Abstract
Background
Percutaneous vertebroplasty is currently an alternative for treating vertebral fractures of the thoracic and lumbar spine, providing both pain control and vertebral stabilization. In the cervical spine, however, percutaneous vertebroplasty is technically challenging because of the complex anatomy of this region.
Questions/purposes
We evaluated the technical feasibility, complication rate, and ability of percutaneous vertebroplasty to provide pain relief in patients with painful metastatic cervical fractures.
Methods
We retrospectively reviewed 62 patients (24 men) who, between May 2005 and May 2009, underwent vertebroplasty to treat painful metastatic cervical fractures. Each patient was evaluated by a visual analog scale for pain, number of pain analgesics, and CT and MRI before, the day after, and at 3 months after the procedure.
Results
Two of the 62 patients had asymptomatic cement leakage in the soft tissues. We observed no delayed complications. Mean pretreatment and 24-hour posttreatment visual analog scale pain scores were 7.9 ± 1.7 and 1.5 ± 2, respectively. Immediately after surgery, the pain completely disappeared in 25 (40%) patients. Administration of analgesics was suspended in 34 (55%) patients whereas in 27 (39%) patients the median analgesics use decreased from two pills per day (range, 0–3) to 0 (range, 0–3). In two (3%) patients, analgesics administration was continued due to the persistence of pain. At 3 months, the patients reported a mean visual analog scale pain score of 1.7 ± 2.
Conclusions
Our data suggest, in selected patients, percutaneous vertebroplasty may be performed with a high technical success rate combined with a low complication rate, providing immediate pain relief lasting at least 3 months and a reduction in the use of analgesic drugs.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Bone is one of the most frequent sites of metastasis and the spine is the most common of painful skeletal sites [25]. Spinal metastases are found in more than 2/3 of patients who die of cancer [15]. Vertebrae are therefore frequently affected by pathologic fractures, in 10% to 20% of cases involving the posterior wall of the vertebral body [16], which can protrude posteriorly and cause spinal canal compromise and neurologic injury [16]. Thoracic vertebrae are the most common sites of disease (60%–80%), followed by lumbar (20%) and cervical spine (10%) [15].
Harrington [16] classified patients with spinal metastases into the following five categories, according to the extent of neurologic compromise and bone destruction: Class 1, no major neurologic involvement; Class 2, involvement of bone without collapse or instability; Class 3, major neurologic impairment (sensory or motor) without major involvement of bone; Class 4, vertebral collapse with pain due to mechanical causes or instability but without major neurologic compromise; and Class 5, vertebral collapse or instability combined with major neurologic impairment. Treatment is palliative and aims to relieve pain, prevent development of any pathologic fracture, improve mobility and function, and, if possible, prolong survival [17, 29]. The initial therapeutic option (Classes 1 and 2) is a nonoperative treatment based on rest, bracing, chemotherapy/hormonal treatment, and analgesics, but in the presence of refractory pain, spinal instability or neurologic deficit from cord compression (Classes 4 and 5), surgical stabilization is necessary. Patients in Class 3 are initially treated with radiotherapy alone, but similar to those in Classes 4 and 5, they do not always respond [27]. Furthermore, life expectancy is an important factor to justify surgical treatment, and generally, indications include a life expectancy of greater than 6 months and isolated metastasis [3, 13, 18, 20]. Radiation therapy provides local pain control in a high percentage of cases and works best if combined with chemotherapy. The aim of radiotherapy for patients with a short life expectancy may be different from that for those who have a better prognosis and require not only pain relief but also spinal function [27]. Since its efficacy is dose-dependent, to obtain longer-lasting results, a high dose of irradiation may be required and the adverse effects of radiation therapy (eg, radiation myelitis) must be considered. Furthermore, the pain-relieving effect of radiation therapy is gradual and local response rates after repeated radiotherapy due to recurrent pain symptoms at the same port drastically decrease after the first course.
