Abstract
Background
Anterior cervical discectomy and fusion (ACDF) represent the standard treatment for cervical spondylolytic radiculopathy and myelopathy. To achieve solid fusion, appropriate compressive loading of the graft and stability are essential. Fusion may lead to adjacent segment degeneration. Artificial discs have been introduced as motion-preserving devices to reduce the risk of fusion-related complications.
Questions/purposes
We therefore asked: (1) Does the use of a plate reduce motion at the operated level and bone graft compression compared to fusion with bone graft alone; and (2) is adjacent-segment motion higher after fusion with a plate?
Methods
Motions and compressive loads in the graft were quantified for intact, C4–C5 ACDF without and with a plate, and total disc arthroplasty in human cadaver spines.
Results
At the surgery level all motions decreased for ACDF with a plate. The motions were similar to intact motions after total disc arthroplasty. The motions across the adjacent segment increased after fusion in all loading modes except lateral bending and were closer to the intact for the total disc arthroplasty case. The plate maintained a compressive load on the graft with a maximum increase in extension.
Conclusions
Unlike fusion, the arthroplasty can restore motion to normal at the surgery and adjacent segments, compared to fusion cases. A cervical plate with a precompression of the graft provides enhanced stability and fusion due to improved compression.
Clinical Relevance
Our findings support the clinical observations that fusion may lead to the degeneration of the adjacent segments. Disc arthroplasty may be able to circumvent the adjacent segment degeneration.
Introduction
Anterior cervical discectomy and fusion (ACDF) represents a widely accepted surgical procedure to manage cervical spondylolytic radiculopathy and myelopathy symptoms. Cloward [11] and Robinson and Smith [34] originally described noninstrumented cervical arthrodesis, but these approaches reportedly had nonunion rates ranging from 8.3% to 12% [22, 35, 36]. Bohler [3] in 1967 reported what was likely the first use of anterior cervical plate and screw fixation in a patient with cervical spinal trauma. In the early 1980s, Caspar et al. [8] popularized anterior cervical plating. The constructs of the procedure had limited fixation at the screw-plate interface, leading to early screw backouts. However, the concept facilitated graft compression, allowing for a better chance of bony fusion.
ACDF is a reliable procedure with a fusion rate of between 85% and 95% [2, 26]. Cervical plates are used to stabilize the segment(s), affording solid fusion of the grafted bone, probably due to enhanced compression of the graft [19]. However, some complications have been reported, such as nonunion graft migration and kyphotic malunion [1, 4, 9, 19]. Reported nonunion rates range from 4.0% to 9.7% after ACDF with different types of cervical plate systems [22, 35, 36]. The reduced physiologic motion of the spine after fusion may lead to a compensatory increase in motion at the adjacent level, leading to an increase in adjacent-segment degeneration [18, 21]. Adjacent-segment degeneration with new, symptomatic radiculopathy reportedly occurs after ACDF in 2% to 3% of patients per year on a cumulative basis [21]. Spine surgeons are now pursuing alternatives to fusion, such as total disc arthroplasty (TDA) to address fusion-related complications [7, 27, 33, 37]. The goal in using these devices is to alleviate pain by replacing the diseased disc while preserving and/or restoring motion at the surgery and adjacent levels.
We therefore asked the following questions: (1) Does the use of a plate reduce motion at the operated level and bone graft compression in all loading modes compared to fusion with bone graft alone?; (2) Is adjacent-segment motion higher after fusion with a plate?
Materials and Methods
We conducted a series of experiments to study the motion at the surgery and adjacent levels after fusion and TDA using six fresh-frozen ligamentous human cervical spines (Fig. 1). The loads across the bone graft after fusion using a load cell were also quantified. The rotations across various levels were compared among the different conditions.
