Abstract
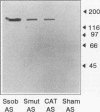
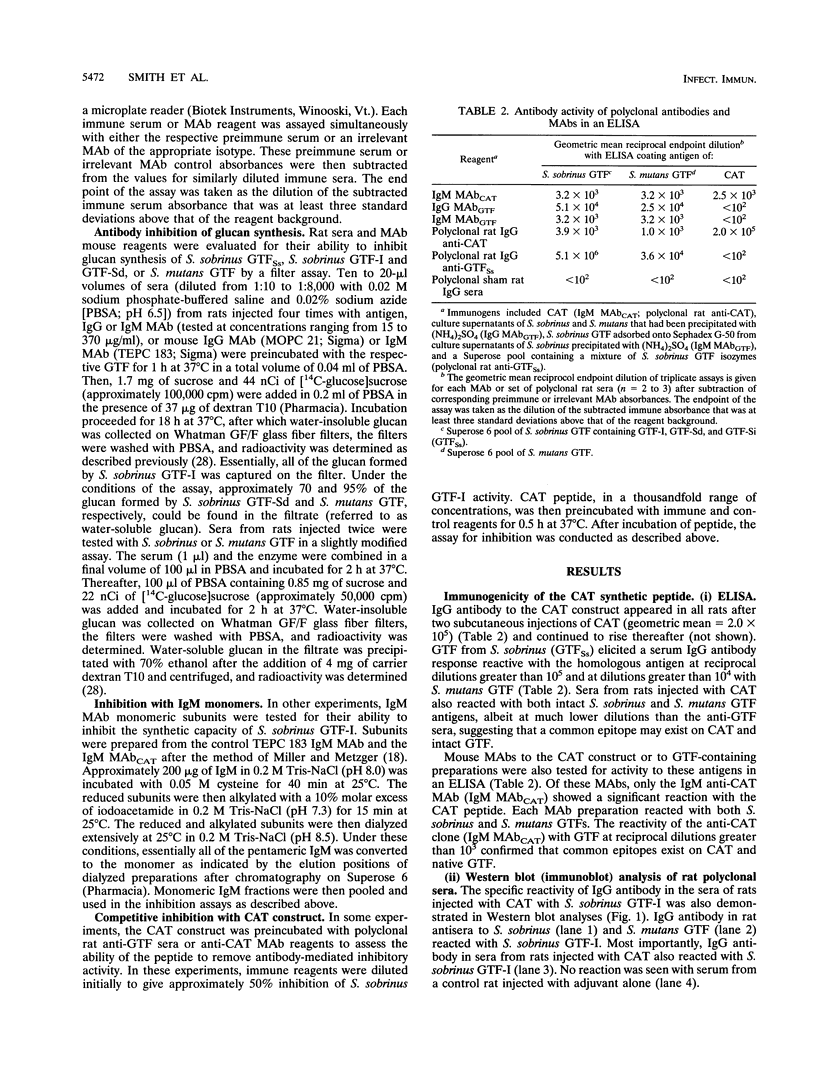
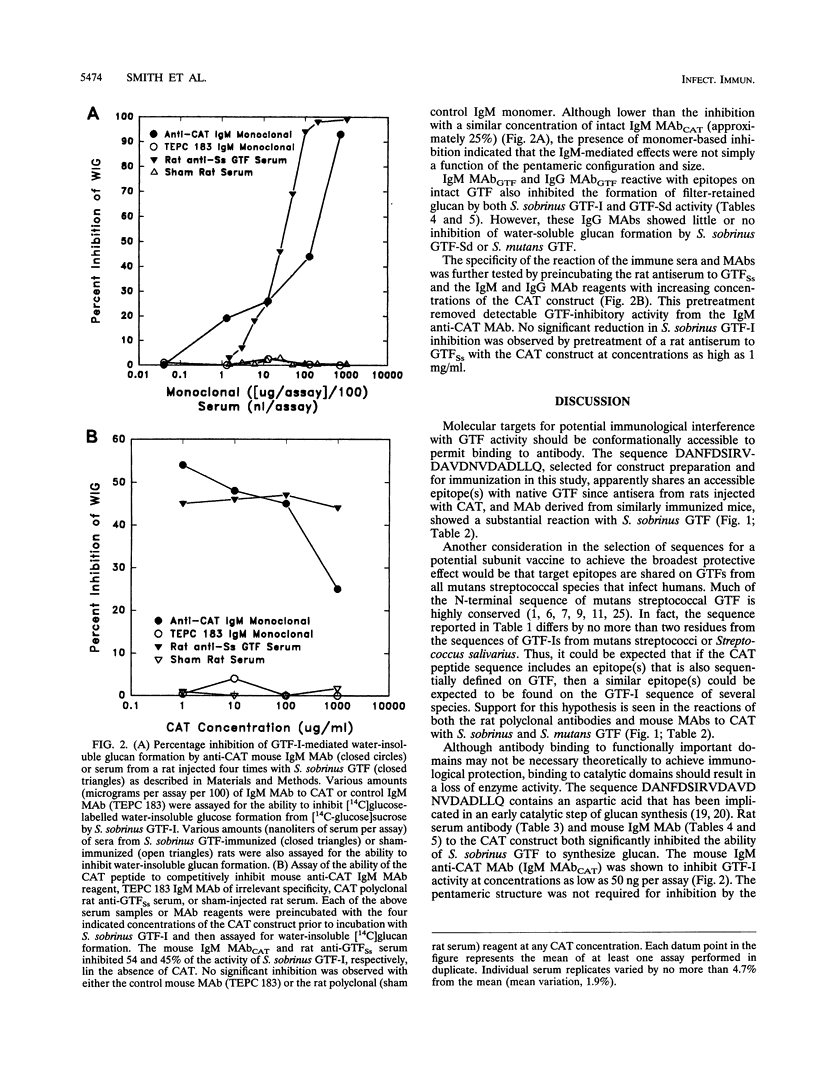
The immunogenicity of a multiple antigenic peptide construct consisting of four copies of the synthetic 21-mer peptide DANFDSIRVDAVDNVDADLLQ was measured. The composition of this peptide was derived from a sequence in the N-terminal region of mutans streptococcal glucosyltransferases (GTFs) containing an aspartic acid implicated in catalysis. The peptide (CAT) construct was synthesized as a tetramer on a lysine backbone and subcutaneously injected into Sprague-Dawley rats for polyclonal antibody formation or intraperitoneally injected into BALB/c mice, and then spleen cell fused with Sp2/0Ag14 murine myeloma cells for monoclonal antibody formation. The resulting rat antisera and mouse monoclonal antibodies reacted with CAT and with native GTF isozymes from Streptococcus sobrinus and Streptococcus mutans (in enzyme-linked immunosorbent assay and Western blot [immunoblot] analyses). Functional inhibition of the water-insoluble glucan synthetic activity of S. sobrinus GTF-I was demonstrated with an immunoglobulin M anti-CAT monoclonal antibody (> 80% inhibited) and with rat sera (approximately 17% inhibited). The monoclonal antibody preparation also modestly inhibited the water-soluble glucan synthetic activity of an S. mutans GTF mixture. These results suggest that the CAT peptide contains B-cell epitopes that are similar to those of intact mutans streptococcal GTFs and has the potential to elicit antibody that can inhibit GTF function. Thus, sequences within this peptide construct may have value for inclusion in a synthetic dental caries vaccine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo H., Matsumura T., Kodama T., Ohta H., Fukui K., Kato K., Kagawa H. Peptide sequences for sucrose splitting and glucan binding within Streptococcus sobrinus glucosyltransferase (water-insoluble glucan synthetase). J Bacteriol. 1991 Feb;173(3):989–996. doi: 10.1128/jb.173.3.989-996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia J. S., Lin R. H., Lin S. W., Chen J. Y., Yang C. S. Inhibition of glucosyltransferase activities of Streptococcus mutans by a monoclonal antibody to a subsequence peptide. Infect Immun. 1993 Nov;61(11):4689–4695. doi: 10.1128/iai.61.11.4689-4695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia J. S., Lin S. W., Hsu T. Y., Chen J. Y., Kwan H. W., Yang C. S. Analysis of a DNA polymorphic region in the gtfB and gtfC genes of Streptococcus mutans. Infect Immun. 