Abstract
Background
Highly cross-linked polyethylene (HXLPE), created by disrupting the molecular structure of polyethylene, then through the application of heat, encourages creation of new cross-links in the process, resulting in a material with improved wear resistance. The impetuses for this new technology were the unsatisfactory wear properties and subsequent osteolysis of noncross-linked polyethylene. A 72% reduction in wear using highly cross-linked polyethylenes (HXLPE) compared with conventional polyethylene at 5 years was described previously. The longest term followup studies on HXLPE range from 2 to 6 years.
Questions/purposes
We therefore addressed the following questions: (1) Does the improvement in wear observed at the earlier followup continue to 7 to 10 years? (2) What is the incidence of osteolysis in this group of patients and in the control group?
Methods
We retrospectively reviewed 38 prospectively followed patients who had 42 hips with an annealed HXLPE who were followed a minimum of 7 years (average, 8.6 years; SD = 1; range, 7–10.3 years). Wear and osteolysis were compared with those of a control group of 39 patients (40 hips) from a US Investigational Device Exemption (IDE) prospective, randomized study begun in 1996 with conventional polyethylene and followed for a minimum of 6 years (average, 7.5 years; SD = 1.1; range, 6–10.2 years). Linear head penetration was measured from AP radiographs at early, 1-year, 5-year, and most recent followups.
Results
At the average followup, annual linear wear was 0.031 mm (SD = 0.014) for the HXLPE and 0.141 mm (SD = 0.080) for the control group, a 78% reduction. No mechanical failure of the polyethylene was noted in either group. Incidence of osteolysis was 50% in the control group (all lesions confined to proximal Gruen Zones 1 and 7) compared with no cases in the investigational group.
Conclusions
We observed an improvement in wear and no mechanical failures with this annealed material.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Since their introduction in the past decade, HXLPEs have been associated with reduced polyethylene wear rates when compared with conventional materials. Reductions between 23% and 95% have been reported by numerous authors [7, 10, 11, 13, 17–20] with limited followups (5.5 years or less). The amount of wear reduction noted varies with the control used. Those articles comparing the highly cross-linked material with controls that were gamma-sterilized showed less wear reduction than those comparing the highly cross-linked material with nonirradiation sterilization methods. Two processes of creating the HXLPE are represented in these reports. In one, the material is irradiated, remelted, and then recrystallized and sterilized by nonradiation means. This method produces a polyethylene that is highly cross-linked and essentially free of free radicals but with compromised mechanical properties. The second process also subjects the polyethylene to radiation but is heated below the melting point (annealed). This process creates a HXLPE that preserves the physical properties but retains free radicals in the crystalline structure. In an earlier report, D’Antonio et al. reported an annealed (but not remelted) polyethylene had a 72% improvement in wear at 5 years when compared with a control group of patients with polyethylene that was gamma-sterilized in an inert atmosphere [5]. Since that publication, other reports have appeared documenting the performance of the same annealed HXLPE [18, 23, 25, 26]. These reports have documented similar improvements in wear rates. In addition, retrieval analysis of components removed for various reasons has looked at these components for evidence of oxidation [14, 15]. Despite these reports, some authors have expressed concerns regarding the long-term performance of this annealed material because of the presence of free radicals [25, 26].
The purpose of this article is to update the earlier publication by D’Antonio et al. [5], and to report the wear rates of this group of patients with HXLPE and, once again, compare them with our control group. In doing so, we will address the following questions: (1) Does the improvement in wear noted at the earlier followup continue to 7 to 10 years? (2) What is the incidence of osteolysis in this group of patients and the control group?
