 serial sections from ≥2 different depths). Averages shown represent ≥3 independent experiments ±S.D. P values, where shown, are calculated by the Mann-Whitney test.
Quantification of tumor volume
Two weeks after transplantation, tumors were photographed every 3-4 days. Tumor volume is calculated by the formula V = L × W × H.
Quantification of tumor differentiation
Tumor sections were analyzed by with H&E staining as well as K1/K10 immunohistochemistry. The total and differentiated areas of the tumors were measured using Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI). R, the ratio of differentiated areas and total tumor area was calculated using the formula R = TD/TA where TD is the K1/K10 positive area and TA is the total area of the tissue containing tumor cells.
RNAi
Lentivirus particles expressing shRNA against RalA, RalB, Exo84, Sec5, and RalBP-1 were previously described (27). Lentiviral vectors overexpressing shRNA against HGF were purchased from Sigma (TRCN clones 3307, 3309, and 3310) and packaged into lentivirus particles as previously described (27).
ELISA
Reagents for human-specific HGF and IL-6 ELISA were purchased from R&D Systems (Minneapolis, MN). 4 × 106 fibroblasts were seeded onto 100mm tissue culture plates and grown in 10ml for two days. Media was assayed in duplicate from ≥3 independent samples. The HGF ELISA recognizes both the uncleaved and mature forms of HGF (29).
Data analysis
Data represented in graphs and analyzed by Mann–Whitney, or Student's t tests were performed using GraphPad Prism 4.0 for Windows.
RESULTS
Suppression of RalA, but not RalB, expression in dermal fibroblasts blocks tumor progression of neighboring keratinocytes by increasing their E-cadherin expression
To test the importance of stromal fibroblast function in the progression of cutaneous squamous cell carcinoma, we used a well-characterized bioengineered human tissue model that mimics early steps of malignant disease, the loss of cell-cell adhesion and the acquisition of invasive behavior (30). This organotypic system uses immortalized human keratinocytes (HaCaT cells) grown on top of collagen gels that are populated with human dermal foreskin fibroblasts (HFF). Expression of activated H-Ras in HaCaT keratinocytes (referred to as II-4 cells) induces a dysplastic phenotype that is not sufficient for invasion into the dermal layer. (31, 32) The acquisition of invasive properties in Ras-expressing II-4 cells can be induced by the suppression of E-cadherin levels. This can be accomplished by increasing its degradation rate by expression of dominant-negative E-cadherin (H-2Kd-Ecad) (30, 33), or by expression of shRNA against E-cadherin (27). Suppression of E-cadherin function in II-4 cells is associated with the formation of a poorly-differentiated, high-grade aggressive carcinoma, following transplantation of bioengineered tissues comprised of these cells to the dorsal fascia of immune-compromised mice (34).
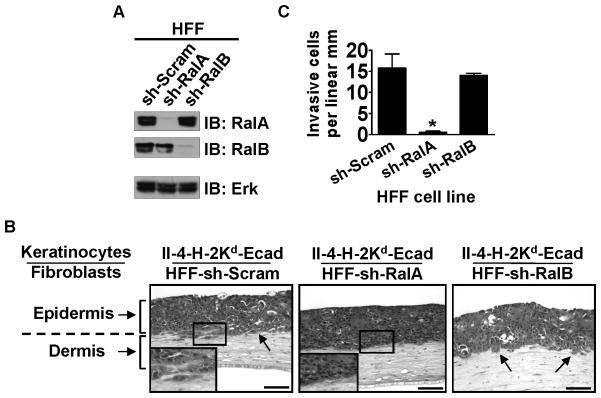
To investigate how Ral proteins in dermal fibroblasts influence the progression of skin squamous cell carcinoma in tissues, RalA or RalB expression was reduced (~90%) in dermal fibroblasts by stable expression of either RalA (sh-RalA) or RalB (sh-RalB) shRNA (Figure 1A). These cells along with scrambled control shRNA (sh-Scram) expressing fibroblasts were used to populate the dermal compartment of engineered skin equivalents that contained II-4-H-2Kd-Ecad keratinocytes in the epidermal compartment. Analysis of these tissues demonstrated that depletion of RalA in the dermal fibroblasts blocked invasion of keratinocytes as seen by the uninterrupted (or intact) basement membrane interface between the surface epithelium and the underlying fibroblast-populated collagen gel (Figure 1B, center panel). This was in contrast to the invasive pattern was seen when keratinocytes were grown on control, fibroblast-populated collagen gels (Figure 1B, left panel). In contrast, comparable knock-down of RalB in fibroblasts (Figure 1B, right panel) did not block tumor cells invasion. Quantification of invading cells in multiple tissue sections from independent experiments revealed that in tissues populated with RalA knock-down fibroblasts invasion of II-4-H-2Kd-Ecad keratinocytes was reduced by ~95% (Figure 1C). A second sequence to knock-down RalA in fibroblasts was also tested (Supplementary Figure 1A), with similar effects on invasion by II-4-H-2Kd-Ecad cells (Supplementary Figure 1B, center panel).
Figure 1. Suppression of RalA expression in dermal fibroblasts prevents the invasive phenotype of Ras-expressing, E-cadherin-suppressed keratinocytes.
