Abstract
Background
Unplanned excision of a soft tissue sarcoma generally requires reexcision to achieve an adequate surgical margin. Many surgeons assume delay of definitive surgery adversely affects patient survival and local recurrence. However, no clear evidence of this assumption can be found in the literature.
Questions/purposes
We asked whether delay in reexcision affects patient survival and local recurrence in reexcision after unplanned excision for soft tissue sarcoma.
Patients and Methods
We retrospectively reviewed 104 patients who underwent definitive surgery after unplanned excision of a localized soft tissue sarcoma. The average age of the patients was 44 years (range, 5–81 years). The most common diagnoses were malignant fibrous histiocytoma (36) and synovial sarcoma (22). Locations of the tumors were the lower extremity (62), upper extremity (32), and trunk (10). The median interval to definitive surgery was 32 days (interquartile range, 22–50 days). The minimum followup was 0.2 years (median, 4.7 years; range, 0.2–16.7 years).
Results
The 5-year disease-specific survival was 88% and 5-year local control rate was 74%. We found no difference in disease-specific survival or local recurrence according to the time until definitive surgery. Higher histologic grade and larger tumor size independently predicted disease-specific survival whereas a positive margin at reexcision and larger tumor size independently predicted local control.
Conclusions
The data suggest any influence of delayed definitive surgery is likely to be of minor clinical importance.
Level of Evidence
Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Soft tissue sarcomas (STS) often are mistaken for benign tumors and initial resection is performed as though they were benign tumors. Unplanned excision of a STS is defined as the operation performed for gross removal of a STS without regard for preoperative imaging or the necessity to remove a margin of normal tissue covering the cancer [5, 15]. It is widely accepted reexcision usually is necessary to remove the possible residual tumor or to obtain an adequate margin of resection [2, 4, 11].
Braunschweiger suggested delay of definitive surgery after unplanned excision of STS results in adverse clinical outcome by allowing remaining cancer cells to proliferate [1]. However, no clear evidence on this presumption can be found in the literature [4]. Although urgent reexcision logically is appropriate for unplanned excision of a STS, an arbitrary time window for treatment to minimize the risk of local recurrence or increase survival cannot be justified based on the existing medical evidence. An examination of the effect of the interval to definitive surgery on disease-specific survival and local recurrence may have practical relevance in this clinical setting.
We therefore asked whether delayed reexcision would have an adverse effect on (1) disease-specific survival and (2) local recurrence in patients with unplanned excision of a STS.
Patients and Methods
We retrospectively reviewed 114 patients referred to our hospital after unplanned excision at other hospitals for treatment of a STS, and who subsequently underwent reexcision between September 1985 and September 2007. Based on the policy of our hospital, all patients treated at our hospital for a STS after unplanned excision, underwent surgical reexcision. Unplanned excision was defined as removal of a STS before the correct histologic diagnosis was made, without regard for the necessity to remove a margin of normal tissue covering the cancer. Histologic diagnosis of STS was made for all patients before referral to our hospital, all of whom were advised to undergo reexcision as soon as possible. The diagnosis of STS was confirmed by a review of the histologic material at our hospital. Histologic diagnoses of well-differentiated liposarcoma or dermatofibrosarcoma protuberance, which show inherently good prognoses, were not included for review in this study. Of the 114 patients, we excluded 10: five who had preoperative radiotherapy or chemotherapy before reexcision, three who presented with metastatic disease, and two who had less than 2 years followup among the patients who were event-free. This left 104 patients for analysis. There were 61 males and 43 females with a median age of 44 years (range, 5–81 years) at the time of referral. Sixty-two patients (60%) had tumors in their lower limb, 32 (31%) had tumors in their upper limb, and 10 (9%) had tumors in their back. Minimum followup was 0.2 years (median, 4.7 years; range, 0.2–16.7 years). No patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. Our institutional review board approved this study.
