Abstract
Cellular Src (c-Src) integrates a large number of signal transduction pathways regulating cell division, migration, and other aspects of cell physiology. Mutations of Src kinase have not been described in human prostate cancer, but evidence for increased levels of expression accompanying cancer progression has been reported. We analyzed over-expression of c-Src in naïve mouse prostate epithelium and observed no change in tubule formation frequency or histological structure. However, when enhanced c-Src expression is coupled with enhanced expression of androgen receptor (AR), it results in a strong activation of Src kinase activity accompanied by activation of the MAPK pathway, and enhanced AR activity. Similar to the pathology induced by constitutively active c-Src(Y529F), the tubules progress to frank carcinoma with invasion and display markers of epithelial to mesenchymal transition. These combined results suggest that non-mutated Src kinase may play a more important role in the genesis and progression of prostate cancer than previously appreciated and that epigenetic changes that enhance the level of AR may select for enhanced expression of c-Src with accompanying activation and a strong drive to malignant progression.
Keywords: Src kinase, Androgen receptor, Invasive prostate adenocarcinoma, Prostate carcinoma
Introduction
Prostate cancer is the second leading cause of cancer-related deaths among males in the United States. The pathological stages of prostate cancer begin with abnormal epithelial proliferation and prostatic intraepithelial neoplasia (PIN) with progression to invasive carcinoma and eventually metastatic disease (1). While early localized disease is usually curable, the survival rate drops to only 34% with progression to invasive and metastatic disease. In advanced patients, a therapeutically intractable castration resistant prostate cancer (CRPC) often emerges (2).
Initiation of prostate cancer has been linked to activation of AKT signaling due to loss or mutation of PTEN, as well as aberrant expression of erythroblast transformation specific (ETS) family transcription factors following chromosomal translocation (3–5). Additionally, over-expression of androgen receptor (AR), c-MYC, polycomb group protein EZH2, and anti-apoptotic protein Bcl-2 also appear to be important to this process (6, 7). In contrast, the genetic and epigenetic alterations that drive progression to invasive and metastatic disease are poorly understood. Alterations in the expression and activation state of AR have been identified in CRPC. Over 80% of CRPC cases present with high levels of AR expression. This is thought to increase the sensitivity of AR to low levels of androgen following castration or by other endogenous steroids (8).
Various experimental and human cancers have shown that Src can be activated by mutation or DNA amplification mechanisms (9, 10). Src kinase is over-expressed and activated in a wide variety of human cancers, including metastatic or hormone-refractory prostate cancer (10, 11). However, since activating mutations of Src kinase are rare in human cancer (12, 13), it is not well-appreciated how the activation of Src kinase contributes to the initiation of invasive carcinoma in prostate cancer.
Src kinase regulates several upstream molecular signaling components including numerous G-protein coupled receptors, integrins, and receptor tyrosine kinases. The activation of Src kinase contributes to prostate tumorigenesis through activation of downstream signaling pathways including PI3K-Akt, RAS, integrin-FAK, MAPK, and STAT3 signaling (14, 15). Therefore, as a pleiotropic activator, Src kinase regulates numerous cell signaling pathways important for survival, proliferation, invasion, migration, and angiogenesis of cancer cells (15, 16).
In addition to the clinical observation that expression of AR and Src are often elevated in CRPC (10), several studies provide further evidence that Src kinase can interact with AR signaling pathways. Guo et al. (2006) reported tyrosine phosphorylation and activation of AR by Src kinase. Cell culture studies using the LNCaP cell line indicate that the association between Src kinase and AR is mediated through the receptor for activated C kinase 1 (RACK1) scaffold protein (17). This Src kinase-AR interaction is modulated by the AR antagonist DOC-2/DAB2 protein (18).
We have previously established a prostate regeneration system in which prostate tissue can be regenerated in vivo by combining dissociated postnatal prostate epithelia with dissociated embryonic urogenital sinus mesenchyme, and engrafting these cells under the kidney capsule of immunodeficient mice (19). The expression of specific oncogenes can be manipulated in this system to monitor both the influence of cell autonomous and extrinsic signals on the initiation and progression of prostate cancer (19, 20). This system allows for chronologic investigation of prostate cancer development, in which prostate tissue can be regenerated from defined cellular populations based on the expression of defined surface markers (4, 21).
We show that over-expression of wild type Src kinase alone does not change prostate tubule structure, while enhanced co-expression of c-Src and AR results in invasive prostate carcinoma with associated epithelial-mesenchymal transition (EMT). This combination leads to strong activation of Src tyrosine kinase activity accompanied by activation of the MAPK pathway, and enhanced AR activity. The in vivo progression to undifferentiated adenocarcinoma and EMT by constitutively active Src(Y529F) kinase closely resembles the observed phenotype from co-expression of wild-type Src kinase and AR. To evaluate the dynamic process of invasive carcinoma, we used a tetracycline inducible system in concert with the prostate regeneration system, and found that the initiation of invasive carcinoma was coupled with dynamic alterations in prostate tubule structure associated with changes of epithelial and EMT markers.
Material and Methods
Plasmids
The open reading frame of murine Src kinase, its constitutively active mutant Src(Y529F), and kinase dead mutant Src(Y529F/K298M) was cloned into lentivector FUCRW (22), in which RFP expression is constitutively active and regulated by the CMV promoter. Src(Y529F) was also cloned into pTK380 (23) lentivector, designated as TRE-Src(Y529F), which the expression of Src(Y529F) was regulated by the addition of Dox.
