Abstract
Objective
Adiponectin, a protein secreted by adipose cells, is inversely associated with endometrial cancer (EC). Our objective was to assess pre-diagnostic adiponectin levels in relation to risk of EC.
Study Design
Prospective nested case-control study within the Nurses’ Health Study; cases 146, controls 377. Adiponectin was measured using enzyme-linked immunoabsorbant assay. Logistic regression analyses were performed adjusting for known EC risk factors.
Results
Mean age at diagnosis was 64.6 years. Mean interval between blood draw and diagnosis was 7.4 years (range 2–13). There was no difference in median adiponectin (cases 12.9 vs controls 12.9µg/mL; p=0.97). Adiponectin >15ul/mL was not associated with EC risk (RR=0.86 95% CI=0.53–1.39; p=0.48), even among postmenopausal women (OR 0.66, CI 0.29–1.5). Results did not vary by time from blood draw to diagnosis (p for heterogeneity = 0.18).
Conclusion
Pre-diagnostic adiponectin was not predictive of EC risk. Further study will better define the relationship between adiponectin and EC.
Keywords: Adiponectin, Endometrial Cancer, Insulin Resistance, Obesity, Risk Factors
Introduction
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among women in the United States. It is estimated that 43,470 new cases and 7,950 deaths from endometrial cancer will have occurred during 2010 1. Obesity is a well-known risk factor for endometrial cancer with the level of risk related to the degree of obesity. Women with a body mass index (BMI) ≥ 32 kg/m2 have a relative risk of 4.0 and women with a BMI ≥ 35 kg/m2 have a relative risk of 6.0 when compared to women with a BMI of less than 23 kg/m2 2. The relationship between obesity and endometrial cancer is complex and likely involves multiple pathways including the sex steroid, insulin and inflammation pathways3.
Adipose tissue secretes a number of metabolically active cytokines and hormones including adiponectin, leptin, resistin, and TNF-α4. Adiponectin, the most abundant adipokine, is secreted exclusively by adipocytes 5, 6. Low levels of adiponectin have been shown to have a high correlation with hyperinsulinemia and the degree of insulin resistance independent of adiposity, suggesting that adiponectin level may serve as surrogate marker for insulin resistance 7. Adiponectin levels have also been shown to be decreased in both obesity and type 2 diabetes, a disease generally considered an independent risk factor for endometrial cancer 7. In addition, adiponectin has a longer half-life than most polypeptide hormones 8, and circulating levels are not affected significantly by either fasting or oral intake 9.
In a previous retrospective case-control study performed at M. D. Anderson Cancer Center, serum adiponectin level was independently associated with endometrial cancer, even after adjustment for other known risk factors such as BMI, age, diabetes and hypertension 10. Several other authors have also evaluated the relationship between adiponectin levels and endometrial cancer and found a similar association 11, 12. More recently, Cust et al. published the first prospective assessment of pre-diagnostic adiponectin levels and risk of endometrial cancer 13. High circulating levels of adiponectin were associated with a significant decrease in risk of endometrial cancer independent of other obesity related risk factors.
We conducted a case-control study nested within the prospective Nurses’ Health Study (NHS) to assess if baseline circulating levels of adiponectin were associated with endometrial cancer risk, independent of other known risk factors including obesity, age, diabetes, and parity. We hypothesized that higher circulating adiponectin levels in healthy women would be independently and inversely associated with risk of endometrial cancer.
Material and Methods
Nurses’ Health Study Population
The Nurses’ Health Study (NHS) began in 1976 when 121,700 female United States registered nurses between the ages of 30–55 completed a self-administered questionnaire on their medical histories and baseline health-related exposures 14. Information regarding endometrial cancer risk factors was obtained from biennial questionnaires as well as a questionnaire completed at the time of blood collection. These questionnaires include data on reproductive variables, oral contraceptive and postmenopausal hormone use, cigarette smoking and (since 1980) dietary intake. Between 1989 and 1990, blood samples were collected from 32,826 women; details regarding the blood collection methods have been published previously 15, 16. Briefly, each woman arranged to have her blood drawn and shipped, via overnight courier with an icepack, where it was processed; 97% of the samples were returned within 26 hours of blood draw. Upon receipt, the samples were centrifuged, aliquoted into plasma, red blood cells and buffy coat fractions, and stored in liquid nitrogen freezers. Subsequent compliance with follow-up has been greater than 98% for this sub-cohort of NHS participants who have given blood.
