Abstract
Background
Smoking is associated with reduced fusion rates after anterior cervical decompression and arthrodesis procedures. Posterior cervical arthrodesis procedures are believed to have a higher fusion rate than anterior procedures.
Questions/purposes
We asked whether smoking (1) would reduce the fusion rate in posterior cervical procedures; and (2) be associated with increased pain, decreased activity level, and a decreased rate of return of work as compared with nonsmokers.
Methods
We retrospectively reviewed 158 patients who had a posterior cervical fusion with lateral mass instrumentation and iliac crest bone grafting between 2003 and 2008. Fusion rates and Odom Criteria grades were compared among smokers and nonsmokers. The minimum followup was 3 months (average, 14.5 months; range, 3–72 months).
Results
Smokers and nonsmokers had similar fusion rates (100%). Although 80% of patients had Odom Criteria Grade I or II, smokers were five times more likely to have Grade III or IV with considerable limitation of physical activity. Age, gender, and diagnosis did not influence fusion rates or the Odom Criteria grade.
Conclusions
In contrast to the effect of smoking on anterior cervical fusion, we found smoking did not decrease posterior cervical fusion with lateral mass instrumentation and iliac crest bone grafting. Posterior cervical fusion with lateral mass instrumentation should be considered over anterior procedures in smokers if the abnormality can appropriately be addressed from a posterior approach.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Fusion is reportedly important for successful outcomes and clinical results in cervical spine procedures [4, 5, 10, 15]. Pseudarthrosis can lead to persistent neck and arm pain syndromes as well as numbness and dysesthesias in the upper extremities [5, 23, 24]. Both local and systemic factors are important for graft healing and solid fusion. Local factors include the biomechanical stability of the spinal fusion segment [3] as well as the local biologic environment, including the blood supply of the soft tissue bed [3] and the bone graft material used [3]. Solid fusion can be negatively affected by systemic factors, including osteoporosis, poor nutrition, and smoking [3].
The role of smoking in causing pseudarthrosis has been well studied in lumbar spine fusions with up to a fourfold increase in nonunion rates from 8% to 40% for lumbar fusions [7]. Nicotine has a direct inhibitory effect on autologous cancellous bone graft revascularization [11] as well as an increased rate of bone graft necrosis in a rabbit model of bone graft implantation [11]. Systemic nicotine has also been linked to nonunion in spinal fusion animal models [3, 32].
For cervical spine surgery, the effects of smoking have been studied only in patients undergoing anterior decompression and arthrodesis. Smoking is associated with a higher rate of delayed fusions and pseudarthrosis [1, 2, 4, 18], greater interspace collapse [2], and increased pain and decreased activity in multilevel anterior interbody grafting [18]. Bishop et al. [2] found these negative effects on anterior interbody fusion with the use of both allogenic and autogenic grafts, but the effect of smoking was most pronounced in attempted fusions with allograft. Despite the reportedly higher rate of pseudarthrosis in smokers for interbody grafting, Hilibrand et al. [18] reported no difference in the rate of fusion between smokers and nonsmokers who underwent corpectomy and anterior strut grafting. The addition of anterior plating in multilevel anterior decompressions and fusions improves fusion rates in smokers [5].
Although the evidence suggests smoking negatively affects the fusion rate and clinical outcomes for both lumbar fusions and multilevel anterior cervical interbody fusions, whether smoking influences posterior cervical spine fusions is unknown. Posterior cervical fusion is used across a wide spectrum of cervical spinal pathology, including multilevel cervical spondylosis with myelopathy, traumatic injuries requiring multilevel stabilization, and for revision cervical decompression and fusion cases. Lateral mass screw fixation has allowed for precise placement of instrumentation in the cervical spine without spinal cord or vertebral artery injury [12]. Biomechanical stability [26], maintenance of alignment [27], and low complication rates [12, 27] have also been reported.
We therefore asked whether (1) smoking correlates with increased rates of pseudarthrosis in posterior spinal fusions performed with autograft and lateral mass screw and rod instrumentation; (2) Odom Criteria grade for functional outcome differed between smokers and nonsmokers undergoing posterior cervical spine fusion; and (3) whether comorbidities influence the clinical score.