1993 Apr;61(4):1563–1566. doi: 10.1128/iai.61.4.1563-1566.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope P. A., Mooser G. Antibodies against active-site peptides common to glucosyltransferases of mutans streptococci. Infect Immun. 1993 Nov;61(11):4814–4817. doi: 10.1128/iai.61.11.4814-4817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertzbaugh M. T., Macrina F. L. Inhibition of Streptococcus mutans glucosyltransferase activity by antiserum to a subsequence peptide. Infect Immun. 1990 Jun;58(6):1509–1513. doi: 10.1128/iai.58.6.1509-1513.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Gilpin M. L., Russell R. R. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J Bacteriol. 1987 Sep;169(9):4271–4278. doi: 10.1128/jb.169.9.4271-4278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard P. M., Simpson C. L., Milward C. P., Jacques N. A. Molecular characterization of a cluster of at least two glucosyltransferase genes in Streptococcus salivarius ATCC 25975. J Gen Microbiol. 1991 Nov;137(11):2577–2593. doi: 10.1099/00221287-137-11-2577. [DOI] [PubMed] [Google Scholar]
- Gilmore K. S., Russell R. R., Ferretti J. J. Analysis of the Streptococcus downei gtfS gene, which specifies a glucosyltransferase that synthesizes soluble glucans. Infect Immun. 1990 Aug;58(8):2452–2458. doi: 10.1128/iai.58.8.2452-2458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988 Aug;56(8):1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989 Jul;57(7):2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda O., Kato C., Kuramitsu H. K. Nucleotide sequence of the Streptococcus mutans gtfD gene encoding the glucosyltransferase-S enzyme. J Gen Microbiol. 1990 Oct;136(10):2099–2105. doi: 10.1099/00221287-136-10-2099. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M., Smith E. E. Purification of dextran-binding protein from cariogenic Streptococcus mutans. Biochem Biophys Res Commun. 1977 Sep 9;78(1):273–278. doi: 10.1016/0006-291x(77)91250-5. [DOI] [PubMed] [Google Scholar]
- McCabe M. M. Purification and characterization of a primer-independent glucosyltransferase from Streptococcus mutans 6715-13 mutant 27. Infect Immun. 1985 Dec;50(3):771–777. doi: 10.1128/iai.50.3.771-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F., Metzger H. Characterization of a human macroglobulin. II. Distribution of the disulfide bonds. J Biol Chem. 1965 Dec;240(12):4740–4745. [PubMed] [Google Scholar]