Few patients with neoplastic vertebral fractures are surgical candidates; therefore, current treatments are aimed at pain palliation and prevention of complications [25]. Standard treatment includes analgesics, mainly opioids, and radiation therapy. However, more than 20% to 30% of patients are nonresponsive [17, 29], and in this case, patients are exposed to a number of deleterious consequences, such as impairment of function and quality of life, decreased mobility, and depression [26]. Percutaneous vertebroplasty (PVP) is currently considered a reasonable alternative for treating vertebral fractures of the thoracic and lumbar spine since polymethylmethacrylate (PMMA) injection inside the vertebral body provides both pain control in approximately 97% of patients and vertebral stabilization [8, 9].
However, in the cervical spine, PVP is technically challenging because of the potential complications related to the complex anatomy of this region (spinal cord, jugular vein, cranial nerves, carotid artery, and vertebral artery). Furthermore, in the case of osteolytic lesions, the risk of cement leakage is increased, which, when involving the spinal canal, may compromise the spinal cord or nerve roots, leading to severe neurologic deficit. At present, cervical spine percutaneous vertebroplasty has been only anecdotally reported to treat lesions in selected patients with cervical lesions (Cortet et al. [8] reported only five cases in the cervical spine), and since most cases have been in the thoracic and lumbar spines it is not known whether the procedure is technically feasible with a low complication rate and whether it reliability relieves pain in patients with cervical spine metastasis.
We therefore evaluated (1) the technical feasibility considering the complications rate; and (2) the ability of PVP to reduce pain and medication usage in cervical fractures due to metastases.
Patients and Methods
We retrospectively analyzed data from 62 patients (24 men, 38 women) who underwent PVP to treat painful cervical fractures from metastatic disease between May 2005 and May 2009 (Table 1). Primary tumors included breast cancer (16), myeloma (11), lung cancer (10), gastric cancer (seven), and other tumors (19). The indication for PVP for each patient was determined by an interdisciplinary consult that included an orthopaedist, an oncologist, a neurosurgeon, and a radiation therapist. All patients were evaluated before the procedure with a multidisciplinary approach that included a thorough interview to obtain the patient’s medical history and a physical examination. Furthermore, diagnostic imaging examinations, CT and MRI, were performed to evaluate the presence of cortical breakthrough, the eventual extravertebral tumor extension, and the type of lesion and to quantify the degree of collapse. Patients were eligible for PVP if they met the following criteria: (1) vertebral fracture resulting from osteolytic metastasis at the cervical spine with integrity of the posterior vertebral wall as shown from CT scan and MRI; (2) severe debilitating pain or loss of mobility that could not be relieved by appropriate oncologic or medical therapy; (3) failed radiation therapy or chemotherapy with a life expectancy of between 3 and 6 months; (4) and/or the presence of comorbidities contraindicating surgery, as evaluated by the oncologists of the multidisciplinary team [19, 22, 23]. Contraindications included noncorrectable coagulation disorders (international normalized ratio > 1.5, platelets < 90.000) and an ongoing systemic infection. The median age of the population was 61.5 years (range, 31–85 years). The cervical fractures were localized in C1 (n = 3), C2 (n = 32), C3 (n = 3), C4 (n = 11), C5 (n = 15), C6 (n = 4), and C7 (n = 2), with a total of 70 vertebrae treated. Each patient was evaluated before, the day after, and at 3 months after the procedure. No patients were recalled specifically for this study; all data were obtained from medical records. All patients gave their informed consent for participation in the study.
Table 1.
