Abstract
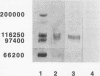
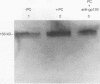
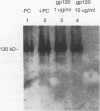
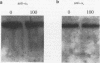
Pneumocystis carinii is an extracellular pathogen which requires attachment to alveolar epithelial cells for growth and replication. Previous studies have demonstrated that the extracellular matrix protein fibronectin (Fn) facilitates attachment of P. carinii to lung cells. This study addresses the role of cell surface Fn receptors (integrins) as mediators of P. carinii attachment and demonstrates the effect of P. carinii attachment on integrin expression on cultured lung cells. To determine the role of Fn-binding integrins in P. carinii attachment, attachment of 51Cr-labelled P. carinii organisms to the lung epithelial cell line A549 was quantified in the presence or absence of anti-integrin antibodies. Antibodies to the alpha v and alpha 5 integrin subunits significantly inhibited P. carinii attachment, while addition of antibody to the alpha subunit of a non-Fn-binding integrin, alpha 2, did not affect P. carinii attachment. To further investigate the role of Fn-binding integrins in P. carinii attachment, the effect of P. carinii attachment on expression of the alpha v and alpha 5 integrin subunits was determined. A549 cells incubated with either P. carinii organisms or with the P. carinii major surface glycoprotein gp120 demonstrated a 3- to 10-fold increase in expression of the alpha 5 integrin subunit; however, neither P. carinii nor gp120 affected the expression of alpha v integrin. Furthermore, the effects of P. carinii on A549 cell alpha 5 integrin expression were attenuated by the addition of an anti-gp120 antibody which blocks P. carinii attachment to A549 cells. Therefore, P. carinii attachment to lung epithelial cells appears to be mediated by alpha v- and alpha 5-containing integrins expressed on the epithelial cell surface, and P. carinii attachment results in increased expression of the alpha 5 integrin subunit.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
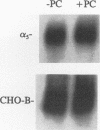
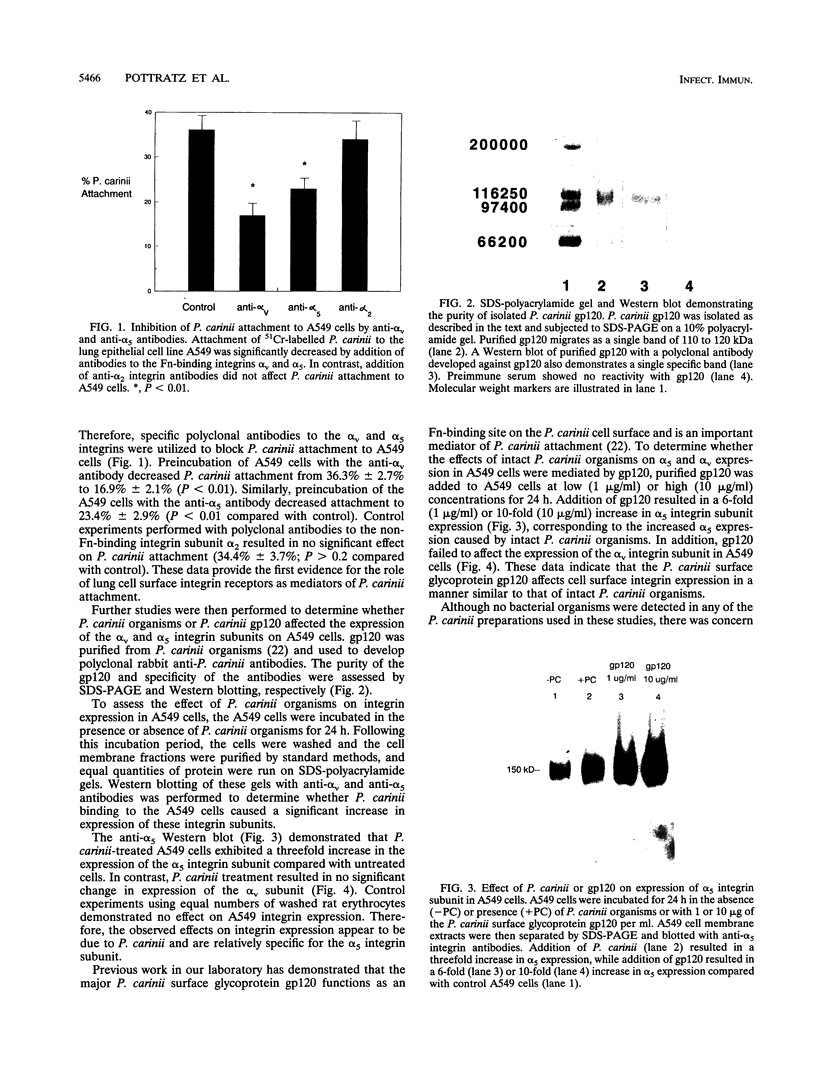
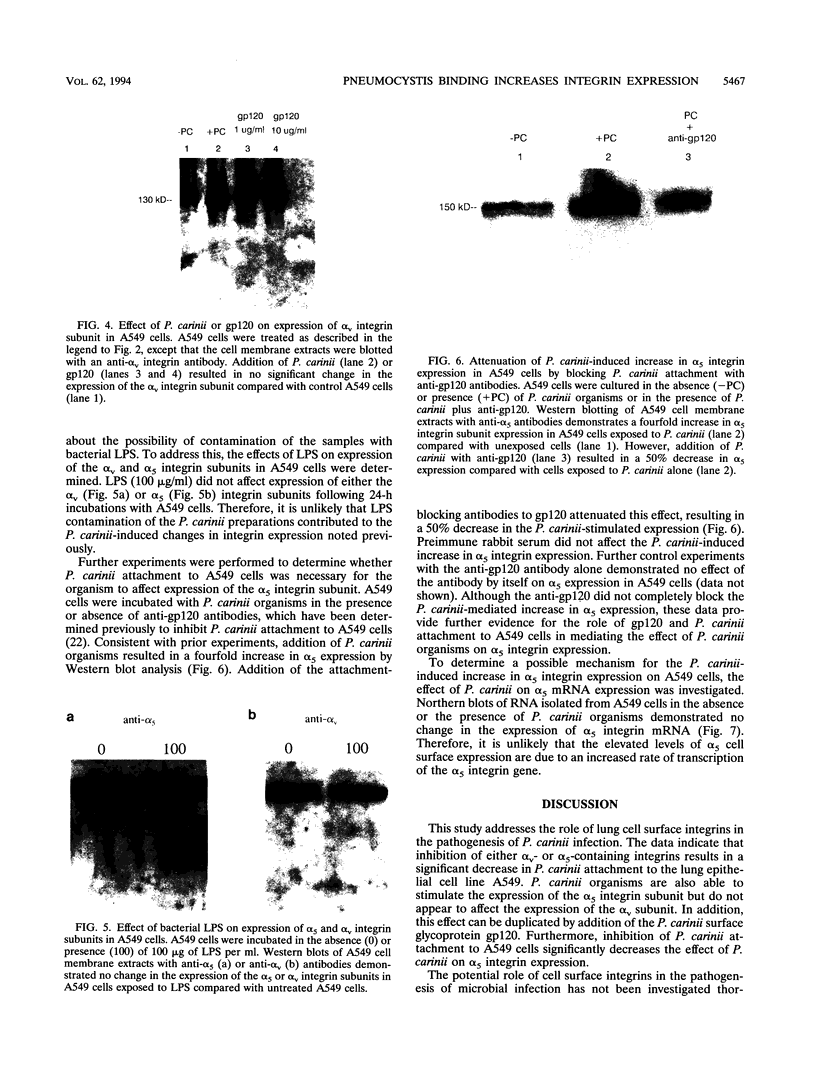
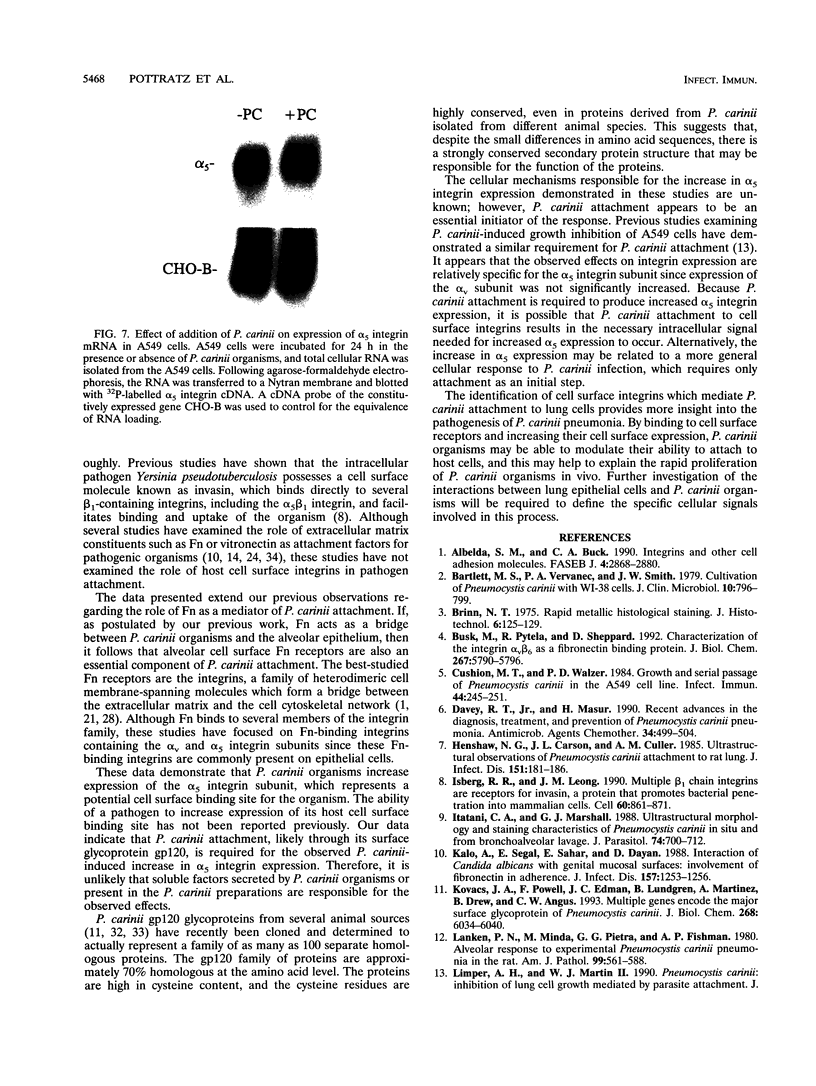
