Abstract
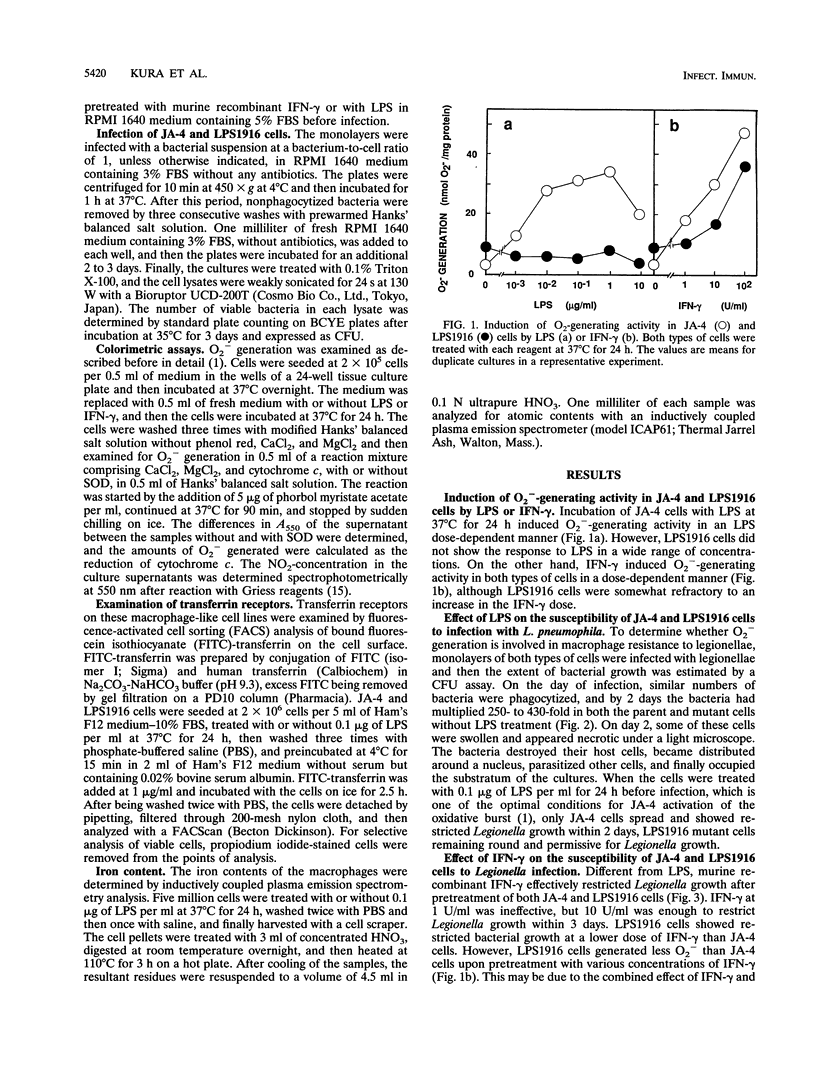
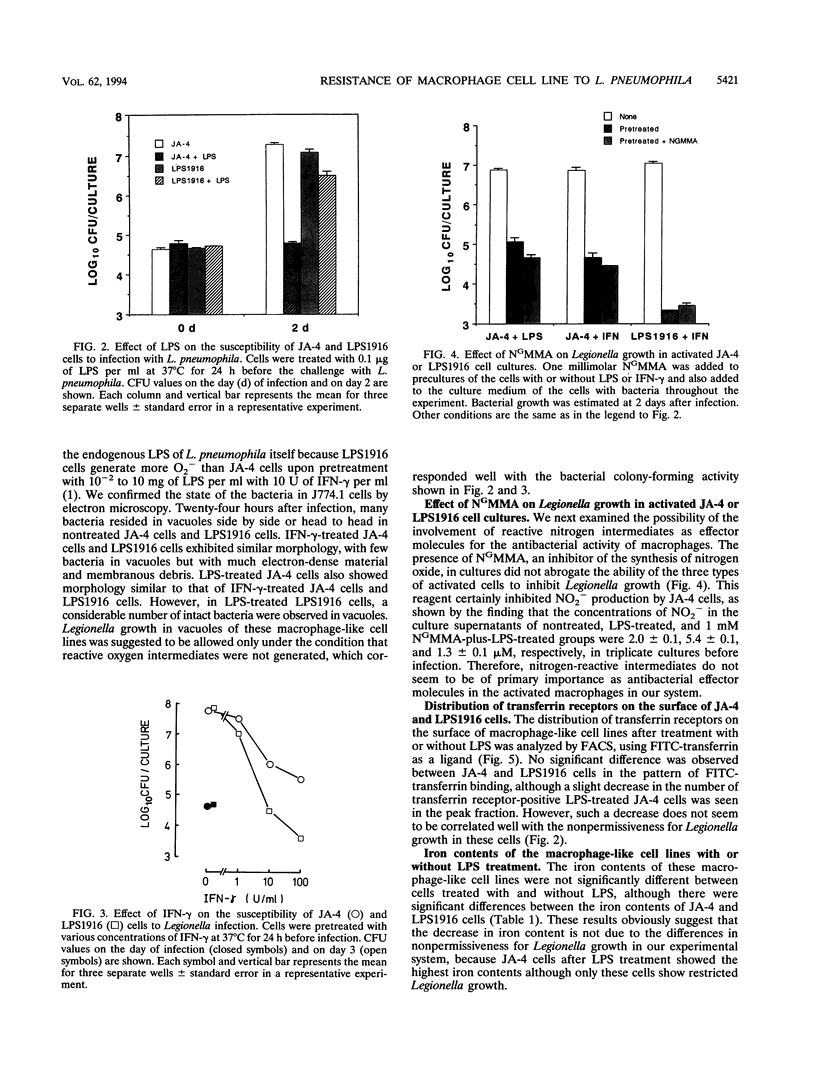
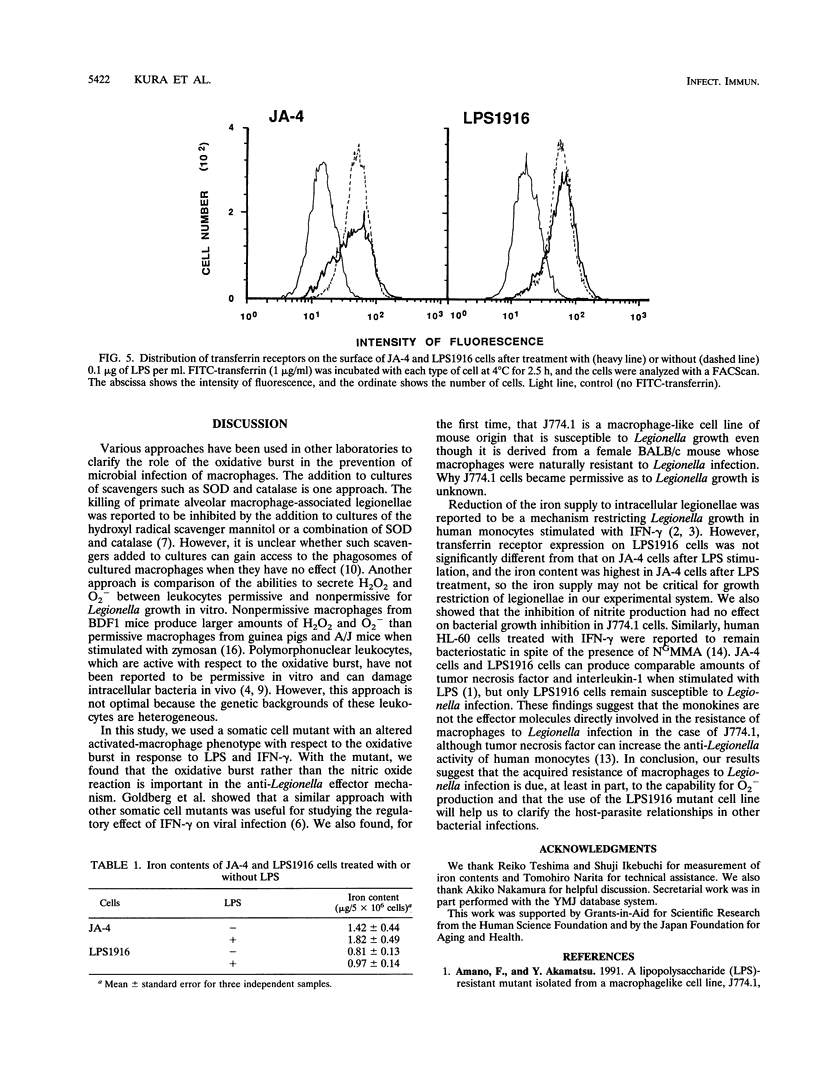
To elucidate the role of the oxidative burst in macrophage resistance to Legionella infection, we examined a murine macrophage-like cell line, J774.1, for permissiveness to Legionella growth, using a mutant that has a selective defect in the oxidative burst after lipopolysaccharide (LPS) stimulation. Legionella pneumophila serogroup 1 was infected into J774.1 monolayers, and then the extent of bacterial growth was estimated by a CFU assay. Both the parental cell line, JA-4, and the LPS-resistant mutant, LPS1916, were permissive for Legionella growth but became nonpermissive after pretreatment with gamma interferon. However, pretreatment of LPS1916 cells with LPS failed to inhibit bacterial growth, although LPS-treated JA-4 cells exhibited inhibited multiplication of the bacteria. The bacterial growth inhibition in JA-4 and mutant LPS1916 cells was correlated with the extent of the oxidative burst in the cells, as judged by cytochrome c reduction but not nitrite production. Neither transferrin receptor expression nor the iron content in JA-4 and LPS1916 cells, with or without LPS treatment, was correlated with suppression of Legionella growth. These results suggest that the restriction of Legionella growth in J774.1 cells is due to a bactericidal effect of the oxidative burst rather than reduction of the iron supply to the intracellular bacteria and that the effectors are reactive oxygen intermediates and not reactive nitrogen intermediates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano F., Akamatsu Y. A lipopolysaccharide (LPS)-resistant mutant isolated from a macrophagelike cell line, J774.1, exhibits an altered activated-macrophage phenotype in response to LPS. Infect Immun. 1991 Jun;59(6):2166–2174. doi: 10.1128/iai.59.6.2166-2174.