Patient demographics and results
| Patient | Gender | Age (years) | Treated levels | Approach | Visual analog scale pain score (0–10) | Analgesics (pills per day) | |||
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | 3 months | Preoperative | Postoperative | |||||
| 1 | Female | 62 | 1 | Transoral | 10 | 9 | 9 | 3 | 2 |
| 2 | Female | 54 | 2 | Transoral | 10 | 10 | 10 | 3 | 3 |
| 3 | Female | 68 | 2 | Transoral | 5 | 0 | 0 | 1 | 0 |
| 4 | Female | 70 | 2 | Monolateral | 9 | 1 | 1 | 1 | 0 |
| 5 | Female | 72 | 1 | Transoral | 9 | 1 | 2 | 3 | 1 |
| 6 | Male | 64 | 1 | Transoral | 9 | 2 | 2 | 3 | 1 |
| 7 | Female | 41 | 1 | Transoral | 7 | 0 | 1 | 3 | 0 |
| 8 | Male | 31 | 1 | Transoral | 10 | 5 | 5 | 2 | 1 |
| 9 | Male | 79 | 1 | Transoral | 10 | 4 | 5 | 3 | 1 |
| 10 | Male | 63 | 1 | Transoral | 10 | 3 | 3 | 3 | 1 |
| 11 | Female | 54 | 1 | Transoral | 6 | 0 | 0 | 1 | 0 |
| 12 | Male | 46 | 1 | Transoral | 6 | 0 | 0 | 2 | 0 |
| 13 | Female | 64 | 1 | Transoral | 8 | 0 | 0 | 1 | 0 |
| 14 | Female | 58 | 1 | Transoral | 6 | 0 | 0 | 1 | 0 |
| 15 | Female | 80 | 1 | Transoral | 10 | 2 | 2 | 3 | 1 |
| 16 | Male | 47 | 1 | Transoral | 6 | 0 | 0 | 1 | 0 |
| 17 | Male | 51 | 1 | Transoral | 6 | 0 | 1 | 1 | 0 |
| 18 | Female | 75 | 2 | Transoral | 4 | 0 | 0 | 1 | 0 |
| 19 | Female | 51 | 1 | Transoral | 7 | 1 | 1 | 3 | 1 |
| 20 | Female | 61 | 1 | Monolateral | 4 | 4 | 4 | 3 | 3 |
| 21 | Female | 49 | 1 | Monolateral | 8 | 1 | 1 | 1 | 0 |
| 22 | Female | 71 | 1 | Monolateral | 9 | 1 | 2 | 1 | 0 |
| 23 | Female | 55 | 1 | Monolateral | 6 | 0 | 0 | 1 | 0 |
| 24 | Female | 53 | 1 | Monolateral | 8 | 1 | 1 | 3 | 1 |
| 25 | Female | 80 | 1 | Monolateral | 7 | 1 | 1 | 1 | 0 |
| 26 | Female | 67 | 1 | Monolateral | 7 | 3 | 3 | 3 | 1 |
| 27 | Male | 72 | 2 | Monolateral | 10 | 2 | 3 | 3 | 1 |
| 28 | Female | 50 | 2 | Monolateral | 10 | 5 | 5 | 3 | 2 |
| 29 | Female | 85 | 2 | Monolateral | 9 | 4 | 4 | 2 | 1 |
| 30 | Male | 50 | 2 | Monolateral | 5 | 0 | 0 | 3 | 1 |
| 31 | Male | 54 | 1 | Monolateral | 4 | 1 | 1 | 2 | 1 |
| 32 | Male | 65 | 1 | Monolateral | 9 | 3 | 3 | 3 | 1 |
| 33 | Male | 51 | 1 | Monolateral | 8 | 2 | 2 | 3 | 0 |
| 34 | Male | 38 | 1 | Monolateral | 9 | 0 | 0 | 2 | 0 |
| 35 | Male | 53 | 1 | Monolateral | 9 | 2 | 2 | 3 | 1 |
| 36 | Male | 64 | 1 | Transoral | 7 | 0 | 1 | 1 | 0 |
| 37 | Female | 72 | 1 | Monolateral | 8 | 0 | 0 | 2 | 0 |
| 38 | Female | 63 | 1 | Monolateral | 9 | 1 | 1 | 3 | 0 |
| 39 | Female | 59 | 1 | Transoral | 9 | 1 | 1 | 3 | 1 |
| 40 | Male | 47 | 1 | Monolateral | 10 | 2 | 3 | 3 | 1 |
| 41 | Male | 65 | 1 | Transoral | 6 | 0 | 0 | 1 | 0 |
| 42 | Female | 82 | 1 | Monolateral | 9 | 0 | 0 | 3 | 0 |
| 43 | Female | 54 | 1 | Transoral | 8 | 3 | 3 | 3 | 1 |