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Bartlett M. S., Verbanac P. A., Smith J. W. Cultivation of Pneumocystis carinii with WI-38 cells. J Clin Microbiol. 1979 Dec;10(6):796–799. doi: 10.1128/jcm.10.6.796-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busk M., Pytela R., Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J Biol Chem. 1992 Mar 25;267(9):5790–5796. [PubMed] [Google Scholar]
- Cushion M. T., Walzer P. D. Growth and serial passage of Pneumocystis carinii in the A549 cell line. Infect Immun. 1984 May;44(2):245–251. doi: 10.1128/iai.44.2.245-251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey R. T., Jr, Masur H. Recent advances in the diagnosis, treatment, and prevention of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1990 Apr;34(4):499–504. doi: 10.1128/aac.34.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshaw N. G., Carson J. L., Collier A. M. Ultrastructural observations of Pneumocystis carinii attachment to rat lung. J Infect Dis. 1985 Jan;151(1):181–186. doi: 10.1093/infdis/151.1.181. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Itatani C. A., Marshall G. J. Ultrastructural morphology and staining characteristics of Pneumocystis carinii in situ and from bronchoalveolar lavage. J Parasitol. 1988 Aug;74(4):700–712. [PubMed] [Google Scholar]
- Kalo A., Segal E., Sahar E., Dayan D. Interaction of Candida albicans with genital mucosal surfaces: involvement of fibronectin in adherence. J Infect Dis. 1988 Jun;157(6):1253–1256. doi: 10.1093/infdis/157.6.1253. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Powell F., Edman J. C., Lundgren B., Martinez A., Drew B., Angus C. W. Multiple genes encode the major surface glycoprotein of Pneumocystis carinii. J Biol Chem. 1993 Mar 15;268(8):6034–6040. [PubMed] [Google Scholar]
- Lanken P. N., Minda M., Pietra G. G., Fishman A. P. Alveolar response to experimental Pneumocystis carinii pneumonia in the rat. Am J Pathol. 1980 Jun;99(3):561–588. [PMC free article] [PubMed] [Google Scholar]
- Limper A. H., Standing J. E., Hoffman O. A., Castro M., Neese L. W. Vitronectin binds to Pneumocystis carinii and mediates organism attachment to cultured lung epithelial cells. Infect Immun. 1993 Oct;61(10):4302–4309. doi: 10.1128/iai.61.10.4302-4309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke M. J., Cushion M. T., Walzer P. D. Properties of the major antigens of rat and human Pneumocystis carinii. Infect Immun. 1989 May;57(5):1547–1555. doi: 10.1128/iai.57.5.1547-1555.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. G., Smith J. S., Meier J. L. Attachment of Pneumocystis carinii to rat pneumocytes. Lab Invest. 1986 Jun;54(6):609–615. [PubMed] [Google Scholar]