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989 May;83(5):1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Regulation of transferrin receptor expression and ferritin content in human mononuclear phagocytes. Coordinate upregulation by iron transferrin and downregulation by interferon gamma. J Clin Invest. 1993 Mar;91(3):969–976. doi: 10.1172/JCI116318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. S., Winn W. C., Jr, Gump D. W., Beaty H. N. The kinetics of early inflammatory events during experimental pneumonia due to Legionella pneumophila in guinea pigs. J Infect Dis. 1983 Nov;148(5):823–835. doi: 10.1093/infdis/148.5.823. [DOI] [PubMed] [Google Scholar]
- Fujio H., Yoshida S., Miyamoto H., Mitsuyama M., Mizuguchi Y. Investigation of the role of macrophages and endogenous interferon-gamma in natural resistance of mice against Legionella pneumophila infection. FEMS Microbiol Immunol. 1992 Apr;4(4):183–191. doi: 10.1111/j.1574-6968.1992.tb04993.x. [DOI] [PubMed] [Google Scholar]
- Goldberg M., Belkowski L. S., Bloom B. R. Regulation of macrophage function by interferon-gamma. Somatic cell genetic approaches in murine macrophage cell lines to mechanisms of growth inhibition, the oxidative burst, and expression of the chronic granulomatous disease gene. J Clin Invest. 1990 Feb;85(2):563–569. doi: 10.1172/JCI114473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. F., Locksley R. M., Wilson C. B., Haas J. E., Klebanoff S. J. Interaction of primate alveolar macrophages and Legionella pneumophila. J Clin Invest. 1984 Jun;73(6):1515–1523. doi: 10.1172/JCI111357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepras R. I., Fitzgeorge R. B. The effect of oxygen-dependent antimicrobial systems on strains of Legionella pneumophila of different virulence. J Hyg (Lond) 1986 Aug;97(1):61–69. doi: 10.1017/s0022172400064354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. M., Hashemi S. Electron microscopic examination of the inflammatory response to Legionella pneumophila in guinea pigs. Lab Invest. 1982 Jan;46(1):24–32. [PubMed] [Google Scholar]