Patients and Methods
We performed a retrospective chart review on all 520 patients who had a multilevel (two or more segments) posterior cervical fusion with lateral mass instrumentation performed by a single surgeon (JDK) from 2003 to 2008 with a minimum followup of 3 months. Stringent exclusion criteria included: combined AP procedures, infection, tumor, trauma, less than two segments fused, instrumentation other than lateral mass screws/rods, and cervical fracture or dislocation. A total of 530 patients were identified with posterior cervical instrumentation. Of these, 307 met the inclusion criteria. Thirty patients had insufficient followup documentation (charts with greater than 3-month followup). This left 277 patients available for final review. A pilot analysis was performed on the first 158 patients in this cohort. All charts and radiographs were reviewed and statistical analyses performed as described subsequently. Because of apparently adequate power in the primary outcome measures in this initial analysis, the remaining 119 records were not reviewed.
Of the 158 patients, 117 (74%) were nonsmokers and 41 (26%) were smokers. We recorded age, gender, diagnosis, procedure performed, number of levels fused, type of instrumentation, comorbidities, perioperative complications, and Workmen’s Compensation status (Table 1). One hundred thirty-eight (87.3%) patients had a primary diagnosis of stenosis and 20 patients (12.7%) had another primary diagnosis, including radiculopathy or pseudarthrosis. Secondary and tertiary diagnoses included myelopathy (n = 36), radiculopathy (n = 72), myeloradiculopathy (n = 46), and nonunion (n = 37). The minimum length of followup was 3 months (average, 14.5 months; range, 3–72 months); the average patient age was 61 years (range, 35–87 years); and the average number of cervical segments fused was 4.2 (range, 2–8) (Table 1). With the numbers available, the differences in age and length of followup between smokers and nonsmokers were not meaningful in regard to the outcomes of interest.
Table 1.
Descriptive statistics of sample by smoking status (N = 158)
| Variable | Entire cohort (N = 158) | Smoking (N1 = 41) | Nonsmoking (N2 = 117) | p Value* |
|---|---|---|---|---|
| Age (years), mean (SD) | 61 | 55.3 (8.6) | 63.1 (11.7) | < 0.0001 |
| Length of followup (months), mean (SD) | 14.5 | 10.7 (6.0) | 15.8 (14.1) | 0.002 |
| Levels fused, mean (SD) | 4.2 | 4.1 (0.87) | 4.2 (1.0) | 0.3 |
| Gender, number (%) | 0.29 | |||
| Males | 93 | 27 (65.8) | 66 (56.4) | |
| Females | 65 | 14 (34.2) | 51 (43.6) | |
| Diagnosis, number (%) | 0.91 | |||
| Stenosis | 138 | 36 (87.8) | 102 (87.2) | |
| Other | 20 | 5 (12.2) | 15 (12.8) |
* p Value from t-test and chi square or Fisher’s exact test
Patients underwent a posterior fusion alone (n = 13), foraminotomy and fusion (n = 8), or a posterior fusion and laminectomy (n = 137). A standard technique was performed for all posterior cervical fusions. We used lateral mass instrumentation (Medtronic Vertex, Memphis, TN, or Stryker Oasys, Allendale, NJ), decortication of facet joints and lateral masses and placement of iliac crest bone graft (with the exception of two patients who had local bone grafting only).
Patients were placed in a hard cervical collar for 3 weeks and then transitioned to a soft collar for another 3 weeks on a prn basis. We instituted no routine physical therapy unless a specific need arose due to a lack of patient motivation. Patients were allowed to return to light duty at 6 weeks and full activity at 12 weeks, depending on the patient’s job description.