(A) Western blot analysis of human foreskin fibroblasts (HFFs) expressing shRNA against RalA (sh-RalA) or RalB (sh-RalB) compared to control (sh-Scram) HFFs. Erk is shown as a loading control. (B) E-cadherin-suppressed, Ras-expressing keratinocytes were grown on top of collagen gels populated with HFFs expressing sh-Scram, sh-RalA, or sh-RalB. Tissue sections were stained with H&E. Bar: 100 μm. Invading cells in representative images from ≥3 independent experiments are indicated with arrows. (C) Invading E-cadherin-suppressed cells were quantified from >100 microscope fields in >20 sections from multiple experiments. * P < 0.01 for invasion of II-4-H-2Kd-Ecad keratinocytes in tissues comprised of sh-RalA HFFs vs. sh-Scram HFFs, using Mann-Whitney test.
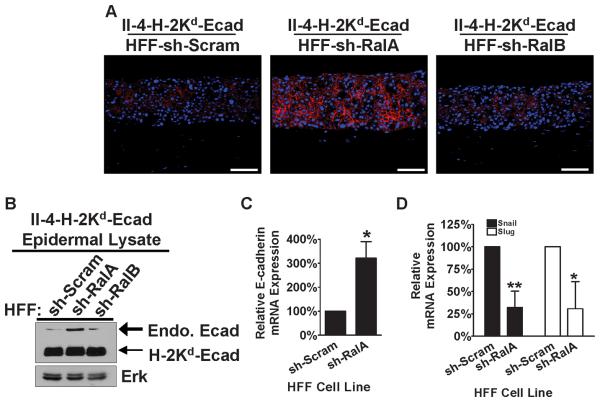
As the invasive phenotype of II-4-H-2Kd-Ecad cells has been previously shown to be dependent upon decreased E-cadherin function (32) we analyzed E-cadherin protein expression in these tissues by immunofluorescence. In contrast to tissues with sh-Scram and sh-RalB dermal fibroblasts (Figure 2A, left and right panels), in which weak E-cadherin staining of invasive II-4-H-2Kd-Ecad keratinocytes was observed, RalA knock-down in dermal fibroblasts led to strong keratinocyte staining consistent with elevated E-cadherin protein in non-invasive II-4-H-2Kd-Ecad keratinocytes (Figure 2A, center panel) and a more normalized tissue architecture (Figure 1B, center panel). This conclusion was confirmed by immunoblotting performed using the epidermal layer that was peeled from the underlying connective tissue and homogenized (Figure 2B). Analysis of mRNA levels showed that knock-down of RalA in fibroblasts led to an increase in E-cadherin gene expression in neighboring II-4-H-2Kd-Ecad keratinocytes (Figure 2C). It also led to a decrease in the expression of Snail and Slug, transcription factors that are known to negatively regulate E-cadherin gene expression (Figure 2D). Thus, these findings imply that RalA in dermal fibroblasts plays a vital tumor-promoting function in the early stages of squamous carcinoma progression by contributing to the down-regulation of E-cadherin gene in neighboring keratinocytes.
Figure 2. Suppression of RalA expression by shRNA in dermal fibroblasts is associated with re-expression of E-cadherin in E-cadherin-suppressed keratinocytes and its accumulation at the plasma membrane.
(A) E-cadherin-suppressed, Ras-expressing keratinocytes were grown on top of organotypic collagen gels populated with HFFs expressing sh-Scram, sh-RalA, or sh-RalB. Frozen tissue sections were immunostained with anti-E-cadherin antibody (red) and counterstained with DAPI (blue). Representative images from ≥3 independent experiments are shown. Bar: 100μm. (B) Western blot analysis from homogenized epidermal layer of tissues shown in (A). Total Erk is shown as a loading control. mRNA expression of E-cadherin (C) and Snail and Slug (D) were measured in the linear range by quantitative real-time PCR (qRT-PCR) from homogenized epidermal layers of bioengineered tissues containing either sh-Scram or sh-RalA fibroblasts. * P < 0.01, ** P < 0.005 for each of the mRNAs tested by Student's t-test.
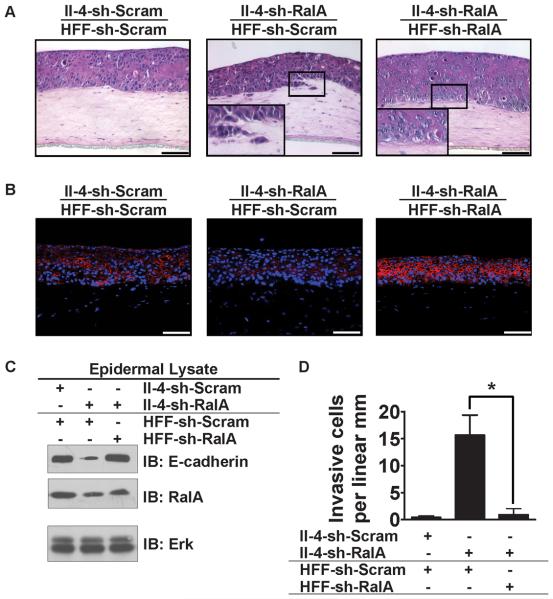
In contrast to this novel tumor-promoting role for RalA in dermal fibroblasts, we recently showed that RalA plays an opposite, tumor-suppressing function in Ras-expressing keratinocytes, since modest inhibition of RalA expression induced an invasive phenotype by reducing E-cadherin stability (27). Thus, we investigated which function of RalA (its tumor-suppressing function in keratinocytes or its tumor-promoting function in dermal fibroblasts) is dominant in this system, by engineering tissues with RalA suppressed in both cell types. We observed that the invasive phenotype associated with RalA depletion in II-4 cells (Figure 3A, compare left and center panels) was blocked when RalA was also depleted in the dermal fibroblasts (Figure 3A, right panel). sh-RalA fibroblasts also increased E-cadherin levels in neighboring RalA knock-down keratinocytes assayed by both immunofluorescence (Figure 3B, right panel) and immunoblotting of isolated epithelium (Figure 3C). Thus, the tumor-promoting function of RalA in dermal fibroblasts is dominant in this bioengineered model of skin SCC.
