Abstract
Microvascular failure largely underlies the damaging secondary events that accompany traumatic brain injury (TBI). Changes in capillary permeability result in the extravasation of extracellular fluid, inflammatory cells, and blood, thereby producing cerebral edema, inflammation, and progressive secondary hemorrhage (PSH). Recent work in rat models of TBI and stroke have implicated two ion transport proteins expressed in brain endothelial cells as critical mediators of edema formation: the constitutively-expressed Na+-K+-2Cl− cotransporter, NKCC1, and the trauma/ischemia-induced SUR1-regulated NCCa-ATP (SUR1/TRPM4) channel. Whereas NKCC1 function requires ATP, activation of SUR1/TRPM4 occurs only after ATP depletion. This opposite dependence on intracellular ATP levels implies that one or the other mechanism will activate/deactivate as ATP concentrations rise and fall during periods of ischemia/reperfusion, resulting in continuous edema formation regardless of cellular energy status. Moreover, with critical ATP depletion, sustained opening of SUR1/TRPM4 channels results in the oncotic death of endothelial cells, leading to capillary fragmentation and secondary hemorrhage. Bumetanide and glibenclamide are two well-characterized, safe, FDA-approved drugs that inhibit NKCC1 and the SUR1/TRPM4 channel, respectively. When used alone, these drugs have documented beneficial effects in animal models of TBI-and ischemia-associated cerebral edema and PSH. Given the mechanistic and temporal differences by which NKCC1 and the SUR1/TRPM4 channel contribute to the pathophysiology of these events, combination therapy with bumetanide and glibenclamide may yield critical synergy in preventing injury-associated capillary failure.
Keywords: Traumatic brain injury, ischemia, capillary, NKCC1, SUR1, TRPM4
The clinical problem of traumatic brain injury (TBI)
Each year, 1.5 million Americans sustain TBI. As a result, 50,000 people die, 230,000 people are hospitalized and survive, and 80,000–90,000 people experience the onset of long-term disability.19,50 TBI is the leading cause of death and disability in children and adults ages 1–44 years. Overall, more than 5 million Americans – 2% of the U.S. population – currently live with disabilities resulting from TBI. The consequences in terms of physical impairments, functional limitations, disabilities, societal restrictions, and economic impact are practically immeasurable. In spite of its importance, there is no effective therapy in clinical use that is specifically directed towards ameliorating secondary brain injury after trauma. An important reason for this unfortunate deficiency in clinical care is an incomplete understanding of cellular and molecular processes that underlie secondary brain injury. One important area of deficiency concerns mechanisms of secondary injury related to microvascular dysfunction, in particular, edema formation and “progressive secondary hemorrhage”.
Early consequences of TBI: cerebral edema and progressive secondary hemorrhage
The pathophysiology of TBI is complex and involves multiple injury mechanisms that are spatially and temporally specific. Primary traumatic injury is invariably complicated by secondary injury, which results in expansion of the original lesion and concomitant worsening of neurological outcome. Mechanisms of secondary injury may include cytotoxic processes, such as excitotoxicity, free radical damage, apoptosis, and inflammation. In addition, secondary injury may result from microvascular dysfunction, including ischemia, edema, and progressive secondary hemorrhage, a phenomenon wherein microvessels gradually lose their structural integrity and become fragmented, resulting in extravasation of blood and formation of petechial hemorrhages.
Cerebral edema
Edema complicates virtually all forms of severe CNS injury. The primary mechanical insults of TBI and its secondary metabolic effects (such as ischemia) alter the permeability of the blood-brain-barrier, blood-CSF barrier, and neuroglial cell membranes, thereby disrupting fluid and solute homeostasis of the brain. Minor changes in the composition of ions in the brain’s extracellular or intracellular fluid can significantly affect the function of neurons, which rely on precise ionic gradients across their plasma membranes to modulate finely-tuned changes in membrane potential that underlie neuronal signaling. Because the contents of the brain are confined by the bony calvarium, even small increases in the total volume of intracranial fluid due to edema can exert mechanical forces that disrupt normal anatomical relationships and increase pressure within the skull. As intracranial pressure approaches that of the brain capillaries, perfusion is compromised, leading to ischemia and further accumulation of edema fluid. These increases in intracranial pressure can result in devastating neurological injury, including infarction, herniation, and death. The degree of cerebral edema after TBI has been classified into four categories of severity, based on studies by the NIH Traumatic Coma Data Bank.25 Using this classification, the degree of brain edema documented on the first head computerized tomography (CT) scan obtained after injury has been shown to be highly correlated with patient outcome.25 Thus, the reduction of cerebral edema after TBI is important, and new pharmacological tools for decreasing it are badly needed.