Patients were seen at 3 weeks, 3 months, 6 months, 1 year, and 2 years with flexion/extension radiographs at each visit except for 3 weeks. Clinical outcome scores were graded by the criteria of Odom and Finney [25]. The Odom Criteria have been used for decades to grade clinical outcomes after cervical spine surgery. It takes into account the patient’s daily symptoms, activity level, and ability to return to work and grades them on a scale of I to IV: Grade I (excellent) = no cervical spine symptoms, daily activities not impaired; Grade II (good) = intermittent discomfort, no substantial interference with work activities; Grade III (fair) = subjective improvement but major limitations of physical activities; and Grade IV (poor) = no improvement or worse compared with the preoperative condition.
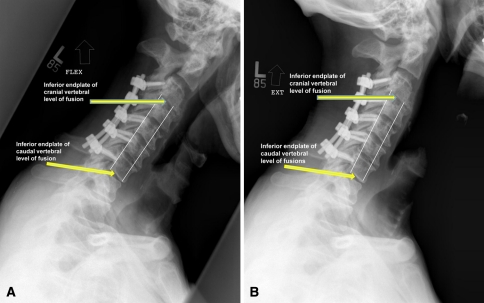
One of us (JDE) determined the presence or absence of arthrodesis by interpretation of flexion/extension radiographs. Fusion was graded on three criteria: (1) absence of obvious hardware loosening; (2) absence of motion (less than 1 mm) between contiguous spinous processes on flexion/extension radiographs [15, 18]; or (3) in cases of laminectomy, absence of motion (less than 1 mm on flexion/extension radiographs) through the fused segments as measured by the vertebral bodies. In this instance, lines were drawn between the most anterior aspects of the inferior end plates of the most cranial and most caudal section of the fusion mass in question. A second line was created at the most posterior aspect of the same end plates (Fig. 1). The length of each line was measured in flexion and then compared with the corresponding line drawn in extension and any difference recorded. To confirm the reliability of this radiographic assessment, flexion and extension radiographs were analyzed by two separate authors (SWT, BAB) and results compared. In a subset of 40 patients reviewed, no difference was found between the two authors’ flexion and extension differences either in anterior lengths (95% confidence interval difference between means −0.174 to 0.614, p = 0.27) or posterior lengths (95% confidence interval difference between means −0.328 to 0.113, p = 0.335) using the statistical analysis software XLSTAT 2010 (Kovach Computing Systems, Anglesey, Wales). In addition, intraclass correlation coefficients were calculated between authors (SWT, BAB) and were greater than 0.9 for all anterior and posterior measured lengths on flexion/extension films (Table 2). This implies the reliability of the assessment of fusion by the method described previously.
Fig. 1A–B.
(A) Flexion and (B) extension radiographs are shown. White lines mark the distances measured in these dynamic films. Differences in measurement would indicate motion.
Table 2.
Intraclass correlation coefficients for determining interobserver variability
| Factor | Intraclass correlation coefficient | 95% confidence interval |
|---|---|---|
| Flexion anterior line R1 & R2* | 0.941 | 0.891–0.968 |
| Flexion posterior line R1 & R2* | 0.954 | 0.915–0.975 |
| Extension anterior line R1 & R2* | 0.947 | 0.903–0.972 |
| Extension posterior line R1 & R2* | 0.955 | 0.916–0.976 |
* R1 & R2 indicates Reviewers 1 and 2.
We conducted statistical analyses using t-test or Wilcoxon rank sum test for continuous variables (age, length of followup, and levels fused) and chi square test for categorical variables (diagnosis and gender). Multivariable logistic regression analyses were conducted to determine the relative influence of smoking on clinical outcome scores (as graded by the Odom Criteria, which then was categorized into two groups of clinical outcome) controlling for age, gender, and diagnosis. Likelihood ratio test was used to fit a parsimonious model. All analyses were conducted using SAS software Version 9.2 (SAS Institute, Inc, Cary, NC).
Results
We observed no difference in radiographic union between smokers and nonsmokers; both groups had 100% fusion rates. Insufficient numbers existed in surgical subgroups to compare differences between posterior fusion (n = 13) alone versus foraminotomy and fusion (n = 8) or laminectomy and fusion (n = 137).