Figure 3. Suppression of RalA expression in dermal fibroblasts prevents the invasive phenotype of RalA knock-down II-4 keratinocytes.
(A) HaCaT-II-4 cells expressing either scrambled shRNA (II-4-sh-Scram) or shRNA against RalA (II-4-sh-RalA) were grown on top of organotypic collagen gels populated with HFFs expressing either scrambled shRNA (HFF-sh-Scram) or shRNA against RalA (HFF-sh-RalA). Tissues were stained with H&E. Bar: 100 μm. Invading cells in representative images from ≥3 independent experiments are indicated with arrows and (D) quantified from >100 microscope fields in >20 sections from multiple experiments. * P < 0.05 for invasion by II-4-sh-RalA keratinocytes in tissues comprised of sh-Scram HFFs vs. sh-RalA HFFs, using Mann-Whitney test. (B) Frozen sections of the tissues shown in (A) were subjected to immunostained with anti-E-cadherin antibody (red) and counterstained with DAPI (blue). (C) Western blot analysis from homogenized epidermal layer of the different tissues shown in (A,B). Total Erk is shown as a loading control.
The anti-tumor properties of RalA knock-down fibroblasts was tested further by populating the epidermal layer of tissues with more advanced tumor cells: an E-cadherin-suppressed, oral squamous cell carcinoma cell line (MSCC-1-Inv-1) isolated from a lymph node metastasis (35) Despite having a different and more-potent oncogenic background than the genetically-defined II-4-H-2Kd-Ecad keratinocytes, the invasive property of these cells was also sensitive to RalA depletion in fibroblasts. MSCC-1-Inv-1 cells grown above sh-Scram fibroblasts showed a robust invasive phenotype (Supplementary Figure 2A, left panel), while their invasive properties were repressed ~90% when grown above RalA depleted fibroblasts (Supplementary Figure 2A, right panel; Supplementary Figure 2B). As before, inhibition of tumor progression was associated with an increase in E-cadherin levels in tumor cells (Supplementary Figure 2C).
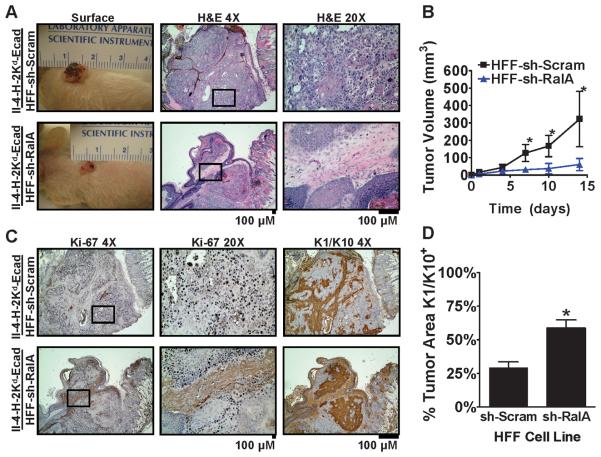
Finally, to test whether RalA knock-down fibroblasts affect later stages of SCC progression in an in vivo microenvironment, bioengineered tissues were transplanted to the dorsum of nude mice. Four weeks after grafting, tissues with II-4-H-2Kd-Ecad cells grown in combination with sh-Scram control fibroblasts formed large, exophytic tumors with raised, irregular borders (Figure 4A, top). H&E staining of tumor sections revealed sheets of pleiomorphic cells, with regions of highly-infiltrative tumor cells that invaded throughout the underlying stroma (Figure 4A, top). Ki-67 staining revealed proliferative cells throughout the tumor mass (Figure 4C, top). Staining for Cytokeratins 1 and 10, markers of keratinocyte differentiation were absent, indicating a poor degree of differentiation and a high-grade tumor cell behavior in the majority (~65%) of tumor area (Figure 4C, top; Figure 4D).
Figure 4. Suppression of RalA expression in dermal fibroblasts suppresses the growth of E-cadherin-suppressed, Ras-induced tumors in vivo.
(A) Tissues comprised of E-cadherin-suppressed II-4-H-2Kd-Ecad cells and HFFs expressing either sh-Scram or sh-RalA were transplanted to the dorsa of immunocompromised mice and tumors were analyzed after 4 weeks (a,d). Representative images of H&E staining from tumor sections at 4x and 20x magnification. Bar: 100 μm. (B) Tumor volume (L × W × H) measured at the indicated time points following the removal of bandages after tissue transplantation. * P < 0.05 comparing tumors harboring sh-Scram vs. sh-RalA fibroblasts using the Student's t-test at days 7, 10 and 14. n=5 for each cohort. (C) Representative Ki-67 and K1/K10 immunohistochemistry images of serial sections from the tumors shown in (A). Bar: 100 μm. (D) Percentage of area containing K1/10-positive, differentiated tumor cells in each group of tumors. * P < 0.05 comparing tumors harboring sh-Scram HFFs vs. sh-RalA HFFs using the Student's t-test.