Prostate regeneration and prostate epithelial viral infections
The regeneration process, lentivirus preparation, titering, and infection of dissociated prostate cells were performed as described previously under University of California, Los Angeles, safety regulations for lentivirus usage (19). Housing, maintenance, and all surgical and experimental procedures were undertaken in compliance with the regulations of the division of Laboratory Animal Medicine of the University of California, Los Angeles. Prostate regeneration in Dox inducible system was adapted from previous reports (19). Dissociated prostate cell suspension was prepared from 6- to 10-week-old R26-M2rtTA mice, which express an optimized form of reverse tetracycline-controlled transactivator (rtTA-M2) protein (24). Dissociated cells were infected with lentivirus TRE-Src(Y529F). Infected cells (1–2 × 105) were mixed with urogenital sinus mesenchymal cells (1–2 × 105). Grafts were implanted under the kidney capsule in SCID mice and allowed to regenerate for 4 weeks. The drinking water contained 0.2% Dox and 1% sucrose and was changed every week until termination of the experiment.
Cellular analysis of co-expression of Src(WT) and AR
To characterize activation of FAK/ERK pathways following expression of constitutively active Src and Src(WT)+AR in vitro, 293T cells (purchased from ATCC) were infected with lentivirus carrying over-expression of AR, Src(WT), Src(Y529F), or co-infected with lentivirus carrying over-expression of AR and Src(WT) together. Two days after infection, 293T cells were harvested and protein lysates were prepared using RIPA buffer supplemented with phosphatase inhibitor cocktail 1 and 2 (P5726 and P2850, SIGMA). The expression of AR, Src, pSrc, pFAK, pERK, and ERK was analyzed by Western blot (Antibodies are listed in Supplemental table 1).
Immunohistochemistry
Transillumination or fluorescent images were taken with a dissecting microscope. Immunohistochemistry (IHC) was as described previously (19, 21). The primary antibodies and dilution are listed in Supplemental table 1.
Dasatinib inhibits active Src kinase induced invasive carcinoma and EMT
The prostate cells infected with vector control or active Src(Y529F) lentivirus were mixed with UGSM and implanted under the kidney capsule of CB.17SCID/SCID mice for prostate regeneration. Three days after implantation, SCID mice were randomized to receive dasatinib or control vehicle. For p.o. administration, dasatinib was dissolved in 80mmol/L citrate buffer (pH3.0) with 5%DMSO. A concentration of 15mg/kg body weight/day was given p.o. at 24 hours intervals, using 20-gauge gavage needle. The control group of mice was given an equal volume of diluent buffer by gavage. Mice were treated for 4 weeks.
Results
Over-expression of wild type Src kinase synergizes with AR in prostate tumorigenesis
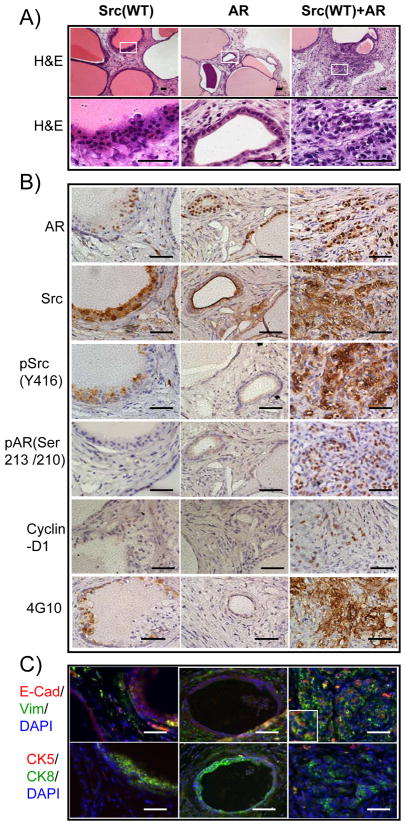
Src kinase and AR are two commonly up-regulated genes in metastatic or castration resistant prostate cancer (8, 10, 11). We asked if forced expression of wild type Src kinase and AR together in naive prostate epithelial cells could promote invasive prostate tumorigenesis in vivo. Cell autonomous over-expression of AR resulted in the formation of histologically normal prostate tubules (Figure 1A), though, as reported previously, regenerated tissues contained fewer tubules than vector controls (data not shown). Regenerated tissues derived from over-expression of Src(WT) resulted in the formation of normal appearing or slightly hyperplastic tubules (Figure 1A). In contrast, regenerated tissues infected with Src(WT)+AR exhibited sheets of undifferentiated cells showing no glandular structure. Compared with the neighboring well-differentiated tubules, which were not infected by Src(WT)+AR genes, these results clearly showed that Src(WT)+AR led to the transformation (Figure 1A). Tumor cells showed hyperchromatic nuclei, high nuclear/cytoplasmic ratio and grew as solid sheets, cords, small nest and single cells, characteristic features of a poorly differentiated or undifferentiated high grade carcinoma (Figure 1A). The expression of Src(WT)+AR correlates with the histological differentiation status of transformed regions. Lower ectopic expression of Src(WT)+AR corresponds to more differentiated lesions while higher levels of Src(WT)+AR were associated with poorly differentiated lesions that exhibited higher levels of pSrcY416 and pAR(Ser210/213) (Figure S1). Additionally, tumorigenic cells expressed high levels of Src(WT)+AR showed extensive invasion into the adjacent stroma (Figure 1A).
Figure 1. Over-expression of wild type Src kinase synergized with AR in prostate cancer.
Dissociated prostate cells from C57BL/6 mice were infected with lentivirus carrying expression of Src kinase or AR alone or in combination.
A) H&E staining of regenerated prostate tissue (top panel, white square indicates the zoomed region in the lower panel) and cellular and nuclear structure of transformed cells (bottom panel) derived from primary prostate cells infected with lentivirus to over-express the wild type Src kinase, AR, or both Src(WT) and AR. Scale bar, 100μm.
B–C) IHC staining of AR, Src kinase, phospho-Src(Y416), phospho-AR(Ser213/210), cyclin-D1, phosphotyrosine (4G10), epithelial/mesenchymal markers E-cadherin (red)/vimentin (green), and basal/luminal cell markers CK5 (red)/CK8 (green) in regenerated prostate tissues. The inserts show the expression of E-cadherin and vimentin in transformed cells. A subset of cells expressed both E-cadherin and vimentin. Scale bar, 100μm.