One hundred twenty-six (80%) patients demonstrated an excellent or good result and 32 (20%) patients displayed a fair or poor result. Clinical outcome scores based on the Odom Criteria were of higher grade (worse functional level) (p < 0.001) in smokers compared with nonsmokers. Smokers were nearly five times (confidence interval, 1.9–11.6) more likely to have a fair or poor outcome compared with nonsmokers after controlling for their age, gender, length of followup, and diagnosis.
There were insufficient numbers to draw any conclusions regarding associations between clinical outcome scores and comorbidities, Workmen’s Compensation status, or the amount of nicotine used. Similarly, insufficient numbers existed to assess any potential effect of smoking status or amount of smoking on complications.
Complications included seven C5 nerve root palsies, four in smokers and three in nonsmokers. The one bilateral palsy was in a smoker. All palsies recovered uneventfully. There was one hardware failure (in a smoker) in the early postoperative (3 weeks) period, which was revised without further complication. There were four infected iliac crest wounds, one infected cervical wound, and one hematoma, which required irrigation and débridement. Both of the cervical wound complications occurred in smokers. There was one patient with a bowel perforation and femoral neck fracture and one patient with postoperative dysphagia unrelated to the surgical procedure. We did not specifically address adjacent segment degeneration over the followup period, but no patients with symptomatic adjacent segment degeneration required subsequent surgery during the followup period.
Discussion
The advent of lateral mass instrumentation has presented a safe and effective method for providing immediate, rigid fixation and maintenance of cervical alignment. In most cases, this instrumentation has negated the historical need for postoperative halo immobilization in complex cervical reconstructive procedures [12, 26, 27, 30]. There is a commonly held belief that posterior cervical fusions have a high fusion rate, ranging from 94% to 100% [6, 9, 15, 19, 21, 22, 29, 31]. The main goal of our study was to answer the question: can the same high fusion rate be achieved in smokers?
We recognize limitations to this retrospective chart review. First, our groups were retrospectively created and not matched for comorbidities. We did not have adequate data on which to provide for such matching although they might affect fusion rates. Ideally, a prospective, randomized and controlled study would provide the most convincing evidence to support the efficacy of posterior cervical fusions in obtaining a solid arthrodesis in the presence of smoking. Second, an ideal approach would include CT scans of all postoperative cervical spines to assess the presence or absence of a solid fusion. However, we were unable perform post hoc CT scans to evaluate fusion masses. This study assessed fusions through flexion and extension radiographs, a well-accepted and published modality [8, 18, 19, 21, 29, 31]. In addition, we have shown interobserver reliability in our method of determining fusion from flexion and extension views. The universal fusion among smokers and nonsmokers alike makes this issue less germane. Third, we used the Odom Criteria which, while convenient for our chart review and used for decades and reported in a number of studies [20, 25, 28], is an unvalidated categorical rating. A more sophisticated outcome measure (ie, SF-36, etc) could have provided more convincing evidence of clinical disparity between smokers and nonsmokers. The retrospective chart review nature of this study did not lend itself to this kind of prospective evaluation because patients were not contacted and were not evaluated for further outcome measures. Furthermore, with these criteria we observed a difference in our patient groups. We believe this is a real finding and one that would likely be confirmed with a more comprehensive and validated outcome tool.
Our data support literature suggesting posterior cervical fusions have a high union rate [6, 9, 15, 19, 21, 22, 29, 31]. However, contrary to the disparity in fusion rates historically noted with anterior cervical arthrodesis [2, 18], we found no difference in fusion rates between smokers and nonsmokers. With these considerations in mind, anterior cervical fusions have a long and successful history in the treatment of radiculopathy and myelopathy [4, 10, 13, 18, 33]. Although fusion rates are generally high, these rates vary depending on a number of variables, including the number of levels involved in the arthrodesis, the type of graft used, the presence or absence of instrumentation, and smoking status [2, 10, 14, 18, 28, 33]. With the advent of anterior plating, fusion rates have increased with reports of 97% to 100% fusion rates in one-level and multilevel anterior cervical decompression and fusion with allograft irrespective of smoking status [5, 28]. Generally, however, anterior cervical arthrodesis shows decreasing radiographic fusion rates associated with an increase in the number of levels fused and the bony surfaces involved in the fusion [14, 18, 33]. Fusion rates can be as low as 56% in multilevel, noninstrumented anterior cervical arthrodesis [14].