The most common histologic subtypes were malignant fibrous histiocytoma (35%), synovial sarcoma (21%), liposarcoma (13%), fibrosarcoma (8%), and leiomyosarcoma (6%). Histologic grading was performed using the Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) differentiation score [16], as proper evaluation for complete histologic grading was not possible in the majority of cases. Mitosis count and presence of necrosis could not be evaluated owing to violation of the original tumor by the unplanned excision, improper processing, or unavailability of the tumor specimen. There were six Score 1 tumors (6%), 54 Score 2 tumors (52%), and 44 Score 3 tumors (42%).
One of us (IH) determined tumor size by reviewing the pathology reports or radiographs of the unplanned excisions. The tumor size was defined as the largest diameter described in the pathology reports or the largest diameter measured on the radiographs. Information regarding tumor size was available for 72 patients (69%), and the median tumor size was 3.2 cm (range, 1.0–12.0 cm). Tumor depth was determined from the radiographic material or operative records of the unplanned excisions. Tumor depth was graded as superficial or deep; tumors located exclusively above the superficial fascia without invasion of the fascia were defined as superficial [6]. Tumor depth could be determined for 96 patients (92%), with 52 superficial and 44 deep tumors.
All patients included in the study underwent reexcision to achieve wide resection margins, with seven patients (6%) requiring amputation of their affected limb. Amputation was performed when wide surgical margins could not be obtained with limb-salvage surgery. The reexcised specimen was examined to determine the presence of residual tumor, and residual tumor was found in 53 patients (51%). Histologically negative surgical margins were achieved in 101 patients (97%). Radiation therapy was administered postoperatively when higher risk of local recurrence was thought to exist based on clinical information, such as histologic grade, tumor size, histologic subtype, and surgical margin. However, no prospectively selected criteria were used for patient selection. Forty-four patients (42%) received radiation therapy. All received external beam radiation and the median dose was 60 Gy (range, 50–65 Gy). Sixteen patients (15%) received postoperative chemotherapy.
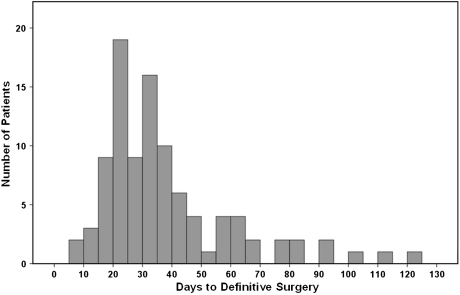
The median interval between unplanned excision to reexcision ranged from 7 to 300 days (median, 32 days; interquartile range, 22–50 days) (Fig. 1). To study the effect of the interval from unplanned excision to definitive surgery on study end points, patients were divided into two groups or four groups according to the median value (≤ 32 days, > 33 days) or interquartile values (≤ 21 days, 22–32 days, 33–50 days, > 50 days), respectively, to have a similar number of patients in each group. The short-interval group (≤ 32 days; n = 52) and the long-interval group (> 33 days; n = 52) had similar patient and disease characteristics, except for the greater proportion of older patients in the long-interval group and the higher proportion of patients receiving postoperative radiation therapy in the short-interval group (Table 1). Flaps or skin grafts for soft tissue coverage were performed with equal frequency (p = 0.250) in the short- and long-interval groups: 12 of 52 patients (23%) versus 17 of 52 patients (32%), respectively.
Fig. 1.
A histogram shows the distribution of patients according to the time from unplanned excision to definitive surgery. The median value was 32 days.
Table 1.