Patients and Materials
This is an update to a previous report in which femoral head penetration was measured in polyethylene inserts of the same design but manufactured using two different methods [5]. We retrospectively reviewed the radiographs of two groups of patients: an experimental group of 38 patients (42 hips) with a Crossfire® (Stryker Orthopaedics, Mahwah, NJ, USA) acetabular liner and a control group of 39 patients (40 hips) with a conventional polyethylene liner to determine the linear wear rates of the Stryker Crossfire® acetabular liners at a minimum of 7 years compared with the conventional polyethylene control. A secondary objective was to assess postoperative serial radiographs at a minimum of 7 years postoperative to evaluate the presence of osteolysis and other radiographic parameters indicative of potential polyethylene wear. Both groups were implanted with 28-mm cobalt-chromium (CoCr) heads and cementless Omnifit® hydroxyapatite (HA) Stems (Stryker Orthopaedics). The acetabular shell in the experimental group was a Secur-Fit® HA PSL® (Stryker Orthopaedics) design with an HA arc deposited titanium surface. The control group was implanted with a titanium MicroStructured® PSL® (Stryker Orthopaedics) shell. Both sockets had the same geometry (dual radius) with a wire in groove locking mechanism.
The Crossfire® liner was manufactured from ram-extruded GUR 1050 UHMWPE that had been irradiated to 7.5 Mrad and annealed for 8 hours at 130° C gamma-sterilized to 3 Mrad in nitrogen and vacuum-packaged (N2VacTM; Stryker Orthopaedics). The conventional liner was manufactured from ram-extruded GUR 1050 UHMWPE gamma-sterilized to 3 Mrad in nitrogen and vacuum-packaged (N2VacTM; Stryker Orthopaedics). When comparing mechanical properties of Crossfire and conventional polyethylene, no differences are noted in yield, ultimate tensile strength, or hardness (Table 1 in [5]).
Table 1.
Demographic characteristics of highly cross-linked and conventional polyethylenes
| Demographics | Crossfire® | Conventional | t-Score | p Value |
|---|---|---|---|---|
| Number of hips | 42 | 40 | ||
| Number of patients | 38 | 39 | ||
| Male:female (%) | 19:19 (50%:50%) | 23:16 (59%:41%) | ||
| Mean age (years) | 55.8 (SD 10.0) | 54.2 (SD 10.4) | 0.69 | > 0.05 |
| Mean weight (kg) | 79.2 (SD 19.6) | 84.5 (SD 16.0) | 1.30 | > 0.05 |
| Mean height (cm) | 168.7 (SD 12.2) | 175.3 (SD 8.4) | 2.76 | < 0.05 |
| Mean body mass index (kg/m2) | 27.4 (SD 4.5) | 27.5 (SD 4.7) | 0.10 | > 0.05 |
| Length of followup (years) | 8.6 (range, 7.0–10.4) | 7.5 (range, 6.0–10.2) | ||
| Diagnosis | 74% osteoarthritis, 8% avascular necrosis, and 18% other | 77% osteoarthritis, 17% avascular necrosis, and 6% other |
Acetabular component outer-diameter sizes ranged from 46 mm to 62 mm for the Crossfire group and 50 mm to 60 mm for the controls. The polyethylene thicknesses in the Crossfire group were 7.8 mm, 9.4 mm, 11.4 mm, 13.1 mm, and 14.6 mm and were determined by the outer diameter of the cup. The control group had three different thicknesses, 9.4 mm, 11.4 mm, and 13.1 mm.
Patients were included in the experimental study group if they met the following criteria: an Institutional Review Board review of the study had been conducted and approved; the patient signed an informed consent form; the Crossfire acetabular insert and a compatible metallic femoral head were implanted in a primary THA; patient data, including gender, diagnosis, height, and weight, were available and AP and lateral radiographs of appropriate quality to assess linear head penetration were available at study intervals.
In the experimental group, 38 patients (42 hips) from three centers were available for evaluation at 7 to 10.3 years followup (Table 1). Initially, 48 patients with 57 hips from three centers were available for evaluation at 5 years in the Crossfire group [5]. However, one surgeon at one center elected not to follow his patients (nine patients, 15 hips) after 2007. This surgeon’s patients with less than 7 years followup at that time were not included in the current study. No other patients were lost to followup. Patients were seen within 7 weeks of surgery and at 1 or 2 years, 5 years, and at latest evaluation from 7 to 10 years. All patients were operated on using a standard posterior lateral approach to the hip. No patient had minimally invasive or computer-assisted surgery.