The contribution of smoking in the development of a pseudarthrosis in spine fusions has been well documented with up to a fourfold increase in nonunion rates and worse clinical outcomes reported [7, 16–18]. With cervical fusions, smoking is associated with a higher rate of delayed unions and pseudarthrosis, greater interspace collapse, and worse clinical outcomes in multilevel anterior interbody grafting [1, 2, 4, 18]. Although these negative effects can be seen with the use of both allogenic and autogenic grafts, the effect of smoking appears most pronounced in attempted allograft arthrodesis [2, 18]. Nicotine has demonstrated a direct inhibitory effect on autologous cancellous bone graft revascularization as well as an increased rate of graft necrosis in a rabbit model [11, 32]. Systemic nicotine has also been linked to nonunion in spinal fusion animal models [3, 32]. The inhibitory effects of nicotine on neovascularization [11] and on fusion [32] appear to be mitigated in our study by the biologic environment created by decortications of the lateral mass and facet joints as well as by the biomechanical advantages of lateral mass constructs.
In a well-known study addressing the role of smoking on cervical fusions, Hilibrand et al. [18] demonstrated a difference in fusion rates among smokers and nonsmokers undergoing multilevel anterior cervical decompression and fusion. All of these procedures included the use of autograft (iliac crest bone graft or fibula) without instrumentation. Compared with those undergoing anterior discectomy and interbody fusions, the authors found no difference in fusion rates between smokers and nonsmokers in patients undergoing a multilevel cervical corpectomy.
Our current study assessed the fusion rate in smokers versus nonsmokers who underwent posterior cervical fusion with lateral mass instrumentation and iliac crest bone graft. Contrary to the findings of Hilibrand et al. [18] with anterior fusion surgery, our study demonstrated no difference in fusion rates between smokers and nonsmokers. A complete clinical arthrodesis was observed with 100% of patients achieving successful radiographic union. Surgical technique is important to this fusion result. Only with decortications of the lateral masses and iliac crest bone graft can we generalize our results. Our result also supports the clinical observation that posterior cervical fusions have a very high arthrodesis rate [6, 9, 15, 19, 21, 22, 29, 31]. It would appear that the negative biologic effects of smoking may not have as much impact during posterior fusion surgery when autograft iliac crest bone graft and lateral mass instrumentation are used. We did not evaluate the use of allograft bone and, therefore, the fusion rates may not be generalized when using a different graft source.
Looking at clinical outcomes, the smokers in our study did worse compared with nonsmokers. This finding is similar to Hilibrand et al. [18] who also found smokers had poorer clinical outcomes during anterior fusion surgery. Based on Odom’s Criteria, smokers were much more likely to have a fair or poor outcome. However, clinical outcome did not seem to correlate with the presence or absence of a solid radiographic fusion. This finding appears to contradict the findings of anterior cervical arthrodesis, in which a solid arthrodesis correlates with a good outcome [4, 5, 10, 15].
Posterior cervical arthrodesis with lateral mass instrumentation and iliac crest bone graft can provide a predictable fusion even in the face of smoking. Clinical outcomes are generally good, but smokers are more likely to have poorer outcomes than nonsmokers. Surgeons and smokers alike should understand that clinical outcomes are inferior when compared with nonsmokers, even in the face of a successful posterior arthrodesis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was waived as no patients were contacted.