| 44 | Male | 76 | 1 | Monolateral | 8 | 0 | 0 | 1 | 0 |
| 45 | Male | 64 | 1 | Transoral | 9 | 1 | 1 | 3 | 1 |
| 46 | Male | 57 | 1 | Transoral | 6 | 0 | 1 | 1 | 0 |
| 47 | Female | 62 | 1 | Monolateral | 7 | 0 | 0 | 3 | 0 |
| 48 | Female | 49 | 1 | Transoral | 10 | 2 | 3 | 3 | 1 |
| 49 | Male | 53 | 1 | Monolateral | 8 | 0 | 0 | 2 | 0 |
| 50 | Female | 55 | 1 | Transoral | 10 | 4 | 4 | 3 | 2 |
| 51 | Male | 68 | 1 | Transoral | 6 | 0 | 0 | 1 | 0 |
| 52 | Female | 63 | 1 | Transoral | 10 | 4 | 4 | 3 | 1 |
| 53 | Female | 65 | 1 | Monolateral | 8 | 0 | 0 | 3 | 0 |
| 54 | Male | 59 | 1 | Transoral | 7 | 0 | 0 | 1 | 0 |
| 55 | Female | 64 | 1 | Monolateral | 7 | 0 | 0 | 1 | 0 |
| 56 | Female | 57 | 1 | Transoral | 9 | 1 | 1 | 1 | 0 |
| 57 | Female | 78 | 1 | Transoral | 10 | 3 | 3 | 3 | 1 |
| 58 | Female | 81 | 1 | Monolateral | 9 | 2 | 3 | 1 | 0 |
| 59 | Female | 59 | 1 | Transoral | 8 | 0 | 1 | 1 | 0 |
| 60 | Female | 61 | 1 | Monolateral | 6 | 1 | 1 | 1 | 0 |
| 61 | Female | 64 | 1 | Transoral | 9 | 1 | 1 | 3 | 1 |
| 62 | Male | 52 | 1 | Monolateral | 9 | 1 | 1 | 3 | 0 |
To minimize the risk of bleeding, anticoagulation/antiplatelet therapy was discontinued before the procedure.
The procedures were performed with the patient in supine position under general anesthesia. In addition, a premedication with intravenous antibiotic therapy (cefazolin 2 g) was administered before treatment and was continued for 3 to 5 days later. For C1 and C2 fractures (n = 35), a transoral approach was used while, for the others cervical sites, a monolateral approach (either anterolateral or posterolateral) was used [13].
In the transoral approach, the needle was advanced across the previously disinfected oropharynx, between the depressed tongue and the uvula toward the posterior pharyngeal wall through which the target lesion was punctured. Transoral and anterolateral approaches were performed either under CT guidance (Fig. 1) or under biplanar (AP and left lateral) fluoroscopic guidance (Fig. 2). Patients were studied before the procedure via CT and/or MRI (Fig. 3) [12, 21, 28, 30]. In the anterolateral approach, a moderate hyperextension of the neck was required and the needle was advanced below the angle of the mandible in an oblique direction aiming at the anterior wall of the target vertebra (Fig. 4) [14]. In the posterolateral approach, the needle was advanced, as in the PVP of the lumbar and thoracic spine, through the posterolateral tissues and the pedicle, into the vertebral body. Once the center of the vertebral body was reached, cement (PMMA) was injected. The median injected PMMA volume was 2.5 mL (range, 1–4 mL).
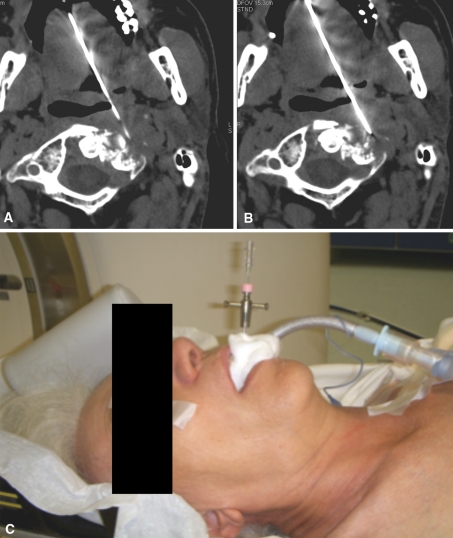
Fig. 1A–C.