In contrast, grafting of tissues constructed with II-4-H-2Kd-Ecad cells grown in combination with RalA knock-down fibroblasts, yielded tumors that grew to less than one fifth the size of those formed with tissues comprised of control fibroblasts (Figure 4A, left; and Figure 4B). Analysis of H&E staining revealed well-demarcated tumor islands that demonstrated well-defined borders with the adjacent stroma in tumors derived from tissues engineered with RalA knock-down fibroblasts (Figure 4A, bottom) These cells also demonstrated a more organized tissue architecture and absence of cellular pleiomorphism. Ki-67 staining showed less cell proliferation in these tumors, of which most was confined to the periphery of the tumor islands (Figure 4C, bottom). Finally, staining for the differentiation marker Cytokeratin 1 was found in a significantly larger number of tumor cells in tissues containing RalA knock-down fibroblasts compared to control fibroblasts, consistent with the presence of more-differentiated tumor cells and a low-grade tumor cell behavior (Figure 4D). Overall, these findings demonstrate that tissues comprised of RalA knock-down fibroblasts generated smaller tumors that contained fewer poorly-differentiated tumor cells with a highly aggressive phenotype than tissues that contained control fibroblasts. Thus, RalA function in dermal fibroblasts is necessary for full tumor progression to occur in overlying and adjacent keratinocytes with tumorigenic potential.
Suppression of exocyst subunits Sec5 and Exo84 in dermal fibroblasts mimics the tumor-blocking effects of RalA knock-down in these cells
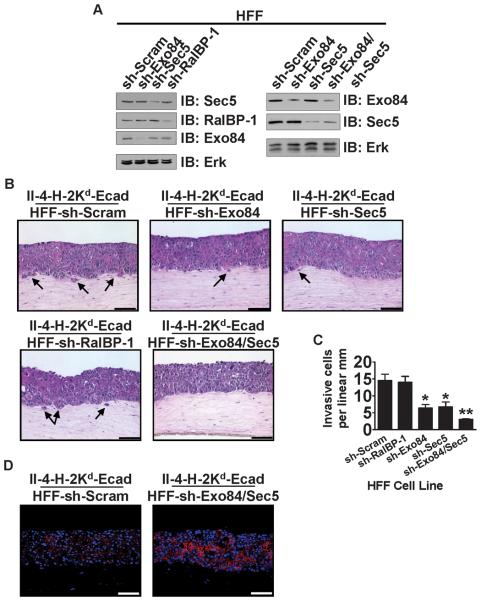
To begin to understand how RalA in dermal fibroblasts promotes the invasive properties of adjacent II-4-H-2Kd-Ecad keratinocytes in the epidermal layer, we tested for RalA effectors whose knock-down could mimic the effect of sh-RalA HFF cells. Each of the three most-investigated RalA effectors, Exo84, Sec5, and RalBP-1 were individually depleted in fibroblasts (Figure 5A). Then, each was used to populate the dermal compartment of bioengineered tissues containing II-4-H-2Kd-Ecad keratinocytes in the epithelial layer. RalBP-1 knock-down had no detectable effect on tumor cell invasion (Figure 5B, bottom left panel) when compared to control tissues (Figure 5B, top left panel). In contrast, Sec5-or Exo84-depleted fibroblasts each yielded partial inhibition of invasion (Figure 5B, top center and right panels and Figure 5C). When both Sec5 and Exo84 were knocked-down in dermal fibroblasts (Figure 5A), inhibition of invasion (Figure 5B, bottom center panel and Figure 5C) was comparable to that seen when RalA was suppressed (Figure 1C). As expected, sh-Exo84/Sec5 HFF cells increased E-cadherin expression at cell-cell junctions in neighboring II-4-H-2Kd-Ecad keratinocytes (Figure 5D) through an increase E-cadherin mRNA caused by a decrease in Snail and Slug transcription factors (Supplementary Figure 3A-C). These findings imply that RalA in fibroblasts influences tumor progression, at least in part, through the function of both of its exocyst effectors, Sec5 and Exo84.
Figure 5. Reduction of Exo84 and Sec5 in dermal fibroblasts prevents the invasive phenotype of E-cadherin-suppressed, Ras-expressing keratinocytes.
(A) Western blot analysis of HFFs expressing shRNA against Exo84 (sh-Exo84), Sec5 (sh-Sec5), RalBP-1 (sh-RalBP-1), or both Exo84 and Sec5 (sh-Exo84/Sec5). Erk is shown as a loading control. (B) E-cadherin-suppressed, Ras-expressing keratinocytes were grown on top of organotypic collagen gels populated with HFFs expressing sh-Scram, sh-Exo84, sh-Sec5, sh-RalBP-1, or sh-Exo84/Sec5. Tissue sections were stained with H&E. Bar: 100 μm. Invading cells in representative images from ≥3 independent experiments are indicated with arrows. Bar: 100μm. (C) Invading E-cadherin-suppressed cells were quantified from >100 microscope fields in >20 sections. * P < 0.05, ** P < 0.01 for invasion of II-4-H-2Kd-Ecad keratinocytes in tissues comprised of sh-Exo84, sh-Sec5, or sh-Exo84/Sec5 HFFs vs. sh-Scram HFFs, using Mann-Whitney test. (D) Frozen sections of the tissues shown in (B) were immunostained with anti-E-cadherin antibody (red) and counterstained with DAPI (blue). Representative images from ≥3 independent experiments are shown. Bar: 100μm.