Progressive secondary hemorrhage
Contusion of brain often results in formation of intraparenchymal petechial hemorrhages, which are frequently complicated by “blossoming” or expansion – i.e., progressive secondary hemorrhage (PSH).9,30,53 PSH is a key mechanism of secondary injury post-TBI.24,52 Early progressive hemorrhage occurs in almost 50% of head-injured patients, usually following contusion injury, and is associated with elevations in ICP.45,56
Although sometimes erroneously attributed to continued bleeding of microvessels fractured by the original trauma, PSH actually represents a secondary pathological process with specific, well-defined molecular antecedents, as has been shown in spinal cord injury.12,42 PSH occurs during the first several hours after a traumatic insult. It results from progressive catastrophic failure of the structural integrity of capillaries, and is characterized by formation of small discrete satellite (petechial) hemorrhages in tissues surrounding the site of primary injury. With time, petechial hemorrhages increase in number and eventually coalesce into a hemorrhagic lesion that encompasses the entire site of primary injury. PSH is particularly damaging because it greatly expands the volume of neural tissue destroyed by the primary injury. The capillary dysfunction underlying PSH also causes tissue ischemia and hypoxia, and the hemorrhage that characterizes PSH is exquisitely toxic to neural tissues,32,54 because it incites free radical formation and inflammatory responses that are especially damaging to myelin of white matter tracks, thereby worsening the overall neurological injury. Together, these processes render PSH the most destructive mechanism of secondary injury involving the CNS. Whereas ischemia and edema have historically been targeted for treatment, PSH has not -- simply because secondary hemorrhage has not previously been viewed as being preventable.
NKCC1: role in edema formation and neurotoxicity in TBI and ischemia
Recent work in rat models of stroke and TBI have implicated two ion transport proteins expressed in brain endothelial cells as critical mediators of edema formation and/or progressive secondary hemorrhage: the constitutively-expressed, bumetanide-sensitive Na+-K+-2Cl− cotransporter NKCC1, and the ischemia-induced, glibenclamide-sensitive SUR1-regulated NCCa-ATP (SUR1/TRPM4) channel.
Derangements in ion transport across brain cell membranes, the blood-brain-barrier, and the choroid plexus underlie the formation of cerebral edema. The Na+-K+-2Cl− cotransporter NKCC1 is expressed in glia, cortical and cerebellar neurons, brain capillary endothelial cells, and epithelial cells of the choroid plexus; in these cells, NKCC1 plays an essential role in cell volume regulation and/or transepithelial ion transport. Ischemia-induced isosmotic cell swelling - cytotoxic edema - is a primary driver of cerebral edema, and alterations in transendothelial ion transport underlie ionic and vasogenic edema. Expressed on the luminal side of brain endothelial cells, NKCC1 plays an important role in the formation of ionic edema by loading sodium into endothelial cells.4,11,18,27–29 The sodium inside endothelial capillary cells is then expelled into the brain’s extracellular space by the activity of Na+-K+ ATPase, which is expressed on the abluminal membrane. Trauma and its associated ischemic injury to brain cells, through various mechanisms, has been shown to increase the expression and activity of NKCC1, resulting in impaired cell volume regulation, pathologic cell swelling, and the alterations in transendothelial capillary permeability that accompany ionic and vasogenic edema. Genetic deficiency or pharmacalogic inhibition of NKCC1 with a low dose of the furosemide-related diuretic, bumetanide, significantly reduce cerebral edema and neuronal injury following traumatic and ischemic brain injury.1,4,5,8,11,18,28,29,35,47,48,57
NKCC1 is a cation-chloride cotransporter (CCC) of the SLC12 gene family. NKCC1 is an intrinsic membrane protein that transports chloride ions, together with sodium and/or potassium ions, across plasma membranes of cells. The stoichiometric coupling and directionality of the cations and chloride ions translocated by the NKCC1 results in an electrically-silent (i.e., electroneutral), secondarily-active transport process that is energetically driven by transmembrane sodium and potassium gradients established by Na+-K+ ATPase. Utilizing the large electrochemically-favorable inward gradient for sodium ions across the plasma membrane, NKCC1 loads chloride ions into the cell, raising the level of intracellular chloride ([Cl−]i) above its electrochemical equilibrium. At low concentrations (2–10 µM), bumetanide is a specific inhibitor of NKCC1.