The transoral approach under CT guidance for the biopsy of an osteolytic C1 metastasis is demonstrated. CT image showing the needle approaching (A) and entering (B) the lesion. (C) An intraoperative photograph illustrates the transoral approach.
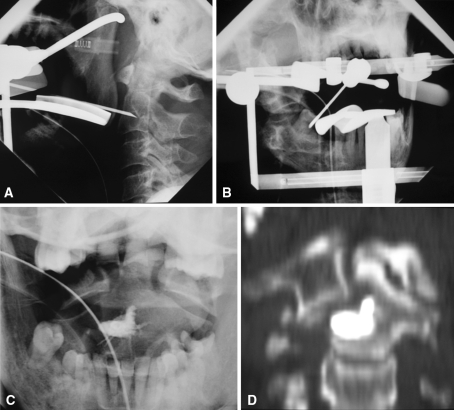
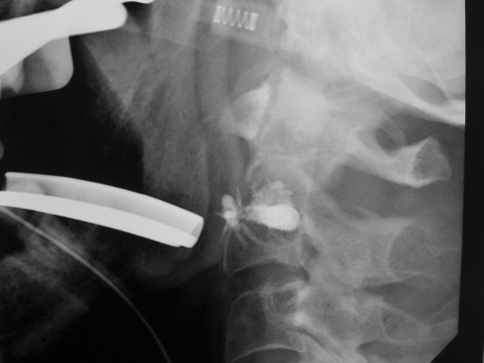
Fig. 2A–D.
Targeting with a 15-gauge needle is performed using the transoral approach under biplanar [(A) AP and (B) left lateral] fluoroscopic guidance. (C) Fluoroscopic and (D) CT postprocedural controls are shown.
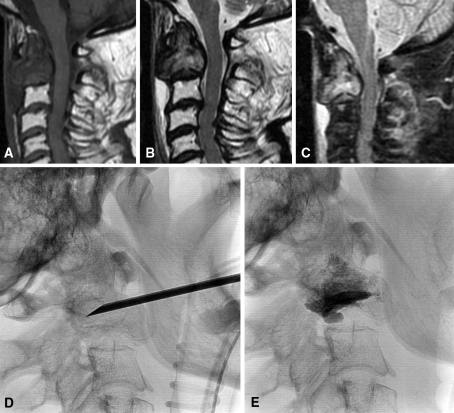
Fig. 3A–E.
Bone metastasis from gastric cancer is demonstrated by (A) T1-weighted, (B) T2-weighted, and (C) STIR MR images. (D) A 13-gauge needle placement using the transoral approach under fluoroscopic guidance is shown. (E) A postprocedural fluoroscopic control shows good PMMA distribution
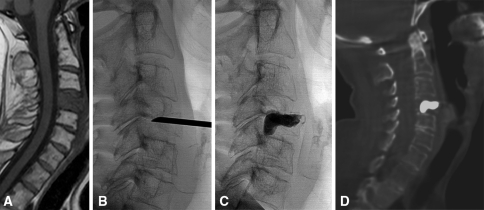
Fig. 4A–D.
C4 bone metastasis from pheocromocytoma is demonstrated by (A) a T1-weighted sagittal MR image. (B) A 13-gauge needle placement using the anterolateral approach under fluoroscopic guidance is shown. (C) Postprocedural fluoroscopic and (D) CT controls show good PMMA distribution.
The day after treatment, all patients were evaluated by CT or MRI to assess eventual extravertebral cement leakage [2]. All patients were asked to remain at rest for 6 hours, after which they were allowed to gradually increase mobility under the supervision of the neurosurgeon, who contextually performed a neurologic examination, and were discharged from the hospital, compatibly with their clinical conditions, 2 days after the treatment. In all patients we used a broad-spectrum antibiotic for 7 days after discharge from hospital. Patients were exposed to different analgesic therapies according to personal drug sensitivity and treatment institution, including oral administration of: ibuprofen (Brufen, Abbott), diclofenac (Voltaren, Innovex), paracetamol + codeine (Coefferalgan; Bristol-Myers Squibb Srl, Roma, Italia), oxycodone (Oxycontin; Mundipharma Pharmaceutic Srl, Milano, Italia), or tramadol (Tradonal, Viatris). The amounts of daily medication intake (number of pills per day) before and after the procedure were recorded (Table 1).