The control patients were part of a US Investigational Device Exemption prospective, randomized study begun in 1996 to compare an alumina bearing couple system with CoCr on polyethylene controls. The study design was described previously [5]. Only patients whose surgery was performed by the two surgeons in the Crossfire study with 7 to 10 years followup were included in the updated control cohort. Thirty-nine patients (40 hips) from the same three surgeons were included in the study (Table 1). The control subjects had followups from 6.0 to 10.2 years with an average followup of 7.5 years. All patients were operated on using a standard posterior lateral approach to the hip. No patient had minimally invasive or computer-assisted surgery.
Sample size calculations were performed to assure that the 40 subjects available for followup in each group were adequate to assess comparative wear values. We used statistics presented in the previous report to determine adequate sample size [5]. To reject the null hypothesis that the linear wear rate at 10 years postoperatively for the Crossfire® liners is equal to that of conventional polyethylene, assuming a two-sample t-test with an alpha of 0.05, it would take 20 subjects per group to obtain 80% power. Thirty-two subjects per group would have been adequate to reject the null hypothesis at 95% power.
Postoperative radiographs were collected and evaluated from the immediate postoperative period, at 1- or 2-, 5-, and 10-year followups. The radiographs also were evaluated by two independent orthopaedic surgeons (PB, SZ) for the presence of radiolucent lines as described by DeLee and Charnley [6] on the acetabular side and Gruen et al. [12] on the femoral side. Osteolysis was reported on the acetabular side as whether the lysis was present in any of the three DeLee and Charnley [6] zones or on the femoral side in any of the proximal Gruen zones (1, 7, 8, or 14) [12] seen on AP and lateral radiographs. Osteolytic lesions were recorded as present if visible on plain radiographs; sizes of the lesions were not recorded.
We used a computer-assisted method, described by Livermore et al. [16], of concentric circles to determine femoral head penetration in the polyethylene with time as observed on an AP radiograph of the pelvis. As stated in the earlier article [5], “This technique has an accuracy of 40 μm in two-dimensional analysis when evaluated with a clinical simulation using an acrylic fixture.” Bragdon et al. tested a phantom model using a radiostereometry (RSA) method and reported an accuracy of 33 μm for the medial direction and 22 μm for the superior direction [3]. All radiographs were digitized with a high-resolution optical scanner (DiagnosticPRO® Advantage; Vidar Corporation, Herndon, VA, USA). The 1-year radiographs were analyzed for head penetration and used to estimate initial bedding-in. Penetration rates from 1 year to last evaluation then were used to estimate the yearly linear wear rate.
Two-sample t-tests were applied to test the mean difference in age, weight, height, and body mass index between Crossfire and conventional groups. Fisher’s exact test was performed to detect the rate difference of osteolysis between the two groups. Statistics were calculated with the SAS® statistical software Version 9 (SAS Institute Inc, Cary, NC, USA).