The activity of NKCC1 determines [Cl−]i. Neurons, glia, endothelial cells, and epithelial cells that line the brain’s ventricular system regulate [Cl−]i to help maintain their cellular volume amidst changes of extracellular osmolality and intracellular solute content, thus preventing the excessive swelling or shrinking that could undermine their structural integrity. Derangements in the expression and activity of NKCC1 can alter [Cl−]i, disrupting the homeostasis of cell volume and altering the absorption or secretion of ions across epithelia.
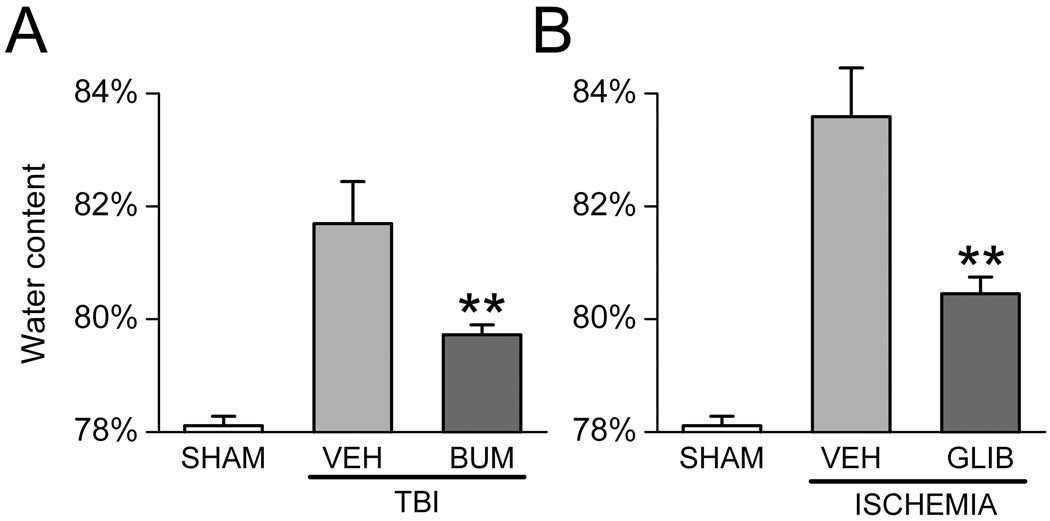
NKCC1 contributes to cerebral edema following TBI.20–22,46 A transient time-dependent up-regulation of NKCC1 mRNA and protein was detected in rodent hippocampus after TBI induced by the calibrated weight drop technique.20 Inhibition of NKCC1 with bumetanide 20 minutes prior to TBI decreased edema and reduced contusion damage.21 This effect of bumetanide was correlated with decreased phosphorylation of Raf/MEK/ERK cascade proteins.20 Cerebral edema from cold trauma is decreased with torasemide, another NKCC1 inhibitor that can also block chloride channels.46 Although torasemide can result in a systemic dehydration that could affect brain edema, in these studies plasma osmolarity was not changed, suggesting that torasemide-mediated effects on brain swelling were through NKCC1 inhibition.46 NKCC1 mRNA and protein levels are also up-regulated on the apical membrane of the choroid plexus 2 hours after TBI, with levels peaking at 8 hours and lasting for 24 hours.21 In this study, while rats in the experimental group displayed severe brain edema and contusion volume 8 hours after TBI, administration of bumetanide significantly attenuated contusion volume and brain edema. We have independently confirmed the potent effect of bumetanide in ameliorating edema following TBI (Fig. 1).