Enhanced expression of wild type Src kinase and AR elevates the activity of AR and Src kinase
One possible mechanism for the observed synergy of Src(WT) and AR could be through cross activation of Src kinase and AR (17). Immunohistochemistry confirmed that tumorigenic areas of the Src(WT)+AR regenerated tissue expressing elevated Src kinase and AR (Figure 1B; Figure S2). While the expression level of Src kinase in the Src(WT)+AR tumor was similar to that in Src(WT) group (Figure 1B; Figure S2), tumor cells over-expressing Src(WT)+AR exhibited elevated levels of activated Src kinase [detected by antibody reactive with pSrc(Y416)], activated AR [detected by antibody reactive with pAR(Ser213/210)], cyclin D1, and phosphotyrosine (detected by 4G10) (Figure 1B; Figure S2), suggesting that wild type Src kinase and AR mutually co-activate.
Synergy of wild type Src kinase with AR displays an expansion of luminal like tumor cells with associated EMT
To identify the epithelial subpopulations present in the neoplastic lesions, the tissue was stained for epithelial markers, cytokeratin CK5 and CK8. Regenerated tubules from Src(WT) or AR groups presented a normal double-layer of CK8+ luminal cells and CK5+ basal cells while tumorigenic cells from Src(WT)+AR grafts displayed only CK8+ cells (Figure 1C). We further stained for E-cadherin and vimentin and observed that some CK8+ tumor cells also expressed both E-cadherin and vimentin. In contrast, E-cadherin, 1C). Collectively, these data indicate that over-expression of wild type Src kinase synergizes with AR, which leads to activation of Src kinase and results in invasive prostate carcinoma and possible EMT.
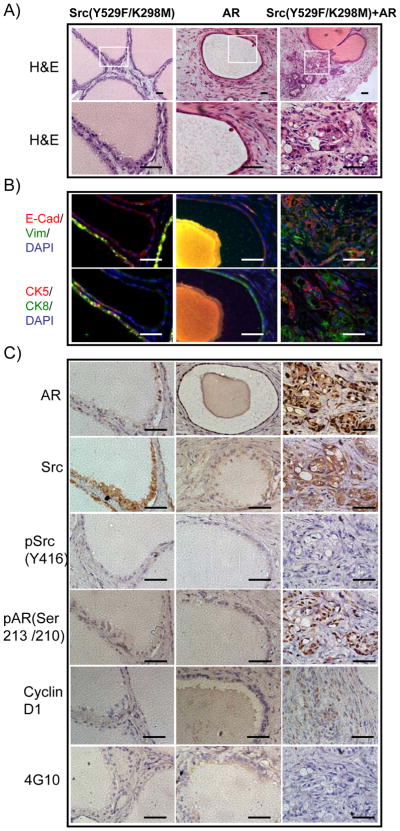
Co-expression of Src(Y529F/K298M) and AR can promote transformation
An open conformation of a Src kinase dead mutant [Src(Y529F/K298M)] was created and co-infected with AR in the prostate regeneration assay. Regenerated tissues derived from over-expression of Src(Y529F/K298M) resulted in the formation of normal appearing tubules. In contrast, regenerated tissues from the Src(Y529F/K298M)+AR group exhibited well differentiated carcinoma displaying expression of CK5/CK8 and E-cadherin (Figure 2A and B). Immunohistochemistry confirmed that expression of total Src kinase was increased in Src(Y529F/K298M) infected tubules and in tumorigenic areas of the Src(Y529F/K298M)+AR grafts (Figure 2C). In contrast to Src(WT)+AR, expression of phospho-Src kinase and phosphotyrosine was not detectable in tumorigenic areas of the Src(Y529F/K298M)+AR grafts (Figure 2B). Nevertheless, phosphorylated AR and cyclin D1, a downstream target gene of AR, were increased in Src(Y529F/K298M)+AR group (Figure 2C) in comparison with Src kinase dead mutant or AR alone, suggesting that when both are co-overexpressed, Src kinase scaffold activity can still promote AR activity and malignant progression, but to a lesser degree than wild type Src plus AR. Similar experiments were conducted with Src(K298M) expressed alone or in concert with AR. A modest degree of synergy was seen in converting normal epithelium towards PIN and well differentiated carcinoma but less dramatically than the Src(Y529F/K298M) plus AR combination (data not shown). These combined results suggest that some of the biological activity derives from the scaffold function of Src while more potent effects require an active kinase.
Figure 2. Over-expression of Src(Y529F/K298M) and AR can promote AR activity.
A) H&E staining (top panel, white square indicating zoomed regions) of regenerated prostate tissue with cellular and nuclear structure (bottom panel) of transformed cells derived from primary prostate cells infected with lentivirus to over-express Src kinase dead mutant Src(Y529F/K298M), AR, or both Src(Y529F/K298M) and AR. Scale bar, 100μm.
B–C) IHC staining of CK5 (red)/CK8 (green), E-cadherin (red)/vimentin (green) AR, Src kinase, phospho-AR(Ser213/210), phospho-Src(Y416), cyclin-D1, and phosphotyrosine in the regenerated prostate tissues. Scale bar, 100 μm.