Patient and disease characteristics according to the interval to definitive surgery
| Variable | Number of patients | p Value | |
|---|---|---|---|
| Short interval | Long interval | ||
| Age | 0.035 | ||
| ≤ 60 years | 45 (86%) | 35 (67%) | |
| > 60 years | 7 (14%) | 17 (33%) | |
| Gender | 0.691 | ||
| Male | 29 (56%) | 32 (61%) | |
| Female | 23 (44%) | 20 (39%) | |
| Site | 0.685 | ||
| Upper extremity | 14 (27%) | 18 (35%) | |
| Lower extremity | 33 (64%) | 29 (56%) | |
| Trunk | 5 (9%) | 5 (9%) | |
| Size | 0.500 | ||
| ≤ 3 cm | 20 (51%) | 16 (49%) | |
| > 3 cm | 19 (49%) | 17 (52%) | |
| Depth | 0.500 | ||
| Superficial | 26 (54%) | 26 (54%) | |
| Deep | 22 (46%) | 22 (46%) | |
| Histologic type | |||
| Malignant fibrous histiocytoma | 20 (39%) | 16 (30%) | |
| Synovial sarcoma | 11 (21%) | 11 (21%) | |
| Liposarcoma | 5 (10%) | 8 (15%) | |
| Fibrosarcoma | 4 (8%) | 4 (8%) | |
| Leiomyosarcoma | 2 (4%) | 4 (85) | |
| Malignant peripheral nerve sheath tumor | 2 (4%) | 1 (2%) | |
| Others | 8 (16%) | 7 (16%) | |
| FNCLCC differentiation score | 0.428 | ||
| 1 | 4 (8%) | 2 (4%) | |
| 2 | 24 (46%) | 30 (57%) | |
| 3 | 24 (46%) | 20 (38%) | |
| Surgical margin | 0.500 | ||
| Negative | 51 (98%) | 50 (96%) | |
| Positive | 1 (2%) | 2 (4%) | |
| Residual tumor | 0.165 | ||
| No | 29 (57%) | 21 (41%) | |
| Yes | 22 (43%) | 30 (59%) | |
| Postoperative radiotherapy | 0.074 | ||
| Not done | 25 (48%) | 34 (67%) | |
| Done | 27 (52%) | 17 (33%) | |
| Postoperative CT | 0.416 | ||
| Not done | 42 (81%) | 46 (88%) | |
| Done | 10 (19%) | 6 (12%) | |
| Followup (years)* | 6.1 ± 4.1 | 6.1 ± 4.3 | 0.974 |
| Total | 52 (100%) | 52 (100%) | |
* Values are expressed as mean ± SD; FNCLCC = Federation Nationale des Centres de Lutte Contre le Cancer.
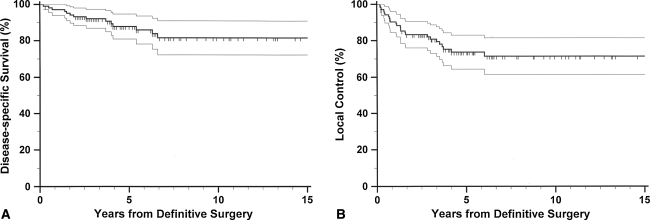
The primary end points of this study were disease-specific death and local recurrence. The time to the occurrence of the event was calculated from the date of definitive surgery to the date when the event was first recorded. Actuarial survival curves were constructed using the Kaplan-Meier method [9]. Deaths confirmed to be caused by the disease were treated as an end point for disease-specific survival and deaths from other causes were regarded as censored observations. Local recurrence was defined as the first recurrence of disease at the site of the primary tumor, occurring after at least 3 months after definitive surgery. Patients who were event-free were considered as censored at the last followup. At the time of analysis, 88 patients were alive and their minimum followup was 2.0 years (mean, 6.6 years; range, 2.0–16.8 years). Fourteen patients died of causes related to the STS and two patients died of other causes. The actuarial 5-year and 10-year disease-specific survival rates were 88% and 81%, respectively (Fig. 2). The disease had recurred locally in 25 patients, and actuarial 5-year and 10-year local control rates were 74% and 71%, respectively (Fig. 2).
Fig. 2A–B.
Kaplan-Meier curves for (A) disease-specific survival and (B) local control of all study patients are shown. The thin lines represent the 95% confidence intervals for the probability rates.
Power analysis showed a sample size of 52 patients in each group would provide an 81% power to detect a difference in survival outcomes based on the log-rank test, assuming a constant hazard ratio of 2.0 during followup and two-sided alpha of 0.05. This means the study had an 81% chance of detecting a twofold increase in the risk of disease-specific death between the two groups.