Suppression of RalA or its effectors Sec5 and Exo84 in dermal fibroblasts blocks tumor progression by inhibiting HGF secretion
The exocyst is part of the cell's secretion machinery (36), suggesting that RalA may influence tumor progression in neighboring keratinocytes with neoplastic potential through the secretion of one or more soluble factor(s). Dermal fibroblasts secrete HGF that influences neighboring keratinocytes (37). For example HGF downregulates E-cadherin gene expression in keratinocytes as part of a wound healing process (3).
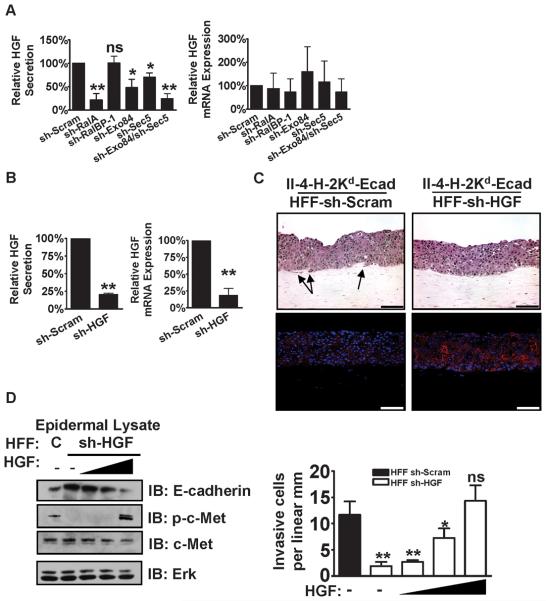
To test whether altered HGF secretion was mediating the effect of RalA knock-down fibroblasts on tumor cell invasion into the underlying matrix, HGF levels were measured by ELISA from growth media of fibroblasts. In sh-RalA fibroblast cultures, HGF levels were approximately four-fold lower than those from control fibroblasts (Figure 6A). In addition, the same RalA effector proteins in fibroblasts that we found contribute to tumor progression in neighboring epithelial cells (see Figure 5) were also found to be involved in the regulation of HGF secretion. In particular, knock-down of both the Sec5 and Exo84 subunits of the exocyst in HFF cells, but not RalBP1, were required to mimic the effect of RalA depletion on HGF secretion in tissue culture (Figure 6A, left panel).
Figure 6. RalA, Exo84, and Sec5 mediate the secretion of HGF by dermal fibroblasts that is necessary for the invasion of Ras-expressing, E-cadherin-suppressed keratinocytes in bioengineered tissues.
HGF protein expression and precursor mRNA expression (A) were measured in the linear range by ELISA of cell culture supernatant or qRT-PCR of cellular RNA, respectively, from the indicated fibroblast cell lines grown as monolayers on tissue culture plates. Data represent the average of at least three independent experiments measured in duplicate and averaged relative to sh-Scram. * P < 0.05, ** P < 0.01 by Student's t-test. Data represent the average of at least three independent experiments measured in duplicate and averaged relative to sh-Scram. (B) Efficiency of shRNA against HGF (sh-HGF) in HFFs was measured in the linear range vs. sh-Scram by ELISA and qRT-PCR. Data represent the average of at least three independent experiments measured in duplicate and averaged relative to sh-Scram. ** P < 0.01 by Student's t-test. (C) E-cadherin-suppressed, Ras-expressing keratinocytes were grown on top of organotypic collagen gels populated with HFFs expressing either sh-Scram or sh-HGF. Tissue sections were stained with either H&E or immunostained with anti-E-cadherin antibody (red) and counterstained with DAPI (blue). Bar: 100 μm. Representative images from ≥3 independent experiments are shown. Bar: 100μm. (D) Specificity of HGF knockdown in dermal fibroblasts was verified by complementation with recombinant human HGF (rh-HGF). The tissues described in (C) were grown in media supplemented with increasing concentrations of rh-HGF. The homogenized epidermal layers of these tissues were evaluated by western blot to measure E-cadherin protein level and phosphorylation of Met in response to rh-HGF treatment. Invading cells in these tissues were quantified from >100 microscope fields in >20 sections. * P < 0.05, ** P < 0.01 for invasion by II-4-H-2Kd-Ecad keratinocytes in tissues comprised of sh-HGF HFFs and vehicle-only control vs. sh-Scram HFFs with increasing concentrations of rh-HGF, using Mann-Whitney test. Data represent the average of three independent experiments measured in duplicate and averaged.