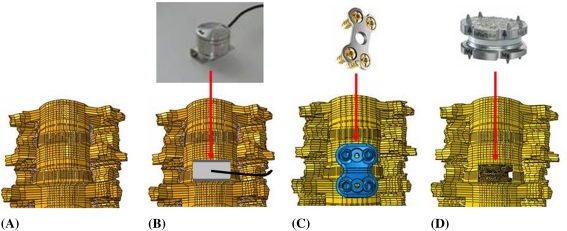
Fig. 1A–D.
Photographs illustrate the four conditions included in the study. (A) Intact C3–T1 specimens were first tested on the kinematic profiler. (B) A load cell was placed at the C4–C5 level. (C) A plate was placed along with the load cell at the C4–C5 level. (D) A Discover™ cervical artificial disc was placed at the C4–C5 level.
The specimens were prepared by fixing the T1 vertebra to the base of the test frame and fixing a frame to the C3 vertebra for the load applications. Three light-emitting diodes (LEDs) were affixed to L-shaped brackets rigidly attached to each vertebral body. The motions of the LEDs in response to the applied loads were tracked using the Optotrak® system (Northern Digital, Waterloo, Canada). The specimens were tested under 0 to 2 Nm of moment in flexion, extension, lateral bending, and axial rotation [5, 6]. These moment values produce physiologic ROM in vivo [18]. The load displacement curves of the cadaver spines and the load passing through the load cell were obtained. The load cell was connected to an amplifier and the amplifier’s signal was recorded using the Optotrak® data acquisition system.
The testing was conducted in the following sequence: (1) intact spine; (2) load cell placement at the C4–C5 level; (3) load cell and cervical plate at the C4–C5 level; and (4) TDA at the C4–C5 level. The load cell (Honeywell, Inc, Morristown, NJ) was custom made for the cervical spine with proper dimensions to fit the C4–C5 disc space (6-mm height, 10-mm diameter). To further ensure a tight fit of the load cell, bone chips were placed along with the load cell. The plate (DePuy Spine, Inc, Raynham, MA) was screwed to the adjacent vertebral bodies such that the bone graft was under compression before testing. For TDA at the C4–C5 level case, a standard anterior cervical discectomy, including removal of the anterior longitudinal ligament, was performed at the surgery level in all specimens. The posterior longitudinal ligament, lateral annulus, and uncinate processes were preserved. An appropriately sized artificial disc (Discover™, DePuy Spine) was implanted at C4–C5 in the specimens (6-mm prostheses in four cases; 8-mm prostheses in two cases). The device consists of two cobalt-chromium-molybdenum endplates with a metal-on-polyethylene bearing surface; the polyethylene insert is fixed to the inferior endplate.
The motion levels at the surgery and adjacent sites were quantified for all cases, intact, stabilized, and TDA. The load cell data were also recorded for the fusion cases. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by a post hoc Fisher test for multiple comparisons (p < 0.05). All data are shown as mean ± 1 SD.
Results
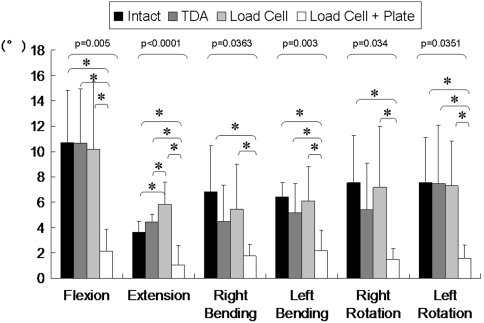
With the load cell and plate implanted at the C4–C5 level, the mean flexion motion decreased (p = 0.005) by about 80% (Fig. 2). Plating at the C4–C5 level restricted (p = 0.0001) the motion during extension as well. The C4–C5 level motions were closer to intact values after TDA, except in extension. The mean extension motion at the C4–C5 level increased by 22% after TDA (Fig. 2). In ACDF with plate fixation, the compressive load increment during cervical motions showed variations, although it was always in compression (Table 1). The graft load decreased in axial rotation, compared to the precompression load. In lateral bending, the graft load was similar to the initial precompression value. On the other hand, graft load increased by approximately a factor of two during extension. In all loading modes, compression of the graft was higher in the presence of the plate, except in flexion. In flexion, graft load was higher in the graft-alone case, illustrating the protective role of the plate in this mode.