Differences between survival curves were evaluated using log-rank testing for univariable influence and Cox stepwise regression for multivariable influence. In all analyses, all covariates were modeled as categorical variables. Comparison of the categorical variables in different groups was performed using Fisher’s exact test. Statistical analyses were performed using SPSS® software (Version 17.0; SPSS Inc, Chicago, IL, USA).
Results
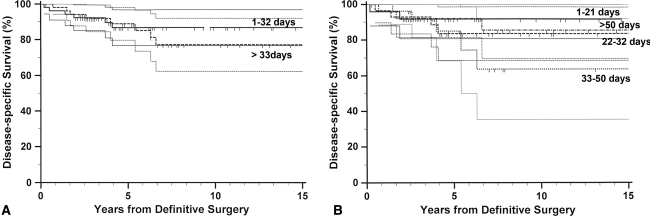
For disease-specific survival, we observed no differences regarding the incidence of disease-specific death between the short- and long-interval groups (Table 2). This lack of difference remained when analyzed in four groups. There was no difference in the actuarial survival curves between the two groups. Further analysis by grouping the patients into four groups revealed no difference in disease-specific survival (Fig. 3). High FNCLCC differentiation score, older age, and larger tumor size independently predicted worse survival (Table 3). No difference in disease-specific survival between the short- and long-interval groups was observed in the subset of patients with worse clinicopathologic factors: higher FNCLCC differentiation score (p = 0.771), older age (p = 0.64), and larger tumor size (p = 0.988).
Table 2.
Interval from unplanned excision to definitive surgery and clinical outcome
| Time to definitive surgery | Number of patients | p Value | |
|---|---|---|---|
| Disease-specific death | Local recurrence | ||
| Short interval (≤ 32 days) | 6 (11.5%) | 11 (21.2%) | 0.775 |
| Long interval (> 33 days) | 8 (15%) | 14 (26.9%) | 0.647 |
Fig. 3A–B.
Kaplan-Meier curves for disease-specific survival according to the interval from unplanned excision to definitive surgery are shown. No difference in survival was observed between (A) the two groups divided by the median value (p = 0.603) or (B) the four groups divided by the interquartile values (p = 0.646). The thin lines represent the 95% confidence intervals for the probability rates.
Table 3.
Multivariate analysis for factors associated with disease-specific survival
| Factor | Disease-specific survival | ||
|---|---|---|---|
| Relative risk | 95% confidence interval | p Value | |
| FNCLCC differentiation score | 0.001 | ||
| 1 or 2 | 1 | ||
| 3 | 11.3 | 2.7–48.0 | |
| Age | 0.002 | ||
| ≤ 60 years | 1 | ||
| > 60 years | 7.5 | 2.1–27.3 | |
| Tumor size | 0.026 | ||
| ≤ 3 cm | 1 | ||
| > 3 cm | 4.4 | 1.2–16.5 | |
FNCLCC = Federation Nationale des Centres de Lutte Contre le Cancer.
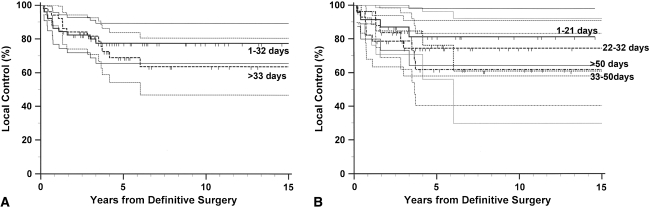
We observed no differences regarding the incidence of local recurrence between the short- and long-interval groups (Table 2). This lack of difference remained when the patients were analyzed in four groups. We also observed no difference in the actuarial survival curves between the two groups. Further analysis by grouping the patients into four groups revealed no difference in local recurrence (Fig. 4). Positive surgical margin and larger tumor size independently predicted higher local recurrence rate (Table 4). No difference in local recurrence between the short- and long-interval groups was observed in the subset of patients with worse clinicopathologic factors: positive surgical margin (p = 0.157) and larger tumor size (p = 0.361).