Most studies have shown that HGF secretion is regulated at the level of gene expression (37). However, that was not the case in this study, where no significant change in HGF precursor mRNA was detected in RalA knock-down fibroblasts (Figure 6A, right panel). This is consistent with the known function of the exocyst as a stimulator of the process of protein secretion. However, in this system RalA does not regulate all protein secretion, since RalA depletion in HFF cells did not suppress the secretion of cytokine IL-6 (Supplementary Figure 4), nor does it suppress contraction the collagen gel (Supplementary Figure 5), which occurs through the secretion of collagenases (38).
We then confirmed that the decrease in HGF secretion by RalA knock-down fibroblasts is responsible, at least in part, for the inhibition of tumor progression by directly reducing HGF secretion to the same degree via stable expression of HGF shRNA in fibroblasts (Figure 6B). A cell line was chosen that generated culture media with HGF levels similar to that found in RalA depleted fibroblasts (compare Figure 6B, left panel to Figure 6A, left panel). When these HGF knock-down fibroblasts were used to populate the dermis of engineered tissues (Figure 6C, top), E-cadherin expression in II-4-H-2Kd-Ecad keratinocytes rose (Figure 6C, bottom) and their invasive properties fell to levels comparable to those found in the tissues populated with RalA depleted fibroblasts (compare Figure 6D to Figure 1C). Specificity of shRNA against HGF in reducing the invasive properties of II-4-H-2Kd-Ecad keratinocytes was confirmed by supplementing growth media with recombinant human HGF (rh-HGF) (Figure 6D). Supplementation of tissues harboring sh-HGF fibroblasts with rh-HGF to produce phosphorylation of c-Met, the HGF receptor, in II-4-H-2Kd-Ecad keratinocytes decreased E-cadherin levels (left) and increased invasion (right) to levels comparable to tissues grown with sh-Scram fibroblasts. Thus, RalA and the exocyst function in dermal fibroblasts to mediate the secretion of HGF, which is necessary for tumor cell invasion in this bioengineered model of skin SCC.
DISCUSSION
This study reveals a key role for the RalA GTPase in how stromal cells support carcinoma development. Using a bioengineered human tissue model of the early steps in skin SCC we show that RalA in dermal fibroblasts promotes tumor progression in epithelial cells of the neighboring epidermis through its regulation of HGF secretion. This conclusion is based on the observation that shRNA-mediated suppression of RalA, but not RalB, expression in dermal fibroblasts blocked tumorigenic keratinocytes from invading into the dermal layer of engineered tissues. Strikingly, it also prevented metastatic oral squamous cell carcinoma cells from invading in this system, despite their having a far more transformed oncogenic background.
Knock-down of RalA expression in dermal fibroblasts also suppressed more advanced tumor progression after these tissues were transplanted onto the dorsa of mice. Suppression of tumor progression in vivo is striking in light of the fact that once the engineered human tissues are transplanted, normal mouse stromal cells are likely to participate in promoting tumor progression. Knock-down of RalA in fibroblasts suppressed HGF secretion approximately four-fold. This degree of HGF inhibition was significant because tumor progression was also blocked when HGF secretion was inhibited to a similar degree by expression of HGF shRNA. Moreover, we found that tumor suppression induced by RalA depletion in fibroblasts was associated with changes in neighboring keratinocytes that are known to occur when HGF levels are reduced, such as an increase in the expression of E-cadherin and a decrease in the expression of Snail and Slug, transcription factors that suppress E-cadherin RNA levels.
HGF plays an important role in dermal fibroblast support of epidermal function. Its secretion by fibroblasts is enhanced during specific stages of development and in response to wounding (3). In these cases and in most other examples of increased HGF release, regulation occurs at the level of increased gene expression (39). In contrast, we show here that HGF mRNA levels are not altered in RalA knock-down fibroblasts, suggesting that regulation of cytokine secretion also occurs at level of the secretion process in dermal fibroblasts. This is consistent with our results implying that RalA functions through, Sec5 and Exo84, the two Ral effectors that are subunits of the exocyst, a regulator of the process of exocytosis and cellular secretion (36). Thus, this study reveals a new level of HGF regulation in stromal fibroblasts that is mediated by RalA, Exo84 and Sec5.
The role of RalA in regulating HGF secretion in fibroblasts may have additional effects on tumor progression, since HGF is also known to promote angiogenesis (40). Thus, future studies using experimental model systems that detect the contribution of angiogenesis to tumor progression may be revealing. Moreover, HGF is known to support tumor progression in other tumor systems such as breast cancer and colon cancer (41, 42) suggesting that RalA in stromal cells may play a tumor supporting role in many types of cancer. Finally, although the addition of HGF to the media was able to restore the invasive properties of tumorigenic keratinocytes grown in tissues populated with HGF knockdown dermal fibroblasts, it did not do the same in comparable tissues populated with RalA knockdown fibroblasts. Thus, while HGF secretion induced by dermal fibroblast RalA is required for SCC tumor progression, it is not sufficient. Experiements are underway to identify the missing RalA-induced, SCC-promoting factor(s).
The tumor-promoting role of RalA in stromal fibroblasts found in this study dramatically contrasts with the tumor-suppressing function of RalA in Ras-expressing keratinocytes found in our previous study that used this same engineered human tissue model of SCC (27). In that study, RalA functioned as a tumor suppressor through its ability to stimulate E-cadherin delivery to the plasma membrane. One possible explanation for these two opposing roles for RalA stems from results from our previous study showing that RalA functioned as a tumor-suppressor in Ras-expressing keratinocytes through Exo84, but not Sec5, whereas in this study RalA functions as a tumor promoter in fibroblasts through both exocyst subunits. Thus, RalA plays opposing roles in two cell types that populate skin tissue by functioning through different sets of effectors.