Fig. 2.
A graph shows surgery-level (C4–C5) motions (mean and SD) at the final loading step (2 Nm). The motion decreased after fusion with a plate compared to the intact. TDA restored the motion to the intact value. The p value using one-way analysis of variance (ANOVA) is shown on the graph. *The asterisks show significant difference using post-hoc Fisher multiple comparison analysis.
Table 1.
Compressive loads on the graft (load cell) at the C4–C5 level under 2-Nm moments
| Motion | Mean compressive load (N) | |
|---|---|---|
| Load cell | Load cell + plate | |
| Initial value (no moment) | 17 | 86 |
| Extension | 37 | 152 |
| Flexion | 102 | 43 |
| Left bending | 49 | 98 |
| Right bending | 70 | 92 |
| Left rotation | 53 | 71 |
| Right rotation | 47 | 63 |
Data are presented for a total of six cases tested with a load cell placed at the C4–C5 level to study the load passing through the anterior column.
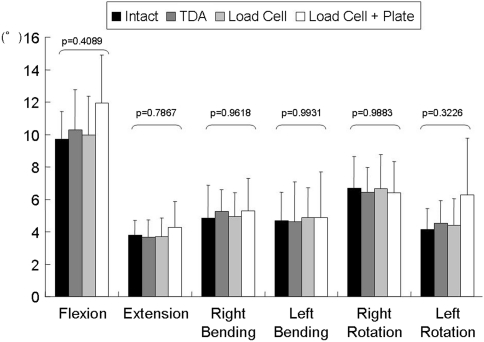
The mean motion after fusion at the adjacent level increased all loading modes except lateral bending, while the motion was closer to intact for the TDA case (Fig. 3). The mean motion at the C5–C6 level with the load cell and plate at the C4–C5 level was similar in flexion (p = 0.4089) and in extension (p = 0.7867). The mean motion at the adjacent level with TDA at the C4–C5 level was near normal in flexion and extension.
Fig. 3.
A graph shows adjacent-level (C5–C6) motions (mean and SD) at 2 Nm. The motion was similar for the bone graft with the plate case in flexion and in extension, compared to the intact case. The motion was close to the intact case for the TDA case. No difference among any groups was seen by one-way analysis of variance (ANOVA).
Discussion
ACDF is the standard to treat patients with cervical spondylolytic radiculopathy and myelopathy. To achieve solid fusion of the grafted bone, the graft should be under compression and undergo very little motion during the healing process. Fusion also leads to adjacent-segment degeneration, due to a compensatory increase in motion across this level as compared to intact values, as well as to other factors. More recently, artificial discs have been developed as motion-preserving devices to address these issues. Besides restoring motion at the surgery level, TDA should lessen the degeneration of the adjacent segment. Using a cadaver model, we asked the following two questions: (1) Does the use of a plate reduce motion at the operated level and bone graft compression in all loading modes compared to fusion with bone graft alone? (2) Is adjacent-segment motion higher after fusion with a plate?