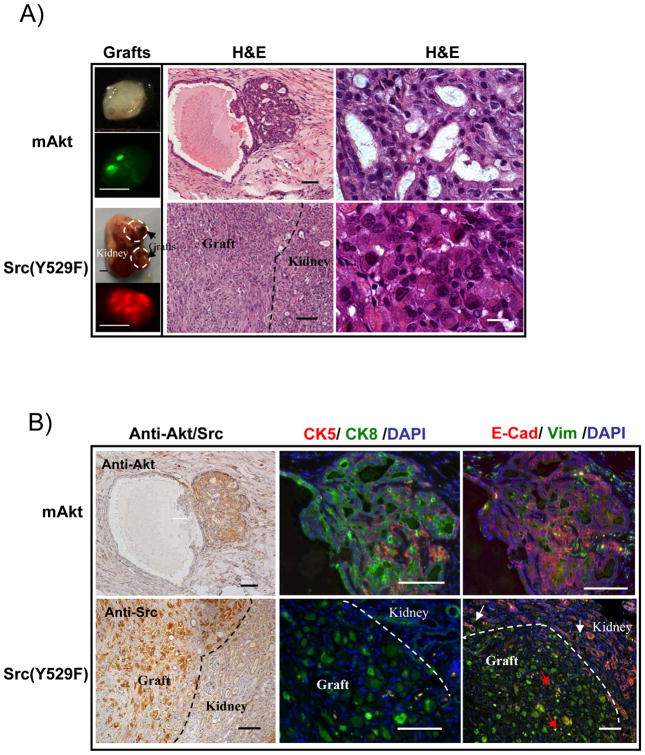
Expression of constitutively active Src kinase in prostate epithelial cells phenotypically resembles co-expression of wild type Src and AR
Since cross-talk between AR and c-Src could lead to the activation of Src kinase signaling, we asked whether constitutively active Src(Y529F) alone phenocopies the synergy of Src(WT)+AR in the initiation of invasive adenocarcinoma and EMT. Over-expression of active Src(Y529F) kinase is known to induce sarcomatous transformation of fibroblast cells (9). Therefore, we isolated prostate basal epithelial cells based on the antigenic profile Lin-CD49f+Sca-1+ (Figure S3), which were previously reported to enrich for prostate basal stem cells (21). Regenerated tissue derived from mAKT infected prostate epithelial cells showed PIN lesions displaying expression of CK8 in luminal cells and CK5 in basal cells, and these epithelial cells also expressed E-cadherin but not vimentin (Figure 3A and B). In contrast, similar to tumors derived from Src(WT)+AR, regenerated tissue derived from Src(Y529F) infected epithelial cells lacked glandular structures and were composed of sheets of poorly differentiated carcinoma cells with focal sarcomatoid areas (Figure 3A). The primary pattern of Src(Y529F)-induced tumors consisted of large tumor cells with abundant eosinophilic cytoplasm, and highly pleomorphic vesicular nuclei (Figure 3A). Cells derived from Src(Y529F) tumor displayed CK8 and vimentin but not CK5 and E-cadherin (Figure 3B), or CK18 but not CK14 (Data not shown). To examine the invasiveness of Src(Y529F)-induced tumors, we further analyzed the junction between the graft and host kidney tissue. CK8/CK18+ tumor cells expressing vimentin were present in the glandular tissue of the kidney at the invasive front, identified by high E-cadherin expression (Figure 3B). This suggests that transformed cells showed luminal epithelial features with mesenchymal differentiation. Collectively, these data indicate that similar to Src(WT)+AR, Src(Y529F) was capable of inducing invasive tumors from naïve murine prostate epithelial cells.
Figure 3. Ectopic expression of active Src(Y529F) in the prostate epithelial cells induces EMT and invasive adenocarcinoma.
A) Regenerated prostate grafts were derived from 5×104 of basal epithelial cells infected with mAKT (GFP marker) or Src (Y529F) (RFP marker) (Left panel, scale bar: 2mm). H&E staining of the regenerated tissue (Middle panel, scale bar: 100μm) and the cellular and nuclear structure of transformed cells (Right panel, scale bar: 15μm) derived from prostate epithelial cells infected with mAKT and active Src kinase.
B) IHC staining of AKT or Src, CK5/CK8, and E-cadherin/vimentin in the regenerated tissue derived from prostate epithelial cells infected with mAKT and active Src kinase. Dashed lines show the junction between kidney tissue and the regenerated tissue. White arrows identify vimentin expressing Src(Y529F) transformed cells at the invasion front that have invaded into the kidney. Red arrows identify E-cadherin expressing kidney cells surrounded by Src(Y529F) induced tumorigenic cells. Scale bar: 100μm
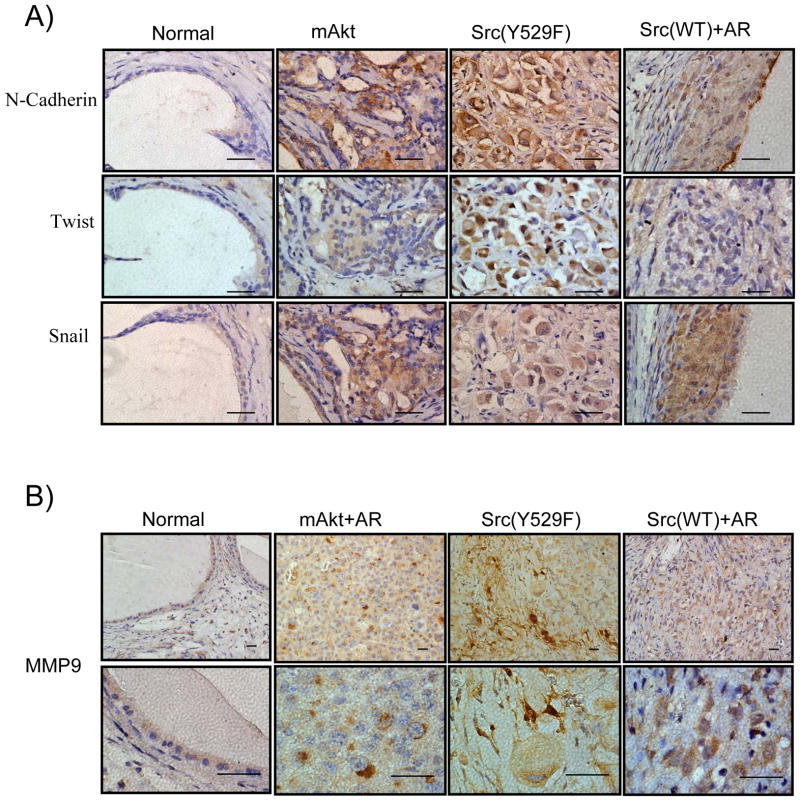
Synergy of Src(WT)+AR or constitutively active Src kinase alters the expression of well-characterized EMT markers and increases the expression of MMP9
EMT is an important step in the initiation of invasive tumorigenesis. During EMT, tumorigenic cells acquire the expression of transcription factors such as Twist, Snail, as well as cell-surface protein N-cadherin (25). Therefore, in addition to the altered expression of E-cadherin and vimentin (Figure 1C and Figure 3B), we further examined the expression of these well characterized markers of EMT in active Src(Y529F) and Src(WT)+AR induced carcinoma. Active Src(Y529F) induced tumors expressed increased level of Twist in the cell nucleus in comparison with the normal regenerated tubules and the mAKT transformed tumors (Figure 4A). In addition, both mAKT and active Src(Y529F) induced tumors expressed increased levels of Snail and N-cadherin (Figure 4A). Src(WT)+AR induced tumor expressed increased levels of N-cadherin, Snail, and to a lesser extent Twist (Figure 4A). These data provide further evidence that Src(WT)+AR or active Src(Y529F) induced invasive adenocarcinoma that can progress to EMT in vivo.