Figure 1. Bumetanide and glibenclamide reduce edema formation.
A,B: Brain water content in rodent models of TBI (A) and cerebral ischemia (B), without (VEH) and with treatment using bumetanide (BUM) (A) or glibenclamide (GLIB) (B). TBI was induced by cortical impact (2.5–3 atm) following craniectomy to expose the dura; bumetanide (15 mg/kg, i.p., plus 200 µg/hr s.q. via mini-osmotic pump) was administered starting within a few minutes after injury, and tissue water was determined at 6 hr (Simard and Gerzanich, unpublished). Ischemia was induced by permanent occlusion of the middle cerebral artery; glibenclamide (75 ng/hr, s.q. via mini-osmotic pump) was administered starting within a few minutes after injury, and tissue water was determined at 8 hr (from Simard et al.37). Brain water was determined using the wet weight/dry weight method; 5 rats per group; **, P<0.01.
Glutamate-mediated neurotoxicity also plays an important role in neuronal damage after traumatic and ischemic injury in the CNS. Acute excitotoxic neurodegeneration after glutamate receptor activation is dependent on sodium and chloride entry.34 Via an unknown mechanism, activation of NMDA and AMPA receptors stimulates NKCC1 activity in neurons.35 Thus, NKCC1 contributes to the overload of sodium and chloride during glutamate-mediated acute excitotoxicity.1 Blocking NKCC1 activity with bumetanide abolishes the glutamate-triggered sodium and chloride accumulation by over 50%.1 Oxygen-glucose deprivation (OGD)-induced neuronal death is also mediated by NMDA ionotropic receptor-triggered excitotoxicity.13 Bumetanide has also been shown to abrogate glutamate and NMDA-mediated excitotoxic cell death. OGD-mediated cell swelling and death is also significantly attenuated by bumetanide.1
During ischemia, the extracellular level of potassium, [K+]o, increases significantly. A few minutes of anoxia/ischemia raises [K+]o to ~60 mM. In astrocytes, NKCC1 has been shown to play an important role in potassium uptake under conditions of high [K+]o. In 75 mM [K+]o, NKCC1-mediated potassium influx was significantly stimulated in astrocytes; this high-[K+]o-induced activation of NKCC1 is completely abolished by either removal of extracellular calcium or blocking of L-type voltage-dependent calcium channels with nifedipine.47,48 These data suggest that NKCC1 activity is stimulated under high [K+]o via calcium-mediated signal transduction pathways. Intracellular accumulation of radiolabeled sodium and chloride is significantly increased in response to 75 mM [K+]o; this increase is abolished by bumetanide or by genetic ablation of NKCC1.47,48 High [K+]o-mediated stimulation of NKCC1 can result in cell swelling via a net increase of intracellular sodium, potassium, and chloride and accompanying water. High [K+]o causes cell swelling in NKCC1+/+ astrocytes, but is absent in NKCC1−/− astrocytes, and is abolished with bumetanide.47,48 High [K+]o-induced astrocyte swelling is also observed in the rat optic nerve model.23 In enucleated nerves, light transmittance progressively increases with high [K+]o, causing cell swelling. Bumetanide can reversibly suppress this high [K+]o-induced cell swelling.23
Multiple in vitro and in vivo studies have also shown that NKCC1 plays a role in ischemic cell damage, and that its pharmacologic inhibition is neuroprotective.57 Administration of bumetanide either prior to ischemia or during ischemia significantly reduces brain edema and infarction after 2-hour middle cerebral artery occlusion (MCAo) and 24-hour reperfusion in rats.57 Similar neuroprotection is observed in NKCC1-null mice following MCAo.5 Focal cerebral ischemia leads to increased NKCC1 protein expression in the ipsilateral cortex and striatum after 2-hour ischemia and 24-hour reperfusion in rats.57 In rats subjected to permanent MCAo, intravenous administration of bumetanide immediately before occlusion attenuates edema formation as determined by brain MR imaging.28,29 This further suggests a role for NKCC1 in edema formation during cerebral ischemia.