We acknowledge limitations to our experiments, as with any other in vitro motion study reported in the literature. First, in our cadaver studies, simulation of the stabilizing effects of in vivo muscle forces was not feasible, although it is desirable to test the spine in the presence of an appropriate preload. The application of muscular forces tends to create a highly unstable spine, with cervical specimens tending to go into extension upon the application of a preload [12, 25]. Second, the literature recommends undertaking in vitro biomechanical studies without the preload so long as the applied moments produce a physiologic ROM [5, 6, 15, 17, 23]. Thus, the experimental studies described here were carried out using pure moments of up to 2.0 Nm since these produce physiologic ROM in a cadaver cervical specimen without a preload [17]. Third, while fresh freezing does not affect the material properties of ligaments, bone, and annulus and the motion of the spine [29], the question does arise of how representative the specimens are of the general population. Fourth, the cervical specimen donors used in this study had an average age of 66 years, which has implications for bone quality, ligament strength, and disc degeneration. All specimens were screened for abnormalities and only those that appeared “normal” were selected for biomechanical testing. Therefore, even though the average donor age was advanced, care was taken to include only specimens of good quality for the present investigation. However, being a cadaver model and despite these precautions and agreement with other cadaver studies in the literature [10, 13–16, 23, 24, 31] (Table 2), it is crucial to show our findings are clinically relevant.
Table 2.
A summary of published in vitro biomechanical studies on cervical arthroplasty
| Study | Study design | Prosthesis* | Mechanical variables | Results |
|---|---|---|---|---|
| Chang et al. [10] (2007) | TDA versus fusion | ProDisc | IDP at the treated and adjacent levels | Adjacent level IDP was similar to intact |
| DiAngelo et al. [14] (2003) | TDA versus fusion | Prestige | Motion at index and adjacent levels | TDA preserved motion and yielded kinematics similar to intact |
| DiAngelo et al. [13] (2004) | TDA versus fusion | ProDisc | Motion at index and adjacent levels | TDA was able to mimic kinematics of the intact spine |
| Dmitriev et al. [15] (2005) | TDA versus fusion | PCM | Motion and IDP | TDA preserved motion and IDP at adjacent level |
| Duggal et al. [16] (2007) | TDA | Bryan | Extension, flexion, and axial rotation motion until failure | Remaining ligaments and annulus sufficient to provide stability with this implant |
| Kotani et al. [23] (2002) | TDA versus fusion | 3D fabric disc | Motion at index and adjacent levels | Increase in extension-flexion motion, no change at adjacent level |
| McAfee et al. [24] (2003) | TDA versus fusion | PCM | Motion, PLL contribution | TDA preserved motion, resection of PLL decreases stability |
| Puttlitz et al. [31] (2004) | TDA | ProDisc | Motion | TDA preserved motion and coupling |
* Prostheses included: ProDisc® (Synthes, Inc, West Chester, PA), Prestige® (Medtronic Sofamor Danek, Memphis, TN), Porous Coated Motion (PCM; Cervitech, Inc, Rockaway, NJ), Bryan® (Medtronic Sofamor Danek); TDA = total disc arthroplasty; IDP = intradiscal pressure; 3D = three-dimensional; PLL = posterior longitudinal ligament.
We found, after ACDF with bone graft and a cervical plate, motion at the surgery level decreased in all directions. The increase in flexion motion across the adjacent segment observed in our study is likely to contribute to adjacent-segment degeneration, in line with clinical observation. The implant-level and the adjacent-level motions were similar to intact after TDA at the C4–C5. The near-normal motions after TDA suggest the adjacent-segment degeneration should be the lesser value. In a meta-analysis of the literature through a MEDLINE search, Harrop et al. [20] reported adjacent-segment degeneration in 34% of the fusion patients as compared to 9% in the TDA patients. Ordway et al. [28], using a stereoradiographic technique, found motions at the TDA level were similar to those of the control group. Likewise, based on plain radiographic analyses, several investigators have reported preservation of motion at the surgery level after TDA at various time intervals postoperatively [30, 32, 33, 37]. Our data support these findings. The near-normal motion in our TDA group is in agreement with the in vivo data. The increase in adjacent-level motion observed in our study after fusion is high compared to the TDA case. This should lead to higher incidences of adjacent-segment degeneration in the fusion group compared to TDA patients, in line with the meta-analysis of Harrop et al. [20].