Figure 4. The expression of EMT markers and MMP9 in Src(WT)+AR or active Src(Y529F) induced carcinoma.
A) IHC staining of N-cadherin, Twist, and Snail in the regenerated tissue derived from control vector, mAKT, Src(Y529F), or Src(WT)+AR. Scale bar: 50μm
B) IHC staining of MMP9 in the regenerated tissue derived from normal regenerated tubules, mAKT+AR, Src(Y529F), or Src(WT)+AR. Scale bar: 20μm
A major characteristic of invasive cancer is the secretion of matrix metalloproteinases, which can degrade extracellular matrix and permit the migration of cancer cells. Therefore, we further examined the expression of matrix metalloproteinase 9 (MMP9) as an indication of invasive adenocarcinoma. MMP9 expression was polarized in the cytoplasm of mAKT+AR tumor cells from our previous study (control), likely localized to the endoplasmic reticulum and the Golgi complex (Figure 4B). Compared with the expression of MMP9 in the normal regenerated tubules, tumorigenic cells derived from Src(WT)+AR show increased levels of intra-cellular MMP9, while Src(Y529F) tumors exhibit both intra- and inter-cellular staining, suggesting the capacity to degrade the extra-cellular matrix (Figure 4B). Collectively, these data suggest that expression of Src(WT)+AR or active Src(Y529F) results in phenotypically similar invasive carcinoma and associated EMT characteristics.
Synergy of Src(WT)+AR or constitutively active Src(Y529F) leads to activation of MAPK signaling pathway
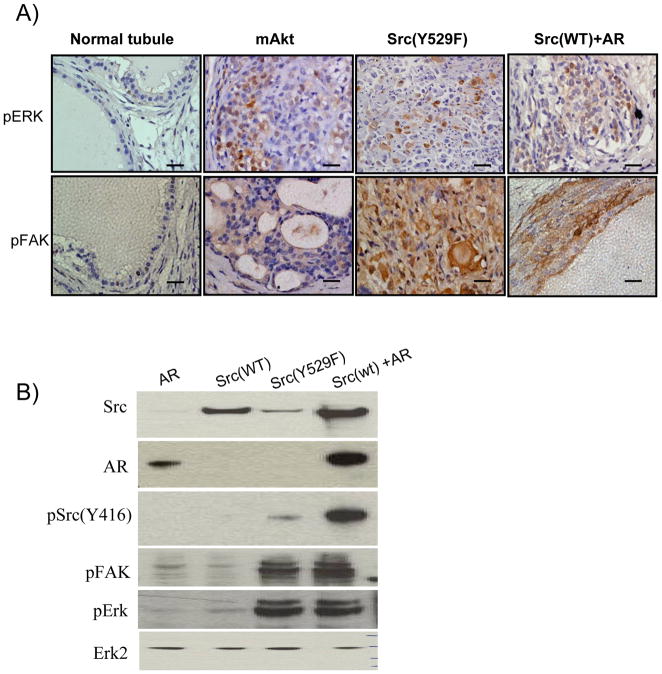
The Src/FAK signaling pathway has been shown to regulate EMT (26). We further investigated if the MAPK signaling pathway is activated in tissues derived from Src(WT)+AR or active Src(Y529F) by examining the expression and activation of proteins within this pathway. Tumors from Src(WT)+AR and active Src(Y529F) expressed increased levels of phospho-FAK as compared to normal regenerated tubules and mAKT transformed tumors (Figure 5A). Src(WT)+AR, active Src(Y529F), and mAKT transformed tumors also expressed elevated pErk in comparison with the regenerated normal prostate tubules (Figure 5A).
Figure 5. Src(WT)+AR or active Src(Y529F) induced invasive adenocarcinoma activates the Src/FAK/ERK pathway.
A) IHC staining of phospho-FAK and phospho-Erk in the regenerated tissue derived from normal tubules, and mAKT, Src(Y529F), and Src(WT)+AR tumor. Scale bar, 200 μm.
B) 293T cells were over-expressed with AR, Src(WT), Src(Y529F), or Src(WT)+AR. The expression of Src, AR, phospho-Src(Y416), phospho-FAK, phospho-Erk and Erk2 (loading control) was examined by Western analysis.
To further characterize functional interactions between Src kinase and AR signaling pathways, we co-overexpressed Src(WT) and AR in 293T cells and interrogated the activation of Src kinase and MAPK pathway (Figure 5B). Cells over-expressing the combination of Src (WT)+AR showed enhanced expression of pSrc416, pFAK, and pErk compared with cells expressing either Src(WT) or AR alone. However, Activation of these signaling cascades is lower in the context of Src(WT)+AR than constitutively active mutant Src(Y529F). This suggests that enhanced co-expression of Src(WT) and AR can activate the MAPK pathway, although to a lesser extent than constitutively active mutant Src(Y529F).