Fig. 4.
Kaplan-Meier curves for local control according to the interval from unplanned excision to definitive surgery are shown. No differences in local recurrence were observed between (A) the two groups divided by the median value (p = 0.492) or (B) the four groups divided by the interquartile values (p = 0.751). The thin lines represent the 95% confidence intervals for the probability rates.
Table 4.
Multivariate analysis for factors associated with local control
| Factor | Local recurrence | ||
|---|---|---|---|
| Relative risk | 95% confidence interval | p Value | |
| Surgical margin | 0.003 | ||
| Negative | 1 | ||
| Positive | 13.4 | 2.5–72.2 | |
| Tumor size | 0.013 | ||
| ≤ 3 cm | 1 | ||
| > 3 cm | 6.5 | 1.5–28.5 | |
Discussion
Although there is a general assumption that delay of reexcision after unplanned excision of a STS is not in the best interest of patients, as far as we know, few published studies exist analyzing this issue. We conducted this study to see whether the delay in reexcision adversely affects (1) disease-specific survival and (2) local recurrence in patients with unplanned excision of a STS.
We acknowledge limitations of our study. First, we had a relatively small number of patients compared with other studies [2, 12]. This raises the question of Type II errors given we found no differences in the groups. However, power analysis showed this study had an 81% power to detect a twofold increase in the risk of disease-specific death. Second, although the patients’ characteristics in the two different interval groups were relatively well matched, we had two notable differences: a greater number of older patients in the long-interval group and fewer patients receiving postoperative radiation therapy in the long-interval group. However, we do not believe these differences would influence the observations because the older age of patients and the lack of postoperative radiation therapy would not work in favor for the long-interval group. Third, the range of time from unplanned excision to definitive surgery was relatively short, as 92% of the patients in this study underwent reexcision in 120 days. This period might have been too short for the delay to have any detrimental effect on disease-specific survival and local recurrence, as some of the eventual STSs may not be recognized as a sarcoma for more than 120 days. However, in a study in which the median interval was 66 days, which is almost double that in our study (32 days), no effect of time to definitive surgery was reported [4].
Undue delay of a definitive operation theoretically would allow proliferation of remaining tumor cells thus increasing the likelihood of local recurrence and disease-related death [7]. Our observations, however, are at odds with this presumption. The delayed definitive surgery might have a prominent adverse impact for patients with worse clinicopathologic factors, such as higher histologic grades and larger tumor size for disease-specific survival and positive resection margin and presence of residual disease for local recurrence. Further analyses of these subsets of patients confirmed no differences in the time to definitive surgery in disease-specific survival and local recurrence.
The reasons for varying intervals to definitive surgery were difficult to determine for most patients. The policy at our hospital has been to recommend that patients undergo reexcision as soon as possible, possibly within 21 days after the unplanned excision. We presume the patients’ delay was one of the main reasons for delay [3, 8].
Although it would be prudent not to delay reexcision in most cases, based on the results of this study, delay of reexcision might be a feasible option in selected cases such as patients with wound-healing problems. It might be beneficial to allow better wound healing from the initial procedure to occur thereby lessening the risk of wound complications after the reexcision. Also, the delayed reexcision might allow a more defined fibroblastic scar to form, providing a better margin by capturing the tumor cells and thus allowing reexcision to provide better local control.
The data for well-established prognostic factors of a STS, such as tumor size or histologic grading, can be limited in an unplanned excision setting, as proper radiographic or histologic evaluations often are not performed at the time of initial surgery [14]. Data regarding tumor size and histologic grading were limited in this study, although the known prognostic factors for a STS remained significant [10, 13].
Synovial sarcomas were more common than liposarcomas in our series compared with other studies [7, 12, 17]. This might be attributable to two reasons. First, synovial sarcomas often present as small superficial soft tissue masses, which are prone to undergo unplanned excision. This characteristic of a synovial sarcoma might have contributed to its high incidence in our series. Second, low-grade liposarcomas were excluded from our series, which would have lowered the incidence of liposarcomas.