- Klein T. W., Yamamoto Y., Brown H. K., Friedman H. Interferon-gamma induced resistance to Legionella pneumophila in susceptible A/J mouse macrophages. J Leukoc Biol. 1991 Jan;49(1):98–103. doi: 10.1002/jlb.49.1.98. [DOI] [PubMed] [Google Scholar]
- Lochner J. E., Friedman R. L., Bigley R. H., Iglewski B. H. Effect of oxygen-dependent antimicrobial systems on Legionella pneumophila. Infect Immun. 1983 Jan;39(1):487–489. doi: 10.1128/iai.39.1.487-489.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Jacobs R. F., Wilson C. B., Weaver W. M., Klebanoff S. J. Susceptibility of Legionella pneumophila to oxygen-dependent microbicidal systems. J Immunol. 1982 Nov;129(5):2192–2197. [PubMed] [Google Scholar]
- Matsiota-Bernard P., Léfèbre C., Sedqui M., Cornillet P., Guenounou M. Involvement of tumor necrosis factor alpha in intracellular multiplication of Legionella pneumophila in human monocytes. Infect Immun. 1993 Dec;61(12):4980–4983. doi: 10.1128/iai.61.12.4980-4983.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summersgill J. T., Powell L. A., Buster B. L., Miller R. D., Ramirez J. A. Killing of Legionella pneumophila by nitric oxide in gamma-interferon-activated macrophages. J Leukoc Biol. 1992 Dec;52(6):625–629. doi: 10.1002/jlb.52.6.625. [DOI] [PubMed] [Google Scholar]
- Takema M., Inaba K., Uno K., Kakihara K., Tawara K., Muramatsu S. Effect of L-arginine on the retention of macrophage tumoricidal activity. J Immunol. 1991 Mar 15;146(6):1928–1933. [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Brown K., Friedman H. Differential morphologic and metabolic alterations in permissive versus nonpermissive murine macrophages infected with Legionella pneumophila. Infect Immun. 1992 Aug;60(8):3231–3237. doi: 10.1128/iai.60.8.3231-3237.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Newton C. A., Widen R., Friedman H. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect Immun. 1988 Feb;56(2):370–375. doi: 10.1128/iai.56.2.370-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