Inducible regulation of active Src kinase reveals progressive phases of invasive prostate carcinoma coupling with EMT
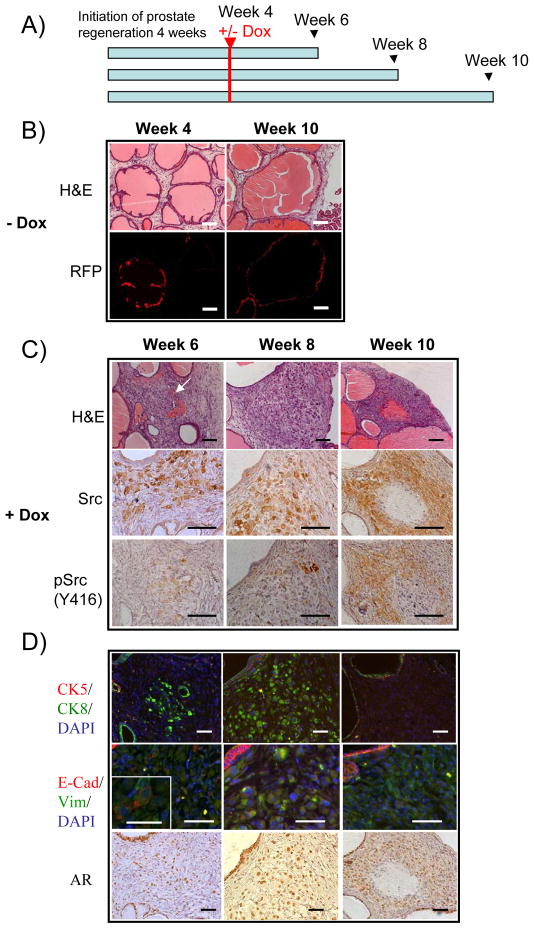
Progression to invasive carcinoma is associated with EMT characteristics in addition to alterations to prostatic glandular architecture (27, 28). To study the dynamic progression of invasive carcinoma in vivo, we used doxycycline (Dox)-regulated expression of active Src(Y529F) in the prostate regeneration model (Figure S4A). The Dox regulated phospho-Src expression was confirmed in cell culture (Figure S4B and C). We allowed normal prostate regeneration to proceed for 4 weeks before inducing the expression of Src(Y529F). Subsequently, the grafts were harvested at 6, 8, or 10-week after Dox induction or without Dox at 10-weeks (Figure 6A). As expected, the transformed cells expressed AR and elevated levels of Src or phospho-Src kinase in the grafts induced by Dox (Figure 6C and D).
Figure 6. Inducible expression of active Src(Y529F) in primary prostate cells reveals progressive phases of EMT.
A) Experimental time frame of prostate regeneration and doxycycline (Dox) induction. Primary prostate cells were infected with TRE-Src (Y529F) and incubated under the kidney capsule of SCID mice for 4 weeks before induction with Dox (0.2%). At week 6, 8, or 10, regenerated grafts were harvested from mice treated with or without Dox. MOI = 10.
B) H&E staining of regenerated tissue derived from primary prostate cells infected with TRE-Src (Y529F) without Dox treatment at week 4 and 10. Without Dox induction, TRE-Src(Y529F) infected tissue contained normal tubules. Scale bar, 100 μm.
C–D) H&E and IHC staining of Src kinase and pSrc (Y416), CK5/CK8, E-cadherin/vimentin, and AR in regenerated tissue derived from primary prostate cells infected with TRE-Src (Y529F) at week 6, 8, and 10 post-Dox treatment. The arrow identifies regenerated tissue that contains a poorly differentiated tumor with focal glandular differentiation (tubular formation) in the group treated with Dox at week 6. Scale bars in C) and D) are 100 μm and 50 μm, respectively. The inset shows the expression of E-cadherin on the cytoplasmic membrane. Scale bar: 25μm.
In the control, TRE-Src(Y529F) infected tubules (RFP+) showed normal histology with dilated tubular structures composed of two cell layers (Figure 6B). In contrast, regenerated tissues with temporal induction of Src developed from poorly differentiated carcinoma with focal glandular differentiation (tubular formation), to no obvious glandular differentiation, and to sarcomatoid transformation with focal areas of spindle cell morphology (Figure 6C). The invasive prostate carcinoma regressed when the expression of active Src(Y529F) was turned off by Dox withdrawal (Figure S5).
Interestingly, the tumor cells of regenerated tissues from the +Dox treated group did not express the basal marker CK5 at any time point analyzed (Figure 6D). In contrast, luminal marker CK8 was expressed in tumor cells of regenerated tissues from 6-week and 8-week groups, but not from the 10-week group (Figure 6D). The induced expression of active Src kinase triggers a progression of EMT as demonstrated by loss of E-cadherin expression on the cellular membrane and increase in vimentin expression in the cytoplasm (Figure 6D). Collectively, these data illustrate that over-expression and activation of Src kinase causes normal tubules to progress to prostate intraepithelial neoplasia, then to frank carcinoma with invasion with markers of epithelial to mesenchymal transition in vivo.
Dasatinib inhibits active Src kinase induced invasive carcinoma and EMT
Dasatinib, a small-molecule tyrosine kinase inhibitor targeting Src family kinases and several receptor kinases showed a favorable inhibitory effect on bone metastasis in advanced castration-resistant prostate cancer patients (29, 30). We examined if dasatinib could inhibit invasive carcinoma and EMT induced by the activation of Src kinase. Mock treatment of grafts from the Src(Y529F) group [Src(Y529F)+mock] generated solid, bulky RFP+ tissue, while dasatinib treated Src(Y529F) grafts [Src(Y529F)+dasatinib] had less weight and rare RFP signal (Figure 7). Our data indicate that dasatinib effectively inhibits active Src kinase induced invasive carcinoma and EMT in vivo (Figure S6 and S7).
Figure 7. Dasatinib inhibits active Src kinase induced invasive carcinoma.
Regenerated prostate grafts (A) and the weight of tissue (B) derived from primary prostate cells infected with lentivirus carrying control vector or Src(Y529F) with mock or dasatinib treatment.