Results
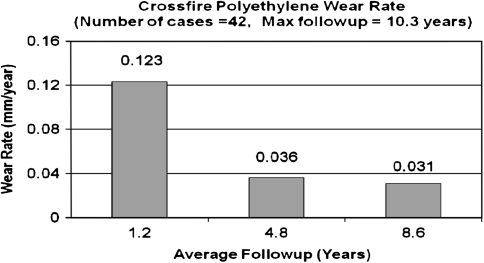
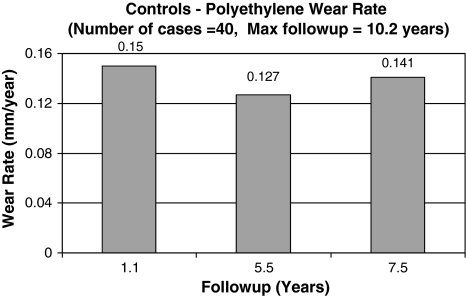
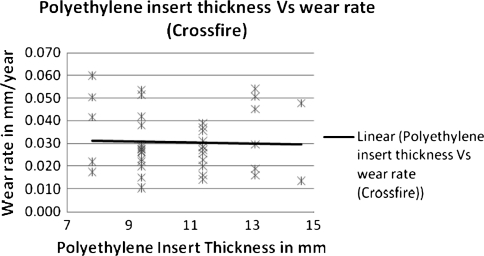
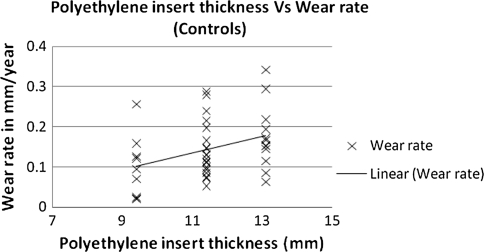
The linear head penetration at 1 year was 0.123 mm for the Crossfire group compared with 0.15 mm for the control group. This represents the bedding-in period. The linear femoral head penetration measured from 1 year to a minimum of 7 years with an average of 8.6 years followup (assumed to be wear) was 0.031 mm/year (SD = 0.014) for the Crossfire group (Fig. 1) and 0.141 mm/year (SD = 0.080) for the control group (Fig. 2) (78% reduction) (p = 0.0167). The majority of hips (36 hips [85.7%]) in the Crossfire group had less than 0.005 mm/year wear rate. The remaining six hips (14.3%) had wear rates between 0.05 mm/year and 0.1 mm/year (maximum 0.06 mm/year) (Fig. 3). The majority of hips in the control group (28 hips [70%]) had greater than 0.1 mm/year of wear. One patient had greater than 0.3 mm/year wear (0.342 mm/year) (Fig. 3). No correlation was found between polyethylene thickness and head penetration wear rates for the experimental group (Fig. 4). There was a tendency of increased wear with increased polyethylene thickness in the control group (Fig. 5).
Fig. 1.
The graph shows Crossfire polyethylene wear rates in 42 patients with a maximum followup of 10.3 years.
Fig. 2.
The graph shows polyethylene wear rates for 40 control subjects with a maximum followup of 10.2 years.
Fig. 3.
The graph shows a comparison of head penetration rate (mm/year) between patients with the Crossfire and the control subjects.
Fig. 4.
The graph shows a comparison of polyethylene thickness and head penetration wear rates for Crossfire inserts for the HXLPE group.
Fig. 5.
The graph shows a comparison of polyethylene thickness and head penetration wear rates for the control group.
No mechanical failures were observed in either the control group or the Crossfire group. In the Crossfire group, radiolucent lines around the acetabular shell were observed in only one case in DeLee and Charnley Zone 3 [6] only. There were no osteolytic lesions around the acetabular component. Two hips (one patient) (4.8%) at 5 years followup showed evidence of small erosions in Gruen Zone 7 [12]. At the latest followup (8.5 years), these small lesions had regressed to less than 5 mm.
In the control group, radiolucent lines around the acetabular shell were observed in eight hips (20%), five in Zone 1 only, two in Zone 3 only, and one in Zones 2 and 3. There were no cases of three-zone radiolucency. Two hips (5.0%) had acetabular erosion, one in Zones 1 and 2 and one in Zone 1 only. Fifty percent of hips (20 hips) receiving the conventional N2Vac polyethylene showed evidence of small osteolytic lesions in the proximal femoral neck resection area, Gruen Zones 1, 7, 8, and/or 14 [12] (p < 0.0001).
In the previous report, there was a difference in the mean age of the subjects in the groups with the mean age of subjects in the Crossfire group being older [5]. In this subset of the original population, there were no statistical differences in age, weight, or body mass index between the Crossfire and control groups (Table 1). There was a difference in height, with subjects in the Crossfire group being slightly shorter (mean 168.7 cm versus mean 175.3 cm); however, this is not considered to be clinically relevant.