Case-control study
For this study, eligible endometrial cancer cases consisted of women with pathologically confirmed invasive endometrioid endometrial adenocarcinoma that had been diagnosed at any time point after blood collection (1989–1990) and up to June 1, 2004, with no history of a prior cancer except for non-melanoma skin cancer. Controls were randomly selected participants who had given a blood sample, had not had a hysterectomy and were free of diagnosed cancer (except non-melanoma skin cancer) up to and including the interval in which the case was diagnosed. Controls were matched to cases according to year of birth, menopausal status at blood draw and at the time of cancer diagnosis, and use of hormone replacement therapy at time of blood draw (current vs. not current users). Controls were also matched to cases by time of day of blood collection, month of blood return and fasting status at blood draw because of planned plasma analyses. A secondary analysis including cases diagnosed with any invasive epithelial endometrial cancer (not just endometrioid) during this time was also performed.. The study protocol was approved by the Committee on Use of Human Subjects of the Brigham and Women’s Hospital, Boston, MA. The Institutional Review Board at University of Texas, M.D. Anderson Cancer Center approved this study with an exemption for informed consent, as all of the received samples and clinical information were de-identified.
Measurement of Adiponectin
Both case and control samples were run on the same microplates and the investigators were blinded to study group. Serum adiponectin levels were measured using a commercially available quantitative sandwich enzyme-linked immunoassay (ELISA) with a sensitivity of 0.246 ng/mL (R&D Systems, Minneapolis, MN). The coefficients of variation from masked quality control samples (embedded with the case-control samples) for intra-assay and inter-assay precision were 4.5–8.5%.
The ELISAs were performed at room temperature, according to the instructions from the manufacturer. Recombinant adiponectin was used as the standard. Study samples were diluted 100-fold with calibrator diluent and placed into 96-well plates which were pre-coated with mouse monoclonal antibody to adiponectin. All samples were run in triplicate. Following a 2-hour incubation period, the microplates were washed thoroughly with buffer. Adiponectin conjugate was then added to each well for an additional 2-hour incubation. Stabilized chromogen (tetramethylbenzidine) was used for color development. Absorbance of the solution in the wells was measured immediately by spectrometer at a wavelength of 450nm, with the correction wavelength set at 540nm. Serum adiponectin concentrations were then determined by comparison to the standard curve generated for each individual microplate. We previously reported a correlation of 0.97 between adiponectin levels from blood samples processed immediately versus after 24–48 hours suggesting our blood collection methods did not adversely influence adiponectin levels 17.
Statistical Analysis
Descriptive statistics were used to report demographic characteristics, clinical and pathologic variables, and serum adiponectin levels for all cases and controls. A Chi-square test was used to assess the significance of differences in categorical variables between the two groups. Continuous variables were compared using the Student’s t-test. In logistic regression models, plasma adiponectin levels were categorized into tertiles based on the distribution of values in the control group (lowest tertile < 10.0 mg/mL, intermediate tertile 10.00 – 14.99 mg/mL, highest tertile ≥ 15.00 mg/mL). In multivariable models, BMI was included as a continuous variable while diabetes, parity and age at last birth were included as categorical variables (see footnote to Table 2 for categories used). Using the lowest tertile of adiponectin level as the reference group, crude and adjusted odds ratios (OR) and 95% confidence intervals (CI) were calculated using conditional logistic regression for the intermediate and highest tertiles. A two-sided p-value of less than 0.05 was considered statistically significant. Tests for trend were assessed by modeling the median value of each category as a single ordinal variable. Tests for interaction were calculated using the product of the median of each category and a binary stratification factor.
Table 2.