Discussion
The sequence of genetic alterations and molecular signal transduction pathways required to drive progression to invasive adenocarcinoma in vivo are largely unknown (1). Studies demonstrate that at least two genetic events are required for efficient progression from high-grade PIN towards locally invasive adenocarcinoma (31, 32). We have previously shown that over-expression of AR in combination with either high levels of ERG, an ETS family transcription factor, or elevated mAKT promotes the development of a poorly differentiated adenocarcinoma (4, 22). Our study reveals that activation of Src kinase pathways by increased co-expression of AR and Src kinase can induce undifferentiated invasive adenocarcinoma in vivo in the absence of activating mutations. We demonstrate that enhanced co-expression of c-Src/AR in vivo phenotypically resembles over-expression of constitutively active Src (Y529F) kinase. Our data indicate that clinical observations of increased AR expression and activation of Src family kinase maybe functionally relevant to the progression of invasive carcinoma of the prostate (10, 15, 33).
Activation of AR responsive pathways likely contribute to the observed synergistic effect of Src/AR co-expression. Our Src/AR co-expression experiments demonstrate elevated levels of serine phosphorylation on AR, which is associated with nuclear translocation and activation of AR responsive transcription (34). While not directly assessed in our model, Guo et al. (2006) reported that Src kinase can mediate tyrosine phosphorylation of AR. This indicates that Src kinase can modulate AR activity directly through tyrosine phosphorylation and indirectly through activation of serine/threonine kinase pathways (35). Additionally, our results suggest that Src kinase may in part promote AR activity input through its function as a scaffold protein. High levels of Src kinase dead mutant can enhance FAK catalytic activity (36) and decrease osteopetrosis in the src−/− animal model (37). Further, AR contains a proline-rich domain that could be a potential binding site for the SH3 domain of Src kinase.
We demonstrate that both constitutively active Src(Y529F) and the synergistic co-expression of Src/AR present features of EMT and strong activation of MAPK pathways. These observations indicate that progression of EMT is associated with alterations in prostatic glandular structure, increased invasion and higher cancer grade (27). Activation of MAPK signaling pathways provide a potential mechanism for EMT driven by Src activity. Induction of the Src/FAK/ERK pathway promotes endocytosis of E-cadherin, which could modulate cellular adhesion ability (38). Activation Ras/MAPK pathway has also been shown to regulate the expression of Snail and Slug transcription factors, which in turn regulate E-cadherin transcription (39).
Progression to CRPC is largely fatal due to strong resistance to currently available therapies (2). Increased levels of activated Src kinase present in a subset CRPC cases (10), in combination with our evidence supporting functional relevance, indicates that inhibition of Src kinase could provide useful therapeutic approach in CRPC treatment. Dasatinib, a small-molecule tyrosine kinase inhibitor was recently shown to efficiently target Src family kinases and several other receptor tyrosine kinases (29, 40). In a clinical study of patients with advanced solid tumors, dasatinib therapy alone was reported to be acceptable and well tolerated with some preliminary evidence of efficacy (41). Additionally, Phase I and II studies of CRPC patients treated with a combination of dasatinib and docetaxel show a reduction in the size and/or number of existing bone lesions (42, 43). Our study shows that dasatinib inhibits invasive carcinoma and EMT induced by expression of constitutively active Src kinase. However, co-overexpression of Src kinase dead mutant and AR could still promote AR activation and lead to well differentiated carcinoma. These data suggest that an increase of AR activity can be partly independent of Src kinase activity.
Various groups are investigating AR antagonists as a means of targeting CRPC. Screening for transcriptional signatures of high AR activity using genomic expression analysis is currently being evaluated in patients with metastatic CRPC. This could be used to identify candidate CRPC patients for AR-targeted therapy using nilutamide, a nonsteroidal antiandrogen (44). The in vivo synergy of c-Src and AR and activation of AR independent of Src kinase activity presented in this study, along with the therapeutic value of independently targeting Src kinase and AR pathways seen in clinical trials and in common practice, suggests that simultaneous targeting of both pathways represents a potentially effective therapy for prostate cancer.
Finally, Mendiratta et al. (2009) reported heterogeneous AR and Src activity in localized prostate cancer and metastatic CRPC samples. The study identifies a subset of patients exhibiting both high Src and AR activity even in localized prostate cancer (45). Characterization of these patient subsets could provide a means to select patients to treat in earlier phases of prostate cancer.
Supplementary Material
Acknowledgments
We thank Li Xin, Devon Lawson, Daniel Smith, Donghui Cheng, Yang Zong, Andrew Goldstein, Justin Drake, and Rita U. Lukacs for technical help and scientific discussions. This work was supported by funds from the U.S. Army Medical Research and Material Command grants W81XWH-08-1-0329 to H.C. and the Prostate Cancer Foundation Challenge Award (O.N.W and J.H.). J.H is also supported by grant RSG-07-092-01-TBE from the American Cancer Society, and Grant PC061456 from the Department of Defense Prostate Cancer Research Program. O.N.W. is an Investigator of the Howard Hughes Medical Institute.