Discussion
Varying methods of producing HXLPE are available, each having its positive and negative features. Basically the remelted products have very low residual free radicals but have somewhat compromised physical properties when compared with controls of nonhighly cross-linked polyethylenes. The annealed HXLPE has a higher density of free radicals but retains the same profile of physical properties as the controls. This report looks at an annealed HXLPE followed to 10 years. The purpose of the study was to compare the head penetration/wear of this material with that of a control group and also report on the instance of osteolysis at this extended period.
This study has numerous limitations. First, the number of patients is not large. Second, although our followup is longer than any previously reported concerning HXLPE, it still is insufficient to categorically state retained free radicals in the material are of no clinical importance. Third, our study is not randomized. The control subjects were part of a randomized study looking at the comparison of metal-on-polyethylene and ceramic-on-ceramic bearings. However, the control group and the experimental group in this study had 28-mm heads, the same stem, the same acetabular component, and polyethylene thicknesses were comparable. The selection process differed between the control and the experimental groups. The control group patients were part of the previously described IDE study in which patients were randomized into an experimental group with a ceramic-on-ceramic bearing or the control group with a metal-on-polyethylene bearing. In the experimental group in the current study, patients were asked to participate based on a retrospective review of the early use of the HXLPE at three institutions. All patients who agreed to participate signed a Patient Informed Consent Form.
A reduction in wear of 72% was reported for patients with this highly cross-linked material compared with that of control subjects with gamma sterilized in an inert atmosphere [5]. Looking at the same group of patients and control subjects, now with followups to 10 years (average, 8.6 years), shows an additional reduction in wear, now 78% when compared with that of control subjects. We also have not seen any mechanical failures or osteolysis in the experimental group. Martell et al., reporting on the same material, reported a 50% reduction at 2 to 3 years followup [18]. Our current study, with much longer followup, shows additional improvement in wear reduction. Röhrl et al., using an RSA method for measuring wear [25] and also studying the same annealed HXLPE, initially reported an 85% reduction in head penetration compared with that of controls at just 2 years. His controls were a group of UHMWPE gamma-sterilized in air. A subsequent study [26], with longer followup (6 years), again noted marked improvement over that of the control group, “10 times less wear than in the control group.” However, they expressed numerous concerns about this nonremelted material. First, their series had only cemented acetabular components in the Crossfire and control groups and they believed it might be “more forgiving and tolerant of stress” than cementless fixation relative to the locking mechanism. Their concern was that the presence of free radicals could lead to oxidation of the material and compromise the locking mechanism in cementless acetabular components. They expressed concern regarding wear of the bearing because of the presence of free radicals and suggested at least a 10-year clinical followup would be necessary to allay these fears. In our current study, the average followup of the Crossfire material is 8.6 years with some patients having followups beyond 10 years. We share the concerns of Röhrl et al. [26], but thus far have seen no evidence of accelerated wear or mechanical failure of the locking mechanism.
Kurtz et al. [14], along the same lines, looking at retrieved Crossfire inserts, did observe oxidation of the components but only at the periphery and noted that neither the locking mechanism nor the bearing surface was involved with oxidation. They concluded that, thus far, concerns regarding adverse effects relative to free radical presence were unfounded [14, 15]. Our current findings are consistent with those of Kurtz et al. [14], although as noted previously, longer followup is necessary before one can categorically state the potential for oxidation has no clinical relevance.
We found a difference in osteolytic lesions between our HXLPE group and the patients with conventional polyethylene implants (0% versus 50%, p < 0.0001). Rajadhyaksha et al., also comparing Crossfire with a control group of N2Vac polyethylene, noted a difference in lysis similar to ours (0% versus 14.8%) [23]. Some studies reported no difference in osteolytic lesions between the HXLPE and control (even with differences in head penetration), but noted their followups were short and that more time may be required for differences to manifest [2, 4, 9, 19, 20, 27].