Adiponectin level and endometrial cancer risk - Simple and adjusted logistic regression models
| Adiponectin (Tertiles, µg/ml) |
Cases /Controls | Univariate Model | BMI-adjusted model | Multivariate model* | |||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| <10.00 | 60/137 | 1.00 | 1.00 | 1.00 | |||
| 10.00–14.99 | 40/123 | 0.71 | 0.43–1.15 | 0.78 | 0.46–1.30 | 0.74 | 0.43–1.28 |
| ≥15.00 | 46/117 | 0.86 | 0.53–1.39 | 1.00 | 0.60–1.66 | 0.98 | 0.57–1.68 |
| p for trend | 0.48 | 0.94 | 0.89 | ||||
| Premenopausal | |||||||
| <10.00 | 24/66 | 1.00 | 1.00 | 1.00 | |||
| 10.00–14.99 | 16/51 | 0.82 | 0.35–1.19 | 0.88 | 0.37–2.07 | 0.80 | 0.30–2.10 |
| ≥15.00 | 22/52 | 1.12 | 0.51–2.49 | 1.29 | 0.56–2.91 | 1.14 | 0.47–2.78 |
| p for trend | 0.79 | 0.57 | 0.76 | ||||
| Postmenopausal | |||||||
| <10.00 | 36/70 | 1.00 | 1.00 | 1.00 | |||
| 10.00–14.99 | 24/72 | 0.51 | 0.25–1.03 | 0.64 | 0.31–1.32 | 0.58 | 0.26–1.39 |
| ≥15.00 | 22/63 | 0.53 | 0.25–1.12 | 0.68 | 0.31–1.46 | 0.66 | 0.29–1.50 |
| p for trend | 0.08 | 0.30 | 0.31 | ||||
Multivariable model with adjustment for BMI at blood draw(continuous), parity[nulliparous (reference), 1–2 & age at last birth<30, 1–2 & age at last birth>=30, 3–4 & age at last birth<30, 3–4 & age at last birth>=30, 5+], diabetes (yes, no)
P for interaction for menopausal status = 0.80
Analyses were also stratified by menopausal status. Women were defined as postmenopausal at the time of blood collection if they reported a natural menopause defined as no menstrual cycle within 12 months of the blood draw. Although many women were peri-menopausal, they were categorized as “pre-menopausal” if they had a period within the previous year. In multivariable models, menopausal status and postmenopausal hormone use were updated until the date of diagnosis for endometrial cancer cases and matched controls.
Results
A total of 146 cases and 337 controls were included in the study. The mean age at the time of blood draw was 57 years (10–90% range 47 – 67) for both cases and controls (this was a matching factor) (Table 1). The median age at cancer diagnosis was 64.6 years (10–90% range 55 – 76). The median interval of time between blood draw and cancer diagnosis was 7.4 years (10–90% range 2–13). The median body mass index (BMI) at the time of blood draw for cases was 27.2 kg/m2 and 25.5 kg/m2 for controls (p<0.01). At blood collection, 45% of cases were in the normal weight range, 30% were overweight and 25% were obese. Among controls, 56% were normal weight, 28% overweight, and 16% obese (p=0.006). There were no differences in race/ethnicity between the two groups.
Table 1.
Demographic Characteristics for Cases and Controls
| Cases (n=146) |
Controls (n=377) |
P-Value | |
|---|---|---|---|
| Mean | |||
| Age at blood draw, years(10–90% range) | 57 (47–67) | 57 (47–67) | * |
| Age at cancer diagnosis (mean, 10–90% range) | 65 (55–76) | ||
| Interval between age at blood draw and age at diagnosis(10–90% range) | 7.4 (2–13) | ||
| BMI at blood draw (kg/m2) | 27.2 | 25.5 | <0.01 |
| BMI at diagnosis (kg/m2) | 27.8 | ||
| Percentile | |||
| BMI Categories at blood draw (%) | 0.06 | ||
| Normal weight (BMI< 25) | 45 % | 56 % | |
| Overweight (BMI 25–30) | 30 % | 28 % | |
| Obese (BMI > 30) | 25 % | 16 % | |
| Ethnicity/Race | 0.99 | ||
| White | 99% | 98% | |
| Black | 0 | 0 | |
| Hispanic | 0 | 1% | |
| Other | 1% | 1% | |
| Nulliparity | 13.6% | 5.1% | <0.01 |
| Parity among parous women | 3.1 | 3.3 | <0.01 |
| Premenopausal at blood draw | 42 % | 45% | * |
| Diabetes | 8.2 % | 2.7 % | <0.01 |
| Hypertension | 28.1% | 27.7% | 0.94 |
matching factor
As expected, cases were more likely to be nulliparous and to have a history of diabetes (p<0.01), while hypertension did not vary by case status (Table 1). Only patients with endometrioid endometrial cancers were included in the primary analysis. 84% were FIGO stage I, 5% stage II, 9% stage III, and 2% were unknown. 52% were grade 1, 29% grade 2, and 14% grade 3. Information on the grade of the tumor was not available in 5%.