For the load cell-graft-alone case, the graft experienced lesser compression during extension, since the C4 vertebral body lost contact with the load cell with increasing extension moment. In the other loading modes, the load cell provided as much stability as the intact spine, suggesting additional stabilization is needed. The plate enhanced stability in extension and ensured compressive load on the graft in all loading modes including extension. The data also suggest, to enhance bone graft healing, the plate should be placed with precompression on the graft. These observations are in agreement with the clinical findings that the fusion rates are higher in patients who receive a cervical plate to augment the bone graft fusion [22, 35, 36].
In summary, use of a cervical plate may increase the rate of fusion but is likely to lead to adjacent-segment degeneration. Under the limited conditions of our experiment, the Discover™ artificial disc replacement system at the surgery level was able to produce motion similar to that of the intact spine. The artificial disc may be able to circumvent adjacent-level disease since it mimics the motion of the native spine at the adjacent level. The long-term clinical implications of these findings remain to be seen, although short-term clinical followups do show a decrease in adjacent-segment degeneration after TDA, compared to fusion cases.
Acknowledgments
We thank Christopher Bono (Department of Orthopaedic Surgery, Boston Medical Center, Boston, MA), Steven Garfin (University of California, San Diego, San Diego, CA), Ashok Biyani and Hossein Elgafy (University of Toledo, Toledo, OH), and Hassan Serhan (DePuy Spine, Inc, Raynham, MA) for their guidance in this study.
Footnotes
One or more of the authors (VKG) has received funding from DePuy Spine, Inc, Raynham, MA.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at The University of Toledo.
References
- 1.Aebi M, Zuber K, Marchesi D. Treatment of cervical spine injuries with anterior plating indications, techniques, and results. Spine (Phila Pa 1976). 1991;16(3 suppl):S38–S45. doi: 10.1097/00007632-199103001-00008. [DOI] [PubMed] [Google Scholar]
- 2.Albert TJ, Eichenbaum MD. Goals of cervical disc replacement. Spine J. 2004;4:292S–293S. doi: 10.1016/j.spinee.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Bohler J. Immediate and early treatment of traumatic paraplegias [in German] Z Orthop Ihre Grenzgeb. 1967;103:512–529. [PubMed] [Google Scholar]
- 4.Branch CL., Jr Anterior cervical fusion: the case for fusion without plating. Clin Neurosurg. 1999;45:22–24. [PubMed] [Google Scholar]
- 5.Brodke DS, Gollogly S, Alexander Mohr R, Nguyen BK, Dailey AT, Bachus KN. Dynamic cervical plates: biomechanical evaluation of load sharing and stiffness. Spine (Phila Pa 1976) 2001;26:1324–1329. doi: 10.1097/00007632-200106150-00010. [DOI] [PubMed] [Google Scholar]
- 6.Brodke DS, Gollogly S, Bachus KN, Alexander Mohr R, Nguyen BK. Anterior thoracolumbar instrumentation: stiffness and load sharing characteristics of plate and rod systems. Spine (Phila Pa 1976) 2003;28:1794–1801. doi: 10.1097/01.BRS.0000083201.55495.0E. [DOI] [PubMed] [Google Scholar]