Followup visits were at 1 and 3 months after surgery. Clinical examinations included a neurological examination and VAS evaluation, while imaging was performed with a simple radiograph at 1 month and an MR or CT at 3 months. The severity of pain was evaluated according to a pain score using a visual analog scale (VAS) ranging from 0 (absence of pain) to 10 (maximal pain) [5]. Measurement of VAS score was recorded before, immediately after, and 3 months after the treatment.
We defined technical success as the injection of cement into the vertebral lesion without leakage, while a difference in VAS of 2 points or more (as reported in previous studies) was considered clinical success [12, 14]. Standard descriptive statistics were expressed as median (range) and/or mean ± SD, when appropriate, absolute counts, and percentages. We compared pretreatment and 24-hour posttreatment VAS scores and pretreatment and 3-month followup VAS scores using the Kolmogorov-Smirnov test. Statistical analysis was run using R Version 2.6.0 open-source software publicly available at http://www.r-project.org.
Results
Technical success was achieved in 60 patients; only two (3.2%) patients presented with an asymptomatic cement leakage in the soft tissues (Fig. 5). No delayed complications were observed.
Fig. 5.
Anterior asymptomatic cement leakage is shown.
Pain was reduced (p < 0.001) by 24 hours postsurgery: mean pretreatment and 24-hour posttreatment VAS scores were 7.9 ± 1.7 and 1.5 ± 2, respectively (Table 1). Within 24 hours, the pain completely disappeared in 25 (40%) patients. Analgesics were suspended in 34 (55%) patients, decreased in 26 (42%), and continued in two. The median analgesics consumption decreased from two pills per day (range, 0–3) to 0 (range, 0–3); in two patients, analgesics administration was continued due to the persistence of pain (Table 1). All patients were directly interviewed at 3 months and reported a mean VAS score of 1.7 ± 2, lower (p < 0.001) than the pretreatment VAS score.
Discussion
PVP is a minimally invasive tool that provides long-lasting pain control in 50% to more than 90% of patients treated for lumbar or thoracic metastases, providing an increased strength and stiffness of the vertebral body, thereby preventing further vertebral collapse as well [6, 10, 11, 31]. Although the details of the mechanisms responsible for pain relief are still not completely clear, it has been suggested the injection of PMMA produces an analgesic effect by stabilizing the treated bone segment and/or by direct effect of cement injection with thermal ablation of local nervous end structures caused by the exothermic reaction produced during the polymerization of PMMA and the neurotoxicity of the monomer. PVP in the cervical spine, which is different from other spinal segments, has only been performed on small numbers of patients or anecdotally to treat patients with spinal metastasis. We therefore evaluated (1) the technical feasibility considering the complications rate and (2) the ability of PVP to reduce pain and medication usage in cervical fractures due to metastases.
We acknowledge limitations to our study. First is the lack of a control group. However, these were patients who failed previous nonoperative treatment and we presume the disease would be progressive in all. Second is the short-term followup, but this is partly limited by the short life expectancy of patients who are candidates for the procedure. Lastly, the study was performed by experienced radiologists with experience in vertebroplasty procedures; Given these are technically difficult we presume there would be a learning curve with the procedure and our results might not be generalizable for radiologists less familiar with the technique.
Consistent with findings in the other spinal segments [4, 26], we observed a short- and long-term drop in VAS pain scores, with a consequent major reduction of analgesic opiate use and improved mobility. Deramond et al. [9] reported on 101 patients with metastatic spinal disease and demonstrated improvements in pain relief in more than 80% of patients. Also, Cortet at al. [7] reported a decrease in pain within 48 hours of vertebroplasty in 97% of their 37 patients (cervical spine involvement in five cases) with osteolytic metastases or multiple myeloma. In their study, pain after the procedure was absent in 13.5%, substantially reduced in 55%, and moderately reduced in 30%. Their complication rate approximated 3%, with two patients developing severe nerve root pain due to leakage of bone cement into the neural foramen. Barr et al. [1] included eight patients with malignancy in their cohort of 47 patients undergoing vertebroplasty, achieving pain relief in four of the eight (50%) patients.