References
- 1.Tomlins SA, Mehra R, Rhodes DR, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39(1):41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441(7092):523–7. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zong Y, Xin L, Goldstein AS, Lawson DA, Teitell MA, Witte ON. ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells. Proc Natl Acad Sci U S A. 2009;106(30):12465–70. doi: 10.1073/pnas.0905931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Gao J, Lei Q, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4(3):209–21. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 6.Sellers WR, Loda M. The EZH2 polycomb transcriptional repressor--a marker or mover of metastatic prostate cancer? Cancer Cell. 2002;2(5):349–50. doi: 10.1016/s1535-6108(02)00187-3. [DOI] [PubMed] [Google Scholar]
- 7.Quinn DI, Henshall SM, Sutherland RL. Molecular markers of prostate cancer outcome. Eur J Cancer. 2005;41(6):858–87. doi: 10.1016/j.ejca.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8(4):440–8. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irby RB, Mao W, Coppola D, et al. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21(2):187–90. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 10.Tatarov O, Mitchell TJ, Seywright M, Leung HY, Brunton VG, Edwards J. SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin Cancer Res. 2009;15(10):3540–9. doi: 10.1158/1078-0432.CCR-08-1857. [DOI] [PubMed] [Google Scholar]
- 11.Guo Z, Dai B, Jiang T, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10(4):309–19. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Wang NM, Yeh KT, Tsai CH, Chen SJ, Chang JG. No evidence of correlation between mutation at codon 531 of src and the risk of colon cancer in Chinese. Cancer Lett. 2000;150(2):201–4. doi: 10.1016/s0304-3835(99)00398-5. [DOI] [PubMed] [Google Scholar]
- 13.Nilbert M, Fernebro E. Lack of activating c-SRC mutations at codon 531 in rectal cancer. Cancer Genet Cytogenet. 2000;121(1):94–5. doi: 10.1016/s0165-4608(00)00226-0. [DOI] [PubMed] [Google Scholar]
- 14.Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2(6):467–75. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 15.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4(6):470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 16.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23(48):7957–68. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 17.Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ. Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Res. 2006;66(22):11047–54. doi: 10.1158/0008-5472.CAN-06-0596. [DOI] [PubMed] [Google Scholar]
- 18.Zhoul J, Hernandez G, Tu SW, Huang CL, Tseng CP, Hsieh JT. The role of DOC-2/DAB2 in modulating androgen receptor-mediated cell growth via the nongenomic c-Src-mediated pathway in normal prostatic epithelium and cancer. Cancer Res. 2005;65(21):9906–13. doi: 10.1158/0008-5472.CAN-05-1481. [DOI] [PubMed] [Google Scholar]
- 19.Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci U S A. 2003;100 (Suppl 1):11896–903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Memarzadeh S, Xin L, Mulholland DJ, et al. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell. 2007;12(6):572–85. doi: 10.1016/j.ccr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A. 2007;104(1):181–6. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xin L, Teitell MA, Lawson DA, Kwon A, Mellinghoff IK, Witte ON. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc Natl Acad Sci U S A. 2006;103(20):7789–94. doi: 10.1073/pnas.0602567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haack K, Cockrell AS, Ma H, et al. Transactivator and structurally optimized inducible lentiviral vectors. Mol Ther. 2004;10(3):585–96. doi: 10.1016/j.ymthe.2004.06.109. [DOI] [PubMed] [Google Scholar]
- 24.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121(3):465–77. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunton VG, Frame MC. Src and focal adhesion kinase as therapeutic targets in cancer. Curr Opin Pharmacol. 2008;8(4):427–32. doi: 10.1016/j.coph.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 28.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66(17):8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 29.Park SI, Zhang J, Phillips KA, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68(9):3323–33. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 30.Saad F, Lipton A. SRC kinase inhibition: Targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev. 2009 doi: 10.1016/j.ctrv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Gao H, Ouyang X, Banach-Petrosky WA, Shen MM, Abate-Shen C. Emergence of androgen independence at early stages of prostate cancer progression in Nkx3.1; Pten mice. Cancer Res. 2006;66(16):7929–33. doi: 10.1158/0008-5472.CAN-06-1637. [DOI] [PubMed] [Google Scholar]
- 32.Lei Q, Jiao J, Xin L, et al. NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell. 2006;9(5):367–78. doi: 10.1016/j.ccr.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 33.Zhao XY, Malloy PJ, Krishnan AV, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6(6):703–6. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 34.Taneja SS, Ha S, Swenson NK, et al. Cell-specific regulation of androgen receptor phosphorylation in vivo. J Biol Chem. 2005;280(49):40916–24. doi: 10.1074/jbc.M508442200. [DOI] [PubMed] [Google Scholar]
- 35.Asim M, Siddiqui IA, Hafeez BB, Baniahmad A, Mukhtar H. Src kinase potentiates androgen receptor transactivation function and invasion of androgen-independent prostate cancer C4-2 cells. Oncogene. 2008;27(25):3596–604. doi: 10.1038/sj.onc.1211016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cary LA, Klinghoffer RA, Sachsenmaier C, Cooper JA. SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading. Mol Cell Biol. 2002;22(8):2427–40. doi: 10.1128/MCB.22.8.2427-2440.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartzberg PL, Xing L, Hoffmann O, et al. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev. 1997;11(21):2835–44. doi: 10.1101/gad.11.21.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17(5):542–7. doi: 10.1016/j.ceb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Saad F. Src as a therapeutic target in men with prostate cancer and bone metastases. BJU Int. 2009;103(4):434–40. doi: 10.1111/j.1464-410X.2008.08249.x. [DOI] [PubMed] [Google Scholar]
- 41.Demetri GD, Lo Russo P, MacPherson IR, et al. Phase I dose-escalation and pharmacokinetic study of dasatinib in patients with advanced solid tumors. Clin Cancer Res. 2009;15(19):6232–40. doi: 10.1158/1078-0432.CCR-09-0224. [DOI] [PubMed] [Google Scholar]
- 42.Edwards J. Src kinase inhibitors: an emerging therapeutic treatment option for prostate cancer. Expert Opin Investig Drugs. 2010 doi: 10.1517/13543781003789388. [DOI] [PubMed] [Google Scholar]
- 43.Galsky MD, Vogelzang NJ. Docetaxel-based combination therapy for castration-resistant prostate cancer. Ann Oncol. 2010 doi: 10.1093/annonc/mdq050. [DOI] [PubMed] [Google Scholar]
- 44.Febbo PG. Genomic approaches to outcome prediction in prostate cancer. Cancer. 2009;115(13 Suppl):3046–57. doi: 10.1002/cncr.24350. [DOI] [PubMed] [Google Scholar]
- 45.Mendiratta P, Mostaghel E, Guinney J, et al. Genomic strategy for targeting therapy in castration-resistant prostate cancer. J Clin Oncol. 2009;27(12):2022–9. doi: 10.1200/JCO.2008.17.2882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.