- 7.Bryan VE., Jr Cervical motion segment replacement. Eur Spine J. 2002;11(Suppl 2):S92–S97. doi: 10.1007/s00586-002-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caspar W, Barbier DD, Klara PM. Anterior cervical fusion and Caspar plate stabilization for cervical trauma. Neurosurgery. 1989;25:491–502. doi: 10.1097/00006123-198910000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Caspar W, Geisler FA, Pitzer T, Johnson TA. Anterior cervical plate stabilization in one- and two-level degenerative disease: overtreatment or benefit? J Spinal Disord. 1998;11:1–11. doi: 10.1097/00002517-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Chang UK, Kim DH, Lee MC, Willenberg R, Kim SH, Lim J. Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine. 2007;7:33–39. doi: 10.3171/SPI-07/07/033. [DOI] [PubMed] [Google Scholar]
- 11.Cloward R. Treatment of acute fractures and fracture dislocations of the cervical spine by vertebral body fusion: a report of 11 cases. J Neurosurg. 1961;18:205–209. doi: 10.3171/jns.1961.18.2.0201. [DOI] [PubMed] [Google Scholar]
- 12.Crawford NR, Peles JD, Dickman CA. The spinal lax zone and neutral zone: measurement techniques and parameter comparisons. J Spinal Disord. 1998;11:416–429. doi: 10.1097/00002517-199810000-00009. [DOI] [PubMed] [Google Scholar]
- 13.DiAngelo DJ, Foley KT, Morrow BR, Schwab JS, Song J, German JW, Blair E. In vitro biomechanics of cervical disc arthroplasty with the ProDisc-C total disc implant. Neurosurg Focus. 2004;17:E7. [PubMed] [Google Scholar]
- 14.DiAngelo DJ, Roberston JT, Metcalf NH, McVay BJ, Davis RC. Biomechanical testing of an artificial cervical joint and an anterior cervical plate. J Spinal Disord Tech. 2003;16:314–323. doi: 10.1097/00024720-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Dmitriev AE, Cunningham BW, Hu N, Sell G, Vigna F, McAfee PC. Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine (Phila Pa 1976) 2005;30:1165–1172. doi: 10.1097/01.brs.0000162441.23824.95. [DOI] [PubMed] [Google Scholar]
- 16.Duggal N, Rabin D, Chamberlain RH, Baek S, Crawford NR. Traumatic loading of the Bryan cervical disc prosthesis: an in vitro study. Neurosurgery. 2007;60:388–392; discussion 392–393. [DOI] [PubMed]
- 17.Goel VK, Panjabi MM, Patwardhan AG, Dooris AP, Serhan H. Test protocols for spinal implants. J Bone Joint Surg Am. 2006;88:103–109. doi: 10.2106/JBJS.E.01363. [DOI] [PubMed] [Google Scholar]
- 18.Goffin J, Casey A, Kehr P, Liebig K, Lind B, Logroscino C, Pointillart V, Van Calenbergh F, van Loon J. Preliminary clinical experience with the Bryan Cervical Disc Prosthesis. Neurosurgery. 2002;51:840–845; discussion 845–847. [DOI] [PubMed]
- 19.Gonugunta V, Krishnaney AA, Benzel EC. Anterior cervical plating. Neurol India. 2005;53:424–432. doi: 10.4103/0028-3886.22608. [DOI] [PubMed] [Google Scholar]
- 20.Harrop JS, Youssef JA, Maltenfort M, Vorwald P, Jabbour P, Bono CM, Goldfarb N, Vaccaro AR, Hilibrand AS. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976) 2008;33:1701–1707. doi: 10.1097/BRS.0b013e31817bb956. [DOI] [PubMed] [Google Scholar]
- 21.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser MG, Haid RW, Subach BR, Barnes B, Rodts GE. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50:229–236. doi: 10.1097/00006123-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Kotani Y, Abumi K, Shikinami Y, Takada T, Kadoya K, Shimamoto N, Ito M, Kadosawa T, Fujinaga T, Kaneda K. Artificial intervertebral disc replacement using bioactive three-dimensional fabric: design, development, and preliminary animal study. Spine (Phila Pa 1976). 2002:27;929–935; discussion 935–936. [DOI] [PubMed]
- 24.McAfee PC, Cunningham B, Dmitriev A, Hu N, Woo Kim S, Cappuccino A, Pimenta L. Cervical disc replacement-porous coated motion prosthesis: a comparative biomechanical analysis showing the key role of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2003;28:S176–S185. doi: 10.1097/01.BRS.0000092219.28382.0C. [DOI] [PubMed] [Google Scholar]