Our data confirm previous finding in the literature on smaller samples of patients and suggest, in experienced hands, PVP provides a minimally invasive way to provide pain relief from metastatic lesions of the cervical spine lesions and a consequent reduction in the consumption of analgesic drugs, with few complications.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
The work was performed at University of Rome “Tor Vergata”, at the Institute for Cancer Research and Treatment, and at Cardarelli Hospital.
References
- 1.Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine (Phila Pa 1976) 2000;25:923–928. doi: 10.1097/00007632-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Barragán-Campos HM, Vallée JN, Lo D, Cormier E, Jean B, Rose M, Astagneau P, Chiras J. Percutaneous vertebroplasty for spinal metastases: complications. Radiology. 2006;238:354–362. doi: 10.1148/radiol.2381040841. [DOI] [PubMed] [Google Scholar]
- 3.Böhm P, Huber J. The surgical treatment of bony metastases of spine and limbs. J Bone Joint Surg Br. 2002;84:521–529. doi: 10.1302/0301-620X.84B4.12495. [DOI] [PubMed] [Google Scholar]
- 4.Calmels V, Vallée JN, Rose M, Chiras J. Osteoblastic and mixed spinal metastases: evaluation of the analgesic efficacy of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2007;28:570–574. [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 6.Chiras J, Depriester C, Weill A, Sola-Martinez MT, Deramond H. [Percutaneous vertebral surgery: technics and indications] [in French] J Neuroradiol. 1997;24:45–59. [PubMed] [Google Scholar]
- 7.Cortet B, Cotten A, Boutry N, Dewatre F, Flipo RM, Duquesnoy B, Chastanet P, Delcambre B. Percutaneous vertebroplasty in patients with osteolytic metastases or multiple myeloma. Rev Rhum Engl Ed. 1997;64:177–183. [PubMed] [Google Scholar]
- 8.Cotten A, Dewatre F, Cortet B, Assaker R, Leblond D, Duquesnoy B, Chastanet P, Clarisse J. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methylmethacrylate at clinical follow-up. Radiology. 1996;200:525–530. doi: 10.1148/radiology.200.2.8685351. [DOI] [PubMed] [Google Scholar]
- 9.Deramond H, Deprestire C, Galibert P, Le Gars D. Percutaneous vertebroplasty with polymethylmethacrylate: technique, indications and results. Radiol Clin North Am. 1998;36:533–546. doi: 10.1016/S0033-8389(05)70042-7. [DOI] [PubMed] [Google Scholar]
- 10.Feydy A, Cognard C, Miaux Y, Sola Martínez MT, Weill A, Rose M, Chiras J. Acrylic vertebroplasty in symptomatic cervical vertebral haemangiomas: report of 2 cases. Neuroradiology. 1996;38:389–391. doi: 10.1007/BF00596600. [DOI] [PubMed] [Google Scholar]
- 11.Fung KY, Law SW. Management of malignant atlanto-axial tumours. J Orthop Surg (Hong Kong) 2005;13:232–239. doi: 10.1177/230949900501300304. [DOI] [PubMed] [Google Scholar]
- 12.Gailloud P, Martin JB, Olivi A, Rüfenacht DA, Murphy KJ. Transoral vertebroplasty for a fractured C2 aneurysmal bone cyst. J Vasc Interv Radiol. 2002;13:340–341. doi: 10.1016/S1051-0443(07)61733-3. [DOI] [PubMed] [Google Scholar]
- 13.Gerszten PC, Welch WC. Current surgical management of metastatic spinal disease. Oncology. 2000;14:1013–1024. [PubMed] [Google Scholar]
- 14.Gupta S, Henningsen JA, Wallace MJ, Madoff DC, Morello FA, Jr, Ahrar K, Murthy R, Hicks ME. Percutaneous biopsy of head and neck lesions with CT guidance: various approaches and relevant anatomic and technical considerations. Radiographics. 2007;27:371–390. doi: 10.1148/rg.272065101. [DOI] [PubMed] [Google Scholar]