Thus far, this is the longest followup of any group of patients with HXLPE implants [13]. We have documented a continued reduction in wear that is comparable to those reported independent of the processing technique. We have not seen any evidence of accelerated wear [1], in fact we have observed the opposite, with a continued reduction of wear noted. We also have observed no mechanical failures or osteolysis at this intermediate followup. Fortunately, some of these concerns have been addressed in newer iterations of HXLPE; the sequentially annealed [8] and those manufactured with vitamin E have minimal free radicals, excellent wear resistance, and minimal compromise in mechanical properties [21, 22, 24].
Acknowledgments
We thank Peter Bonutti MD (Bonutti Clinic, Effingham, IL) for radiographic review of the control group and Steven Zelicof MD (New York Medical College, White Plains, NY) for radiographic review of the Crossfire group.
Footnotes
Each author certifies that he (RR) or she (MN) has or may receive payments or benefits from a commercial entity related to this work.
Two of the authors (WNC, JAD) receive royalties and have consulting agreements with Stryker Orthopaedics, Mahwah, NJ
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethic principles of research, and that informed consent was obtained.
This work was performed at Indiana University School of Medicine, Indianapolis, IN, USA.
References
- 1.Beksaç B, Salas A, Della Valle AG, Salvati EA. Wear is reduced in THA performed with highly cross-linked polyethylene. Clin Orthop Relat Res. 2009;467:1765–1772. doi: 10.1007/s11999-008-0661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bragdon CR, Kwon YM, Geller JA, Greene ME, Freiberg AA, Harris WH, Malchau H. Minimum 6-year followup of highly cross-linked polyethylene in THA. Clin Orthop Relat Res. 2007;465:122–127. doi: 10.1097/BLO.0b013e31815760b1. [DOI] [PubMed] [Google Scholar]
- 3.Bragdon CR, Malchau H, Yuan X, Perinchief R, Kärrholm J, Börlin N, Estok DM, Harris WH. Experimental assessment of precision and accuracy of radiostereometric analysis for the determination of polyethylene wear in a total hip replacement model. J Orthop Res. 2002;20:688–695. doi: 10.1016/S0736-0266(01)00171-1. [DOI] [PubMed] [Google Scholar]
- 4.Calvert GT, Devane PA, Fielden J, Adams K, Horne JG. A double-blind, prospective, randomized controlled trial comparing highly cross-linked and conventional polyethylene in primary total hip arthroplasty. J Arthroplasty. 2009;24:505–510. doi: 10.1016/j.arth.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 5.D’Antonio JA, Manley MT, Capello WN, Bierbaum BE, Ramakrishnan R, Naughton M, Sutton K. Five-year experience with Crossfire highly cross-linked polyethylene. Clin Orthop Relat Res. 2005;441:143–150. doi: 10.1097/00003086-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 6.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 7.Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasul highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87:1816–1821. doi: 10.2106/JBJS.D.01915. [DOI] [PubMed] [Google Scholar]
- 8.Dumbleton JH, D’Antonio JA, Manley MT, Capello WN, Wang A. The basis for a second-generation highly cross-linked UHMWPE. Clin Orthop Relat Res. 2006;453:265–271. doi: 10.1097/01.blo.0000238856.61862.7d. [DOI] [PubMed] [Google Scholar]
- 9.Garvin KL, Hartman CW, Mangla J, Murdoch N, Martell JM. Wear analysis in THA utilizing oxidized zirconium and crosslinked polyethylene. Clin Orthop Relat Res. 2009;467:141–145. doi: 10.1007/s11999-008-0544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glyn-Jones S, Isaac S, Hauptfleisch J, McLardy-Smith P, Murray DW, Gill HS. Does highly cross-linked polyethylene wear less than conventional polyethylene in total hip arthroplasty? A double-blind, randomized, and controlled trial using roentgen stereophotogrammetric analysis. J Arthroplasty. 2008;23:337–343. doi: 10.1016/j.arth.2006.12.117. [DOI] [PubMed] [Google Scholar]