References
- 1.An HS, Simpson JM, Glover JM, Stephany J. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine. 1995;20:2211–3326. doi: 10.1097/00007632-199510001-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bishop RC, Moore KA, Hadley MN. Anterior cervical interbody fusion using autogenic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg. 1996;85:206–210. doi: 10.3171/jns.1996.85.2.0206. [DOI] [PubMed] [Google Scholar]
- 3.Boden SD, Sumner DR. Biologic factors affecting spinal fusion and bone regeneration. Spine (Phila Pa 1976) 1995;20(Suppl):102S–112S. [PubMed] [Google Scholar]
- 4.Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75:1298–1307. doi: 10.2106/00004623-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Bose B. Anterior cervical instrumentation enhances fusion rates in multilevel reconstruction in smokers. J Spinal Disord. 2001;14:3–9. doi: 10.1097/00002517-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Brodsky AE, Khalil MA, Sassard WR, Newman BP. Repair of symptomatic pseudoarthrosis of anterior cervical fusion. Spine. 1992;17:1137–1143. doi: 10.1097/00007632-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Brown CW, Orme TJ, Richardson HD. The rate of pseudoarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine. 1986;11:942–943. doi: 10.1097/00007632-198611000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Cannada LK, Scherping SC, Yoo JU. Pseudoarthrosis of the cervical spine: a comparison of radiographic diagnostic measures. Spine. 2003;28:46–51. doi: 10.1097/00007632-200301010-00012. [DOI] [PubMed] [Google Scholar]
- 9.Carreon L, Glassman SD, Campbell MJ. Treatment of anterior cervical pseudoarthrosis: posterior fusion versus anterior revision. Spine J. 2006;6:154–156. doi: 10.1016/j.spinee.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Cuathen JC, Kinard RE, Vogler JB, Jackson DE, DePaz OB, Hunter OL, Wasserburger LB, Williams VM. Outcome analysis of noninstrumented anterior discectomy and interbody fusion in 348 patients. Spine. 1998;23:188–192. doi: 10.1097/00007632-199801150-00008. [DOI] [PubMed] [Google Scholar]
- 11.Dafarti TK, Whitesides TE, Heller JG, Goodrich AC, McCarey, Hutton WC. Nicotine on the revascularization of bone graft. An experimental study in rabbits. Spine. 1994;19:904–911. doi: 10.1097/00007632-199404150-00007. [DOI] [PubMed] [Google Scholar]
- 12.Deen HG, Birch BD, Wharen RE, Reimer R. Lateral mass screw-rod fixation of the cervical spine: a prospective clinical series with a 1-year follow-up. Spine J. 2003;3:489–495. [PubMed] [Google Scholar]
- 13.Emery SE, Bohlman HH, Bolesta MJ, Jones PK. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy. J Bone Joint Surg Am. 1998;80:941–951. doi: 10.1302/0301-620X.80B6.9517. [DOI] [PubMed] [Google Scholar]
- 14.Emery SE, Fisher JR, Bohlman HH. Three level anterior cervical discectomy and fusion: radiographic and clinical results. Spine. 1997;22:2622–2624. doi: 10.1097/00007632-199711150-00008. [DOI] [PubMed] [Google Scholar]
- 15.Farey ID, McAffee PC, Davis RF, Long DM. Pseudoarthrosis of the cervical spine after anterior arthrodesis. J Bone Joint Surg Am. 1990;72:1171–1177. [PubMed] [Google Scholar]
- 16.Hadley MN, Reddy SV. Smoking and the human vertebral column: a review of the impact of cigarette use on vertebral bone metabolism and spinal fusion. Neurosurgery. 1997;41:116–124. doi: 10.1097/00006123-199707000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Hanley EN, Levy JA. Surgical treatment of isthmic lumbosacral spondylolisthesis. Analysis of variables influencing results. Spine. 1989;14:48–50. doi: 10.1097/00007632-198901000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Hilibrand AS, Fye MA, Emery SE, Palumbo MA, Bohlman HH. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody strut grafting. J Bone Joint Surg Am. 2001;83:668–673. doi: 10.1302/0301-620X.83B5.11585. [DOI] [PubMed] [Google Scholar]