- Majda J. A., Gerner E. W., Vanlandingham B., Gehlsen K. R., Cress A. E. Heat shock-induced shedding of cell surface integrins in A549 human lung tumor cells in culture. Exp Cell Res. 1994 Jan;210(1):46–51. doi: 10.1006/excr.1994.1007. [DOI] [PubMed] [Google Scholar]
- Millard P. R., Wakefield A. E., Hopkin J. M. A sequential ultrastructural study of rat lungs infected with Pneumocystis carinii to investigate the appearances of the organism, its relationships and its effects on pneumocytes. Int J Exp Pathol. 1990 Dec;71(6):895–904. [PMC free article] [PubMed] [Google Scholar]
- Murray J. F., Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1356–1372. doi: 10.1164/ajrccm/141.5_Pt_1.1356. [DOI] [PubMed] [Google Scholar]
- Pottratz S. T., Martin W. J., 2nd Mechanism of Pneumocystis carinii attachment to cultured rat alveolar macrophages. J Clin Invest. 1990 Nov;86(5):1678–1683. doi: 10.1172/JCI114891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottratz S. T., Martin W. J., 2nd Role of fibronectin in Pneumocystis carinii attachment to cultured lung cells. J Clin Invest. 1990 Feb;85(2):351–356. doi: 10.1172/JCI114445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottratz S. T., Paulsrud J. R., Smith J. S., Martin W. J., 2nd Evidence for Pneumocystis carinii binding to a cell-free substrate: role of the adhesive protein fibronectin. J Lab Clin Med. 1994 Feb;123(2):273–281. [PubMed] [Google Scholar]
- Pottratz S. T., Paulsrud J., Smith J. S., Martin W. J., 2nd Pneumocystis carinii attachment to cultured lung cells by pneumocystis gp 120, a fibronectin binding protein. J Clin Invest. 1991 Aug;88(2):403–407. doi: 10.1172/JCI115318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Mosher D. F., Olbrantz P. J. Fibronectin binding to Staphylococcus aureus. J Biol Chem. 1982 Dec 25;257(24):14788–14794. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Radding J. A., Armstrong M. Y., Ullu E., Richards F. F. Identification and isolation of a major cell surface glycoprotein of Pneumocystis carinii. Infect Immun. 