- Mooser G., Hefta S. A., Paxton R. J., Shively J. E., Lee T. D. Isolation and sequence of an active-site peptide containing a catalytic aspartic acid from two Streptococcus sobrinus alpha-glucosyltransferases. J Biol Chem. 1991 May 15;266(14):8916–8922. [PubMed] [Google Scholar]
- Mooser G., Iwaoka K. R. Sucrose 6-alpha-D-glucosyltransferase from Streptococcus sobrinus: characterization of a glucosyl-enzyme complex. Biochemistry. 1989 Jan 24;28(2):443–449. doi: 10.1021/bi00428a006. [DOI] [PubMed] [Google Scholar]
- Mooser G., Wong C. Isolation of a glucan-binding domain of glucosyltransferase (1,6-alpha-glucan synthase) from Streptococcus sobrinus. Infect Immun. 1988 Apr;56(4):880–884. doi: 10.1128/iai.56.4.880-884.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Shiroza T., Kuramitsu H. K., Ferretti J. J. Homology of glucosyltransferase gene and protein sequences from Streptococcus sobrinus and Streptococcus mutans. J Dent Res. 1988 Mar;67(3):543–547. doi: 10.1177/00220345880670030401. [DOI] [PubMed] [Google Scholar]
- Shiroza T., Ueda S., Kuramitsu H. K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987 Sep;169(9):4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Holmberg C. F., Eastcott J., King W. F., Ali-Salaam P. Antigenicity and immunogenicity of a synthetic peptide derived from a glucan-binding domain of mutans streptococcal glucosyltransferase. Infect Immun. 1993 Jul;61(7):2899–2905. doi: 10.1128/iai.61.7.2899-2905.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A. Oral immunization of humans with Streptococcus sobrinus glucosyltransferase. Infect Immun. 1987 Nov;55(11):2562–2569. doi: 10.1128/iai.55.11.2562-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in rats and hamsters. J Immunol. 1977 Feb;118(2):710–720. [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J., King W. F., Eastcott J. W., Bergey E. J., Levine M. J. Immune properties of glucosyltransferases from S. sobrinus. J Oral Pathol. 1988 Nov;17(9-10):466–470. doi: 10.1111/j.1600-0714.1988.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Ueda S., Shiroza T., Kuramitsu H. K. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene. 1988 Sep 15;69(1):101–109. doi: 10.1016/0378-1119(88)90382-4. [DOI] [PubMed] [Google Scholar]
- Weinstein Y., Givol D., Strausbauch P. H. The fractionation of immunoglobulins with insolubilized concanavalin A. J Immunol. 1972 Dec;109(6):1402–1404. [PubMed] [Google Scholar]