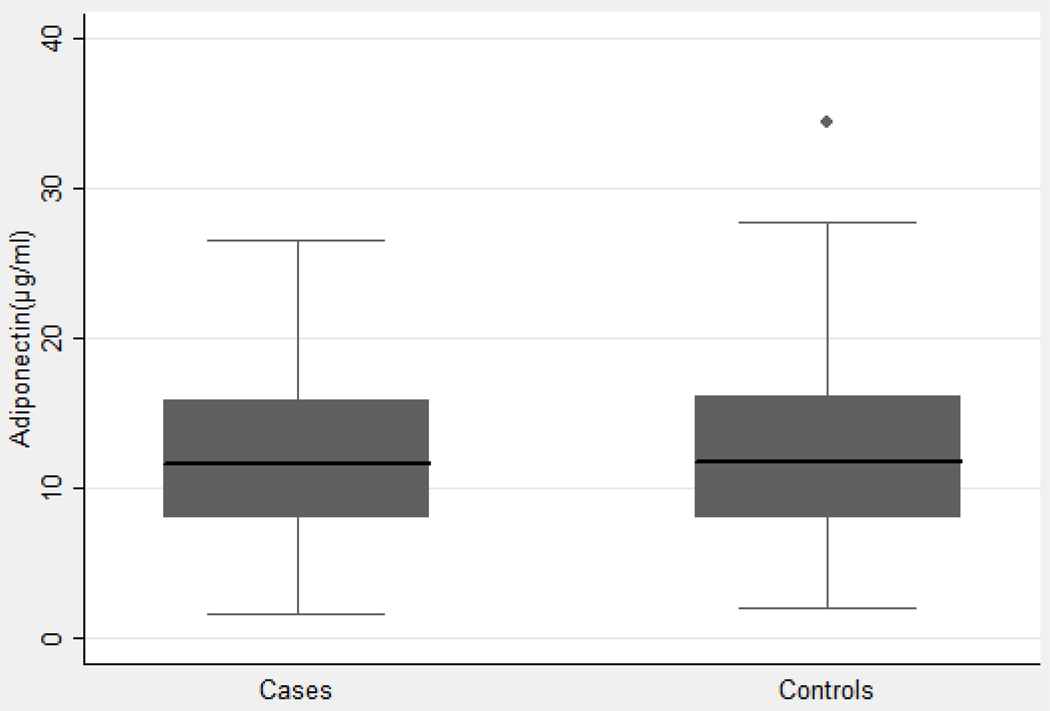
The mean adiponectin level for cases was 12.88 µg/mL and mean adiponectin level for controls was 12.85 µg/mL (Figure 1). There was no statistically significant difference (p=0.97).
Figure 1. Distribution of serum adiponectin levels among cases and controls.
The mean adiponectin level for cases was 12.88 µg/mL and mean adiponectin level for controls was 12.85 µg/mL. There was no statistically significant difference (p=0.97).
Simple and adjusted odds ratios for the relationship between adiponectin level and endometrial cancer are shown in Table 2. Among all cases and controls, no association was observed between adiponectin levels and endometrial cancer risk (top vs bottom tertile of levels RR=0.86 95% CI=0.53–1.39; p trend=0.48). Results were further attenuated after control for BMI, parity and diabetes (comparable RR=0.98 95% CI 0.57–1.68; p trend=0.89).
In a subset analysis based on menopausal status, there was no association between adiponectin level and endometrial cancer in premenopausal women in any of the models (Table 2). While an inverse relationship between adiponectin level and endometrial cancer was suggested in postmenopausal women, these findings were not significant (OR 0.66, 95% CI 0.29 – 1.5, p = 0.31).
To evaluate the relationship between time of blood draw and endometrial cancer risk, the cases were divided into two groups based on the median interval of time between blood draw and endometrial cancer diagnosis (Table 3). For cases with an interval of time greater than 7 years, there was no association between adiponectin level ≥ 15.00 µg/mL and cancer risk (OR 1.28, CI 0.63–2.62 p=0.16). For those less than 7 years, while the OR was 0.55 for adiponectin level ≥ 15.00 µg/mL, this was not statistically significant (p=0.21).
Table 3.
Adiponectin levels and endometrial cancer risk by time from blood draw to diagnosis.
| Adiponectin (Tertiles, µg/ml) | Cases /Controls | Multivariate model* | |
|---|---|---|---|
| OR | 95% CI | ||
| <7 years | |||
| <10.00 | 30/59 | 1.00 | |
| 10.00–14.99 | 16/64 | 0.53 | 0.21–1.30 |
| ≥15.00 | 14/49 | 0.55 | 0.18–1.69 |
| p for trend | 0.21 | ||
| ≥ 7 years | |||
| <10.00 | 27/67 | 1.00 | |
| 10.00–14.99 | 23/56 | 0.84 | 0.40–1.49 |
| ≥15.00 | 30/63 | 1.28 | 0.63–2.62 |
| p for trend | 0.16 | ||
Multivariable model with adjustment for BMI at blood draw(continuous), parity[nulliparous (reference), 1–2 & age at last birth<30, 1–2 & age at last birth>=30, 3–4 & age at last birth<30, 3–4 & age at last birth>=30, 5+], diabetes (yes, no)
P for interaction = 0.18
In the secondary analyses among all epithelial endometrial cancer cases, findings were similar (data not shown). For example, comparing the top to bottom quartile in adiponectin levels, the OR was 1.12 (0.72 – 5.68, p=0.29) for all women and 0.61 (0.27 – 1.37, p=0.20) for postmenopausal women.