- 19.Huang RC, Girardi FP, Poynton AR, Cammisa FP., Jr Treatment of multilevel cervical spondylotic myeloradiculopathy with posterior decompression and fusion with lateral mass plate fixation and local bone graft. J Spinal Disord Tech. 2003;16:123–129. doi: 10.1097/00024720-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Korinth MC, Kruger A, Oertel MF, Gilsbach JM. Posterior foraminotomy or anterior discectomy with polymethyl methacrylate interbody stabilization for cervical soft disc disease: results in 292 patients with monoradiculopathy. Spine. 2006;31:1207–1214. doi: 10.1097/01.brs.0000217604.02663.59. [DOI] [PubMed] [Google Scholar]
- 21.Kuhns CA, Geck MJ, Wang JC, Delemarter RB. An outcomes analysis of the treatment of cervical pseudoarthrosis with posterior fusion. Spine. 2005;30:2424–2429. doi: 10.1097/01.brs.0000184314.26543.7d. [DOI] [PubMed] [Google Scholar]
- 22.Lovely T, Carl A. Posterior cervical spine fusion with tension-band wiring. J Neurosurg. 1995;83:631–635. doi: 10.3171/jns.1995.83.4.0631. [DOI] [PubMed] [Google Scholar]
- 23.Mutoh N, Shinomiya K, Furuya K, Yamaura I, Satoh H. Pseudoarthrosis and delayed union after anterior cervical fusion. Int Orthop. 1993;17:286–289. doi: 10.1007/BF00181700. [DOI] [PubMed] [Google Scholar]
- 24.Newman M. The outcome of pseudoarthrosis after cervical anterior fusion. Spine. 1993;18:2380–2382. doi: 10.1097/00007632-199312000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Odom GL, Finney W, Woodhall B. Cervical disk lesions. JAMA. 1958;166:23–28. doi: 10.1001/jama.1958.02990010025006. [DOI] [PubMed] [Google Scholar]
- 26.Papagelopoulos PJ, Currier BL, Neale PG, Hokari Y, Berglund LJ, Larson DR, Fisher DR, An KN. Biomechanical evaluation of posterior screw fixation in cadaveric cervical spines. Clin Orthop Relat Res. 2003;411:13–24. doi: 10.1097/01.blo.0000068359.47147.bd. [DOI] [PubMed] [Google Scholar]
- 27.Pateder DB, Carbone JJ. Lateral mass screw fixation for cervical spine trauma: associated complications and efficacy in maintaining alignment. Spine J. 2006;6:40–43. doi: 10.1016/j.spinee.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Samartzis D, Shen FH, Goldberg EJ, An HS. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine. 2005;30:1756–1761. doi: 10.1097/01.brs.0000172148.86756.ce. [DOI] [PubMed] [Google Scholar]
- 29.Sawin PD, Traynelis VC, Menezes MD. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88:255–265. doi: 10.3171/jns.1998.88.2.0255. [DOI] [PubMed] [Google Scholar]
- 30.Schultz KD, McLaughlin MR, Haid RW, Comey CH, Rodts GE, Jr, Alexander J. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(Suppl):214–221. doi: 10.3171/spi.2000.93.2.0214. [DOI] [PubMed] [Google Scholar]
- 31.Sembrano JN, Mehbod AA, Garvey TA, Denis F, Perra JH, Schwender JD, Transfeldt EE, Winter RB, Wroblewski JM. A concomitant posterior approach improves fusion rates but not overall reoperation rates in multilevel cervical fusion for spondylosis. J Spinal Disord Tech. 2009;22:162–169. doi: 10.1097/BSD.0b013e318175d821. [DOI] [PubMed] [Google Scholar]
- 32.Silcox DH, Dafarti T, Boden SD, Schimadle JH, Hutton WC, Whitesides TE. The effect of nicotine on spinal fusion. Spine. 1995;20:1549–1553. doi: 10.1097/00007632-199507150-00001. [DOI] [PubMed] [Google Scholar]
- 33.Zdeblick TA, Hughes SS, Riew D, Bohlman HH. Failed anterior cervical discetomy and arthrodesis. Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79:523–532. doi: 10.1302/0301-620X.79B4.6940. [DOI] [PubMed] [Google Scholar]