- 11.Glyn-Jones S, McLardy-Smith P, Gill HS, Murray DW. The creep and wear of highly cross-lined polyethylene: a three year randomised, controlled trial using radiostereometric analysis. J Bone Joint Surg Br. 2008;90:556–561. doi: 10.1302/0301-620X.90B5.20545. [DOI] [PubMed] [Google Scholar]
- 12.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiologic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 13.Jacobs CA, Christensen CP, Greenwald AS, McKellop H. Clinical performance of highly cross-linked polyethylenes in total hip arthroplasty. J Bone Joint Surg Am. 2007;89:2779–2786. doi: 10.2106/JBJS.G.00043. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz SM, Austin MS, Azzam K, Sharkey PF, MacDonald DW, Medel FJ, Hozack WJ. Mechanical properties, oxidation, and clinical performance of retrieved highly cross-linked Crossfire liners after intermediate-term implantation. J Arthroplasty. 2010;25:614–623.e1–2. [DOI] [PMC free article] [PubMed]
- 15.Kurtz SM, Hozack W, Turner J, Purtill J, MacDonald D, Sharkey P, Parvizi J, Manley M, Rothman R. Mechanical properties of retrieved highly cross-linked crossfire liners after short-term implantation. J Arthroplasty. 2005;20:840–849. doi: 10.1016/j.arth.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72:518–528. [PubMed] [Google Scholar]
- 17.Manning DW, Chiang PP, Martell JM, Galante JO, Harris WH. In vivo comparative wear study of traditional and highly cross-linked polyethylene in total hip arthroplasty. J Arthroplasty. 2005;20:880–886. doi: 10.1016/j.arth.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Martell JM, Verner JJ, Incavo SJ. Clinical performance of a highly cross-linked polyethylene at two years in total hip arthroplasty: a randomized prospective trial. J Arthroplasty. 2003;18(7 suppl 1):55–59. doi: 10.1016/S0883-5403(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 19.McCalden RW, MacDonald SJ, Rorabeck CH, Bourne RB, Chess DG, Charron KD. Wear rate of highly cross-linked polyethylene in total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2009;91:773–782. doi: 10.2106/JBJS.H.00244. [DOI] [PubMed] [Google Scholar]
- 20.Olyslaegers C, Defoort K, Simon JP, Vandenberghe L. Wear in conventional and highly cross-linked polyethylene cups: a 5-year follow-up study. J Arthroplasty. 2008;23:489–494. doi: 10.1016/j.arth.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Oral E, Christensen SD, Malhi AS, Wannomae KK, Muratoglu OK. Wear resistance and mechanical properties of highly cross-linked ultrahigh-molecular weight polyethylene doped with vitamin E. J Arthroplasty. 2006;21:580–591. doi: 10.1016/j.arth.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oral E, Godleski Beckos CA, Lozynsky AJ, Malhi AS, Muratoglu OK. Improved resistance to wear and fatigue fracture in high pressure crystallized vitamin E-containing ultrahigh molecular weight polyethylene. Biomaterials. 2009;30:1870–1880. doi: 10.1016/j.biomaterials.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajadhyaksha AD, Brotea C, Cheung Y, Kuhn C, Ramakrishnan R, Zelicof SB. Five-year comparative study of highly cross-linked (crossfire) and traditional polyethylene. J Arthroplasty. 2009;24:161–167. doi: 10.1016/j.arth.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Renó F, Cannas M. UHMWPE and vitamin E bioactivity: an emerging prospective. Biomaterials. 2006;27:3039–3043. doi: 10.1016/j.biomaterials.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Röhrl S, Nivbrant B, Mingguo L, Hewitt B. In vivo wear and migration of highly cross-linked polyethylene cups: a radiostereometry analysis study. J Arthroplasty. 2005;20:409–413. doi: 10.1016/j.arth.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 26.Röhrl SM, Li MG, Nilsson KG, Nivbrant B. Very low wear on non-remelted highly cross-linked polyethylene cups: an RSA study lasting up to 6 years. Acta Orthop. 2007;78:739–745. doi: 10.1080/17453670710014509. [DOI] [PubMed] [Google Scholar]
- 27.Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467:2059–2065. doi: 10.1007/s11999-008-0697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]