1989 Jul;57(7):2149–2157. doi: 10.1128/iai.57.7.2149-2157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkrans W. A., Jr, Albright J. T., Hausman R. E., Penney D. P. Ultrastructural immunocytochemical localization of fibronectin in the developing rat lung. Cell Tissue Res. 1983;234(1):165–177. doi: 10.1007/BF00217410. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Sueishi K., Hisano S., Sumiyoshi A., Tanaka K. Scanning and transmission electron microscopic study of human pulmonary pneumocystosis. Chest. 1977 Aug;72(2):213–216. doi: 10.1378/chest.72.2.213. [DOI] [PubMed] [Google Scholar]
- Tamkun J. W., DeSimone D. W., Fonda D., Patel R. S., Buck C., Horwitz A. F., Hynes R. O. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986 Jul 18;46(2):271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Vogel B. E., Tarone G., Giancotti F. G., Gailit J., Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1). J Biol Chem. 1990 Apr 15;265(11):5934–5937. [PubMed] [Google Scholar]
- Wada M., Kitada K., Saito M., Egawa K., Nakamura Y. cDNA sequence diversity and genomic clusters of major surface glycoprotein genes of Pneumocystis carinii. J Infect Dis. 1993 Oct;168(4):979–985. doi: 10.1093/infdis/168.4.979. [DOI] [PubMed] [Google Scholar]
- Wright T. W., Simpson-Haidaris P. J., Gigliotti F., Harmsen A. G., Haidaris C. G. Conserved sequence homology of cysteine-rich regions in genes encoding glycoprotein A in Pneumocystis carinii derived from different host species. Infect Immun. 1994 May;62(5):1513–1519. doi: 10.1128/iai.62.5.1513-1519.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Sypek J. P., McDonald J. A. In vitro parasite-monocyte interactions in human leishmaniasis: possible role of fibronectin in parasite attachment. Infect Immun. 1985 Aug;49(2):305–311. doi: 10.1128/iai.49.2.305-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K., Walzer P. D. Attachment of Pneumocystis carinii to type I alveolar cells studied by freeze-fracture electron microscopy. Infect Immun. 1983 May;40(2):812–815. doi: 10.1128/iai.40.2.812-815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K., Walzer P. D. Mechanism of pulmonary alveolar injury in experimental Pneumocystis carinii pneumonia in the rat. Br J Exp Pathol. 1981 Aug;62(4):339–346. [PMC free article] [PubMed] [Google Scholar]