Comment
In this prospective study with up to 13 years of follow-up, we observed no overall association between levels of adiponectin and risk of invasive endometrial adenocarcinoma. This is in contrast to the first prospective study of adiponectin and endometrial cancer published in the European EPIC cohort, with an average 5.1 years of follow-up. Women in the highest quartile of adiponectin level had a 50% reduced risk of endometrial cancer when compared to women in the lowest quartile13. The relative risk was attenuated but remained statistically significant after further control for BMI (0.56; 95% CI 0.37–0.86). These findings were more evident in postmenopausal women.
Several retrospective case-control studies have also shown an independent relationship between adiponectin and endometrial cancer. In a previous study, we found that cases were significantly more likely to have adiponectin levels in the lowest (OR 10.5, 95% CI 4.49 to 24.57, p<0.001) and intermediate tertiles (OR 2.5, 95% CI 1.01 to 6.21, p=0.05) versus the highest tertile when compared to controls 10. Petridou et al. found that an increase in one standard deviation of adiponectin was associated with more than a 50% reduction in the risk for endometrial cancer among women <65 years old, even after controlling for BMI 11. Dal Maso et al. found that BMI and adiponectin level were independently associated with endometrial cancer 12. These retrospective studies all found that low adiponectin levels, a marker for insulin resistance, were independently associated with endometrial cancer.
Although it is surprising that we did not observe a statistically significant association between pre-diagnostic adiponectin level and endometrial cancer risk given the proposed underlying biology and prior epidemiologic studies, there are several factors that may explain this finding. First, the length of time between the blood draw and the diagnosis of cancer was approximately seven years; median age at blood draw was 57.4 years compared to 64.6 years at diagnosis. It is not clear how long insulin resistance, measured as low adiponectin levels, would need to be present to result in an increase in risk of endometrial cancer. The shorter interval of time between blood draw and cancer diagnosis in the Cust study may provide a more accurate reflection of insulin resistance immediately preceding the diagnosis of endometrial cancer 13. In addition, we had a higher proportion of premenopausal women in our study where a weaker, if any, association may exist. Finally, while the NHS is a large prospective cohort study, only 146 endometrial cancer cases were available for analysis. This relatively small sample size may have limited our ability to detect a small to moderate difference in adiponectin levels between cases and controls.
Based on our current study as well as others in the literature, there is accruing evidence of a relationship between insulin resistance and endometrial cancer. While we did not find a significant association between pre-diagnostic adiponectin level and endometrial cancer risk, further studies are needed to determine the length of time insulin resistance must be present to increase the risk of endometrial cancer among women.
Acknowledgments
Funding – NIH Grants CA49449 and CA87969
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Brinton LA, Berman ML, Mortel R, et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol. 1992;167:1317–1325. doi: 10.1016/s0002-9378(11)91709-8. [DOI] [PubMed] [Google Scholar]
- 3.Gunter MJ, Hoover DR, Yu H, et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:921–929. doi: 10.1158/1055-9965.EPI-07-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 5.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 6.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 8.Hoffstedt J, Arvidsson E, Sjolin E, Wahlen K, Arner P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J Clin Endocrinol Metab. 2004;89:1391–1396. doi: 10.1210/jc.2003-031458. [DOI] [PubMed] [Google Scholar]
- 9.Gavrila A, Chan JL, Yiannakouris N, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003;88:4823–4831. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 10.Soliman PT, Wu D, Tortolero-Luna G, et al. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106:2376–2381. doi: 10.1002/cncr.21866. [DOI] [PubMed] [Google Scholar]
- 11.Petridou E, Mantzoros C, Dessypris N, et al. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab. 2003;88:993–997. doi: 10.1210/jc.2002-021209. [DOI] [PubMed] [Google Scholar]
- 12.Dal Maso L, Augustin LS, Karalis A, et al. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab. 2004;89:1160–1163. doi: 10.1210/jc.2003-031716. [DOI] [PubMed] [Google Scholar]
- 13.Cust AE, Kaaks R, Friedenreich C, et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab. 2007;92:255–263. doi: 10.1210/jc.2006-1371. [DOI] [PubMed] [Google Scholar]
- 14.Colditz GA, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 15.Hankinson SE, Willett WC, Manson JE, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson SE, Willett WC, Manson JE, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297–1302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 17.Tworoger SS, Eliassen AH, Kelesidis T, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]