- 25.Miura T, Panjabi MM, Cripton PA. A method to simulate in vivo cervical spine kinematics using in vitro compressive preload. Spine (Phila Pa 1976). 2002;27:43–48. doi: 10.1097/00007632-200201010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Moreland DB, Asch HL, Clabeaux DE, Castiglia GJ, Czajka GA, Lewis PJ, Egnatchik JG, Cappuccino A, Huynh L. Anterior cervical discectomy and fusion with implantable titanium cage: initial impressions, patient outcomes and comparison to fusion with allograft. Spine J. 2004;4:184–191. doi: 10.1016/j.spinee.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Nabhan A, Ahlhelm F, Shariat K, Pitzen T, Steimer O, Steudel WI, Pape D. The ProDisc-C prosthesis: clinical and radiological experience 1 year after surgery. Spine (Phila Pa 1976) 2007;32:1935–1941. doi: 10.1097/BRS.0b013e31813162d8. [DOI] [PubMed] [Google Scholar]
- 28.Ordway NR, Fayyazi AH, Abjornson C, Calabrese J, Park S, Fredrickson B, Yonemura K, Yuan HA. Twelve month follow up of lumbar spine range of motion following intervertebral disc replacement using radiostereometric analysis. SAS J. 2008;2:9–15. doi: 10.1016/S1935-9810(08)70012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panjabi MM, Krag M, Summers D, Videman T. Biomechanical time-tolerance of fresh cadaveric human spine specimens. J Orthop Res. 1985;3:292–300. doi: 10.1002/jor.1100030305. [DOI] [PubMed] [Google Scholar]
- 30.Pickett GE, Rouleau JP, Duggal N. Kinematic analysis of the cervical spine following implantation of an artificial cervical disc. Spine (Phila Pa 1976). 2005;30:1949–1954. doi: 10.1097/01.brs.0000176320.82079.ce. [DOI] [PubMed] [Google Scholar]
- 31.Puttlitz CM, Rousseau MA, Xu Z, Hu S, Tay BK, Lotz JC. Intervertebral disc replacement maintains cervical spine kinetics. Spine (Phila Pa 1976) 2004;29:2809–2814. doi: 10.1097/01.brs.0000147739.42354.a9. [DOI] [PubMed] [Google Scholar]
- 32.Rabin D, Pickett GE, Bisnaire L, Duggal N. The kinematics of anterior cervical discetomy and fusion versus artificial disc: a pilot study. Neurosurgery. 2007:61,100–104; discussion 104–105. [DOI] [PubMed]
- 33.Robertson JT, Metcalf NH. Long-term outcome after implantation of the Prestige I disc in an end-stage indication: 4-year results from a pilot study. Neurosurg Focus. 2004;17:E10. doi: 10.3171/foc.2004.17.3.10. [DOI] [PubMed] [Google Scholar]
- 34.Robinson R, Smith G. Anterolateral cervical disk removing and interbody fusion for cervical disk syndrome. Bull Johns Hopkins Hosp. 1955;96:223–224. [Google Scholar]
- 35.Samartzis D, Shen FH, Lyon C, Phillips M, Goldberg EJ, An HS. Does rigid instrumentation increase the fusion rate in one-level anterior cervical discectomy and fusion? Spine J. 2004;4:636–643. doi: 10.1016/j.spinee.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Wang JC, McDonough PW, Endow KK, Kanim LE, Delamarter RB. The effect of cervical plating on single-level anterior cervical discectomy and fusion. J Spinal Disord. 1999;12:467–471. doi: 10.1097/00002517-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Yang S, Wu X, Hu Y, Li J, Liu G, Xu W, Yang C, Ye S. Early and intermediate follow-up results after treatment of degenerative disc disease with the Bryan cervical disc prosthesis: single- and multiple-level. Spine (Phila Pa 1976) 2008;33:E371–E377. doi: 10.1097/BRS.0b013e31817343a6. [DOI] [PubMed] [Google Scholar]