- 15.Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am. 2000;31:515–528. doi: 10.1016/S0030-5898(05)70171-1. [DOI] [PubMed] [Google Scholar]
- 16.Harrington K. Metastatic tumors of the spine: diagnosis and treatment. J Am Acad Orthop Surg. 1993;1:76–86. doi: 10.5435/00124635-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Janjan N. Bone metastases: approaches and management. Semin Oncol. 2001;28:28–34. doi: 10.1016/S0093-7754(01)90229-5. [DOI] [PubMed] [Google Scholar]
- 18.Jónsson B, Jónsson H, Jr, Karlström G, Sjöström L. Surgery of cervical spine metastases: a retrospective study. Eur Spine J. 1994;3:76–83. doi: 10.1007/BF02221444. [DOI] [PubMed] [Google Scholar]
- 19.Levine SA, Perin LA, Hayes D, Hayes WS. An evidence-based evaluation of percutaneous vertebroplasty. Manag Care. 2000;9:56–60, 63. [PubMed]
- 20.Klimo P, Jr, Schimdt MH. Surgical management of spinal metastases. Oncologist. 2004;9:188–196. doi: 10.1634/theoncologist.9-2-188. [DOI] [PubMed] [Google Scholar]
- 21.Martin JB, Gailloud P, Dietrich PY, Luciani ME, Somon T, Sappino PA, Rüfenach DA. Direct transoral approach to C2 percutaneous vertebroplasty. Cardiovasc Intervent Radiol. 2002;25:517–519. doi: 10.1007/s00270-001-0122-7. [DOI] [PubMed] [Google Scholar]
- 22.Masala S, Mammucari M, Angelopoulos G, Fiori R, Massari F, Faria S, Simonetti G. Percutaneous vertebroplasty in the management of vertebral osteoporotic fractures: short-term, mid-term and long-term follow-up of 285 patients. Skeletal Radiol. 2009;38:863–869. doi: 10.1007/s00256-009-0712-z. [DOI] [PubMed] [Google Scholar]
- 23.Masala S, Massari F, Fiori R, Mammucari M, Bartolucci DA, Simonetti G. Future directions in percutaneous vertebroplasty. Radiol Med. 2009;114:976–983. doi: 10.1007/s11547-009-0418-2. [DOI] [PubMed] [Google Scholar]
- 24.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/S0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 25.Perrin RG, McBroom RJ, Perrin RG. Metastatic tumors of the cervical spine. Clin Neurosurg. 1991;37:740–755. [PubMed] [Google Scholar]
- 26.Pilitsis JG, Rengachary SS. The role of vertebroplasty in metastatic spinal disease. Neurosurg Focus. 2001;11:1–4. doi: 10.3171/foc.2001.11.6.10. [DOI] [PubMed] [Google Scholar]
- 27.Saarto T, Janes R, Tenhunen M, Kouri M. Palliative radiotherapy in the treatment of skeletal metastasis. Eur J Pain. 1989;6:323–330. doi: 10.1016/S1090-3801(02)00028-9. [DOI] [PubMed] [Google Scholar]
- 28.Sachs DC, Inamasu J, Mendel EE, Guiot BH. Transoral vertebroplasty for renal metastasis involving the axis. Spine (Phila Pa 1976). 2006;31:E925–E928. [DOI] [PubMed]
- 29.Schachar NS. An update on the nonoperative treatment of patients with metastatic bone disease. Clin Orthop Relat Res. 2001;328:75–81. doi: 10.1097/00003086-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Tong FC, Cloft HJ, Joseph GJ, Rodts GR, Dion JE. Transoral approach to cervical vertebroplasty for multiple myeloma. AJR Am J Roentgenol. 2000;175:1322–1324. doi: 10.2214/ajr.175.5.1751322. [DOI] [PubMed] [Google Scholar]
- 31.Weill A, Chiras J, Simon JM, Rose M, Sola-Martinez T, Enkaoua E. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199:241–247. doi: 10.1148/radiology.199.1.8633152. [DOI] [PubMed] [Google Scholar]