We evaluated the effect of the time until definitive surgery on disease-specific survival and local recurrence for patients who had an unplanned excision of a STS. Our findings suggest no definable adverse effect on disease-specific survival and local recurrence is associated with delay in the definitive operation. Our data suggest any influence of a delayed definitive operation, within the limitations of this study, is likely to be of minor clinical importance.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
One or more of the authors (HSK) have received funding from the Seoul National University Hospital Fund (Number 04-2008-042).
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This study was performed at the Seoul National University College of Medicine.
References
- 1.Braunschweiger P. Proliferative and vascular responses to cytoreduction in solid tumor models. In: Ragaz J, Simpson-Herren L, Lippman M, eds. Effects of Therapy on Biology and Kinetics of the Residual Tumor, Part A: Pre-clinical Aspects. New York, NY: Wiley-Liss; 1990:31–46. [PubMed]
- 2.Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br. 2008;90:203–208. doi: 10.1302/0301-620X.90B2.19760. [DOI] [PubMed] [Google Scholar]
- 3.Clark MA, Thomas JM. Delay in referral to a specialist soft-tissue sarcoma unit. Eur J Surg Oncol. 2005;31:443–448. doi: 10.1016/j.ejso.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Fiore M, Casali PG, Miceli R, Mariani L, Bertulli R, Lozza L, Collini P, Olmi P, Mussi C, Gronchi A. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006;13:110–117. doi: 10.1245/ASO.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano AE, Eilber FR. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J Clin Oncol. 1985;3:1344–1348. doi: 10.1200/JCO.1985.3.10.1344. [DOI] [PubMed] [Google Scholar]
- 6.Greene FL. American Joint Committee on Cancer, American Cancer Society. AJCC Cancer Staging Manual. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 7.Gronchi A, Miceli R, Fiore M, Collini P, Lozza L, Grosso F, Mariani L, Casali PG. Extremity soft tissue sarcoma: adding to the prognostic meaning of local failure. Ann Surg Oncol. 2007;14:1583–1590. doi: 10.1245/s10434-006-9325-0. [DOI] [PubMed] [Google Scholar]
- 8.Johnson GD, Smith G, Dramis A, Grimer RJ. Delays in referral of soft tissue sarcomas. Sarcoma. 2008; doi:10.1155/2008/378574. [DOI] [PMC free article] [PubMed]
- 9.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 10.Kotilingam D, Lev DC, Lazar AJ, Pollock RE. Staging soft tissue sarcoma: evolution and change. CA Cancer J Clin. 2006;56:282–291; quiz 314–315. [DOI] [PubMed]
- 11.Lewis JJ, Leung D, Espat J, Woodruff JM, Brennan MF. Effect of reresection in extremity soft tissue sarcoma. Ann Surg. 2000;231:655–663. doi: 10.1097/00000658-200005000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noria S, Davis A, Kandel R, Levesque J, O’Sullivan B, Wunder J, Bell R. Residual disease following unplanned excision of a soft-tissue sarcoma of an extremity. J Bone Joint Surg Am. 1996;78:650–655. doi: 10.2106/00004623-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1, 041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 14.Siebenrock KA, Hertel R, Ganz R. Unexpected resection of soft-tissue sarcoma: more mutilating surgery, higher local recurrence rates, and obscure prognosis as consequences of improper surgery. Arch Orthop Trauma Surg. 2000;120:65–69. [PubMed] [Google Scholar]
- 15.Springfield DS, Rosenberg A. Biopsy: complicated and risky. J Bone Joint Surg Am. 1996;78:639–643. [PubMed] [Google Scholar]
- 16.Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, Mascarel A, Goussot JF, David M, Bonichon F, Lagarde C. Soft-tissue sarcomas of adults: study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42. doi: 10.1002/ijc.2910330108. [DOI] [PubMed] [Google Scholar]
- 17.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS, Evans HL. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97:2530–2543. doi: 10.1002/cncr.11365. [DOI] [PubMed] [Google Scholar]