Interestingly, when tissues were engineered with RalA suppressed in both fibroblasts and Ras-expressing keratinocytes, no invading keratinocytes were observed. This indicates that the tumor-suppressing effect of knocking-down RalA in fibroblasts was dominant over the tumor-promoting effect of knocking down RalA in Ras-expressing keratinocytes. In a previous study on mice with functional inactivation of the gene for the Ral activator, RalGDS (43), induction of squamous cell carcinoma of the skin by application of tumor promoters that lead to Ras activation was severely suppressed (43). It was assumed that this phenotype was a consequence of the effects of RalGDS loss in keratinocytes. However, the experiments described in this study suggest that this suppression of tumor progression may have also been due to depletion of RalGDS in dermal fibroblasts.
Finally, a variety of previous studies have highlighted how RalA signaling in tumor cells may be an attractive anti-cancer target (44). Our study suggests that targeting the RalA/exocyst signaling cascade that regulates HGF secretion in stromal cells may complement this approach, with the benefit that, unlike tumor cells, fibroblasts are genetically stable with a low propensity for drug resistance.
Supplementary Material
ACKNOWLEDGMENTS
We thank Y. Szwec-Levin, T. DesRochers, C. Rombaoa, H. Hatch, A. Maoine, X. Tian, and S. Dong for excellent technical assistance. This work was supported by grants to LAF from NIGMS and to JAG from NIDCR. We thank N. Fusenig for HaCaT cells and their derivatives and F. Watt for the H-2Kd-Ecad vectors.
REFERENCES
- 1.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–31. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 2.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–8. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem Biophys Res Commun. 1997;239:639–44. doi: 10.1006/bbrc.1997.7517. [DOI] [PubMed] [Google Scholar]
- 4.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer. 2006;119:477–83. doi: 10.1002/ijc.21808. [DOI] [PubMed] [Google Scholar]
- 6.Feig LA. Ral GTPases: Approaching their 15 minutes of fame. Trends Cell Biol. 2003;13:419–25. doi: 10.1016/s0962-8924(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 7.Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. The exocyst is a Ral effector complex. Nat Cell Biol. 2002;4:66–72. doi: 10.1038/ncb728. [DOI] [PubMed] [Google Scholar]
- 8.Moskalenko S, Tong C, Rosse C, et al. Ral GTPases regulate exocyst assembly through dual subunit interactions. J Biol Chem. 2003;278:51743–8. doi: 10.1074/jbc.M308702200. [DOI] [PubMed] [Google Scholar]
- 9.Brymora A, Valova VA, Larsen MR, Roufogalis BD, Robinson PJ. The brain exocyst complex interacts with RalA in a GTP-dependent manner: identification of a novel mammalian Sec3 gene and a second Sec15 gene. J Biol Chem. 2001;276:29792–7. doi: 10.1074/jbc.C100320200. [DOI] [PubMed] [Google Scholar]
- 10.Polzin A, Shipitsin M, Goi T, Feig LA, Turner TJ. Ral-GTPase influences the regulation of the readily releasable pool of synaptic vesicles. Mol Cell Biol. 2002;22:1714–22. doi: 10.1128/MCB.22.6.1714-1722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantor S, Urano T, Feig LA. Identification and characterization of RalBP1, a potential downstream target of Ral GTPases. Mol Cell Biol. 1995;15:4578–84. doi: 10.1128/mcb.15.8.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jullien-Flores V, Dorseuil O, Romero F, et al. Bridging Ral GTPase to Rho pathways. J Biol Chem. 1995;270:22473–7. doi: 10.1074/jbc.270.38.22473. [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Weinberg RA. A putative effector of Ral has homology to Rho/Rac GTPase activating proteins. Oncogene. 1995;11:2349–55. [PubMed] [Google Scholar]
- 14.Frankel P, Aronheim A, Kavanagh E, et al. RalA interacts with ZONAB in a cell density-dependent manner and regulates its transcriptional activity. Embo J. 2005;24:54–62. doi: 10.1038/sj.emboj.7600497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shipitsin M, Feig LA. RalA but Not RalB Enhances Polarized Delivery of Membrane Proteins to the Basolateral Surface of Epithelial Cells. Mol Cell Biol. 2004;24:5746–56. doi: 10.1128/MCB.24.13.5746-5756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim KH, Baines AT, Fiordalisi JJ, et al. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell. 2005;7:533–45. doi: 10.1016/j.ccr.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Cascone I, Selimoglu R, Ozdemir C, et al. Distinct roles of RalA and RalB in the progression of cytokinesis are supported by distinct RalGEFs. Embo J. 2008;27:2375–87. doi: 10.1038/emboj.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalli G, Hall A. Ral GTPases regulate neurite branching through GAP-43 and the exocyst complex. J Cell Biol. 2005;171:857–69. doi: 10.1083/jcb.200507061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han K, Kim MH, Seeburg D, et al. Regulated RalBP1 binding to RalA and PSD-95 controls AMPA receptor endocytosis and LTD. PLoS Biol. 2009;7:e1000187. doi: 10.1371/journal.pbio.1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez JA, Kwan EP, Xie L, He Y, James DE, Gaisano HY. The RalA GTPase is a central regulator of insulin exocytosis from pancreatic islet beta cells. J Biol Chem. 2008;283:17939–45. doi: 10.1074/jbc.M800321200. [DOI] [PubMed] [Google Scholar]
- 21.Chien Y, Kim S, Bumeister R, et al. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127:157–70. doi: 10.1016/j.cell.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 22.Chien Y, White MA. RAL GTPases are linchpin modulators of human tumour-cell proliferation and survival. EMBO Rep. 2003;4:800–6. doi: 10.1038/sj.embor.embor899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balasubramanian N, Meier JA, Scott DW, Norambuena A, White MA, Schwartz MA. RalA-exocyst complex regulates integrin-dependent membrane raft exocytosis and growth signaling. Curr Biol. 2010;20:75–9. doi: 10.1016/j.cub.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim KH, O'Hayer K, Adam SJ, et al. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr Biol. 2006;16:2385–94. doi: 10.1016/j.cub.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Sablina AA, Chen W, Arroyo JD, et al. The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell. 2007;129:969–82. doi: 10.1016/j.cell.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panner A, Nakamura JL, Parsa AT, et al. mTOR-independent translational control of the extrinsic cell death pathway by RalA. Mol Cell Biol. 2006;26:7345–57. doi: 10.1128/MCB.00126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowalsky AG, Alt-Holland A, Shamis Y, Garlick JA, Feig LA. RalA suppresses early stages of Ras-induced squamous cell carcinoma progression. Oncogene. 2010;29:45–55. doi: 10.1038/onc.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson MW, Alt-Holland A, Egles C, Garlick JA. Three-dimensional tissue models of normal and diseased skin. Curr Protoc Cell Biol. 2008 doi: 10.1002/0471143030.cb1909s41. Chapter 19: Unit 19 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuta K, Adachi E, Matsumoto K, Nakamura T. Different reactivities of enzyme-linked immunosorbent assays for hepatocyte growth factor. Clin Chim Acta. 2009;402:42–6. doi: 10.1016/j.cca.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Alt-Holland A, Shamis Y, Riley KN, et al. E-cadherin suppression directs cytoskeletal rearrangement and intraepithelial tumor cell migration in 3D human skin equivalents. J Invest Dermatol. 2008;128:2498–507. doi: 10.1038/jid.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailleul B, Surani MA, White S, et al. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell. 1990;62:697–708. doi: 10.1016/0092-8674(90)90115-u. [DOI] [PubMed] [Google Scholar]
- 32.Margulis A, Zhang W, Alt-Holland A, Crawford HC, Fusenig NE, Garlick JA. E-cadherin suppression accelerates squamous cell carcinoma progression in three-dimensional, human tissue constructs. Cancer Res. 2005;65:1783–91. doi: 10.1158/0008-5472.CAN-04-3399. [DOI] [PubMed] [Google Scholar]
- 33.Zhu AJ, Watt FM. Expression of a dominant negative cadherin mutant inhibits proliferation and stimulates terminal differentiation of human epidermal keratinocytes. J Cell Sci. 1996;109(Pt 13):3013–23. doi: 10.1242/jcs.109.13.3013. [DOI] [PubMed] [Google Scholar]
- 34.Margulis A, Zhang W, Garlick JA. In vitro fabrication of engineered human skin. Methods Mol Biol. 2005;289:61–70. doi: 10.1385/1-59259-830-7:061. [DOI] [PubMed] [Google Scholar]
- 35.Kudo Y, Kitajima S, Ogawa I, et al. Invasion and metastasis of oral cancer cells require methylation of E-cadherin and/or degradation of membranous beta-catenin. Clin Cancer Res. 2004;10:5455–63. doi: 10.1158/1078-0432.CCR-04-0372. [DOI] [PubMed] [Google Scholar]
- 36.Sugita S. Mechanisms of exocytosis. Acta Physiol (Oxf) 2008;192:185–93. doi: 10.1111/j.1748-1716.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tingstrom A, Heldin CH, Rubin K. Regulation of fibroblast-mediated collagen gel contraction by platelet-derived growth factor, interleukin-1 alpha and transforming growth factor-beta 1. J Cell Sci. 1992;102(Pt 2):315–22. doi: 10.1242/jcs.102.2.315. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto K, Nakamura T. Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Crit Rev Oncog. 1992;3:27–54. [PubMed] [Google Scholar]
- 40.Conway K, Price P, Harding KG, Jiang WG. The molecular and clinical impact of hepatocyte growth factor, its receptor, activators, and inhibitors in wound healing. Wound Repair Regen. 2006;14:2–10. doi: 10.1111/j.1743-6109.2005.00081.x. [DOI] [PubMed] [Google Scholar]
- 41.Kammula US, Kuntz EJ, Francone TD, et al. Molecular co-expression of the c-Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett. 2007;248:219–28. doi: 10.1016/j.canlet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207–14. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Garcia A, Pritchard CA, Paterson HF, Mavria G, Stamp G, Marshall CJ. RalGDS is required for tumor formation in a model of skin carcinogenesis. Cancer Cell. 2005;7:219–26. doi: 10.1016/j.ccr.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 44.Bodemann BO, White MA. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev Cancer. 2008;8:133–40. doi: 10.1038/nrc2296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.