SUR1/TRPM4 channel in ischemia and TBI
The SUR1-regulated NCCa-ATP (herein called “SUR1/TRPM4”) channel is a critical mediator of cerebral edema formation. This newly discovered channel is formed from two subunits, a regulatory subunit, SUR1 and a pore-forming subunit, TRPM4.6,12,37,41,43 The properties of this channel have been reviewed.39,41,43 It is a 35 pS cation channel that conducts inorganic monovalent cations, but is impermeable to Ca2+ and Mg2+.7 Channel opening requires nanomolar concentrations of Ca2+ on the cytoplasmic side, and is prevented by intracellular ATP (EC50, ~1 µM). Although no specific blocker exists for the pore-forming subunit, TRPM4, fortunately the receptor-channel complex, SUR1/TRPM4, is blocked with high affinity and specificity by the sulfonylurea antagonist, glibenclamide (EC50, 48 nM).6
The SUR1/TRPM4 channel is not constitutively expressed, but is expressed in the CNS under conditions of injury or hypoxia. The channel was first discovered in reactive astrocytes obtained from the hypoxic inner zone of the gliotic capsule after stab injury and foreign body implantation.6,7 Since then, it has been identified using patch clamp electrophysiology in neurons from the core of an ischemic stroke37 and in cultured human and mouse endothelial cells subjected to hypoxia.42
Apart from patch clamp recordings to demonstrate presence of the channel, CNS tissues have been analyzed to detect the regulatory subunit, SUR1, at protein and mRNA levels. Normally, SUR1 is expressed in some neurons, but not in astrocytes or capillaries. Following injury, SUR1 is upregulated in several rodent models of CNS injury, including cerebral ischemia (MCAo),37,44 penetrating brain injury with foreign body,6 subarachnoid hemorrhage,38 spinal cord injury42 and TBI (Simard and colleagues, unpublished). In humans, SUR1 is upregulated in brain parenchyma adjacent to an intracerebral hemorrhage,43 in germinal matrix of premature infants who sustain or are at risk to sustain germinal matrix hemorrhage,36 in spinal cord injury40 and TBI (Simard and colleagues, unpublished). Upregulation of SUR1 is found in all members of the neurovascular unit, i.e., neurons, astrocytes and capillary endothelial cells.
The consequences of opening the SUR1/TRPM4 channel have been studied in cells by depleting ATP to mimic injury conditions. ATP depletion induces a strong inward current that depolarizes the cell completely to 0 mV. Cells subsequently undergo oncotic cell swelling (cytotoxic edema). Eventually, ATP-depletion leads to cell death, predominantly by non-apoptotic, propidium iodide-positive oncotic (necrotic) cell death. Oncotic cell death is significantly inhibited by glibenclamide.37
SUR1/TRPM4 channel: role in edema formation in ischemia and TBI
The effect of block with the potent SUR1 inhibitor, glibenclamide, on edema formation and brain swelling has been studied in four rat models of ischemic stroke – malignant cerebral edema,37 thromboembolic stroke,37,44 permanent MCAo44 and temporary MCAo with reperfusion.44 In the malignant cerebral edema model, glibenclamide reduced mortality and cerebral edema by half (Fig. 1).37 In non-lethal stroke models, glibenclamide reduced lesion volume by half, and its use was associated with cortical sparing that was attributed to improved leptomeningeal collateral blood flow due to reduced mass effect from edema.37,44 The therapeutic window for glibenclamide in MCAo extends beyond 6 hr after onset of ischemia.44 In a rat model of subarachnoid hemorrhage, block of SUR1 with glibenclamide significantly reduced inflammation, vasogenic edema and caspase-3 activation.38
Ionic versus vasogenic edema
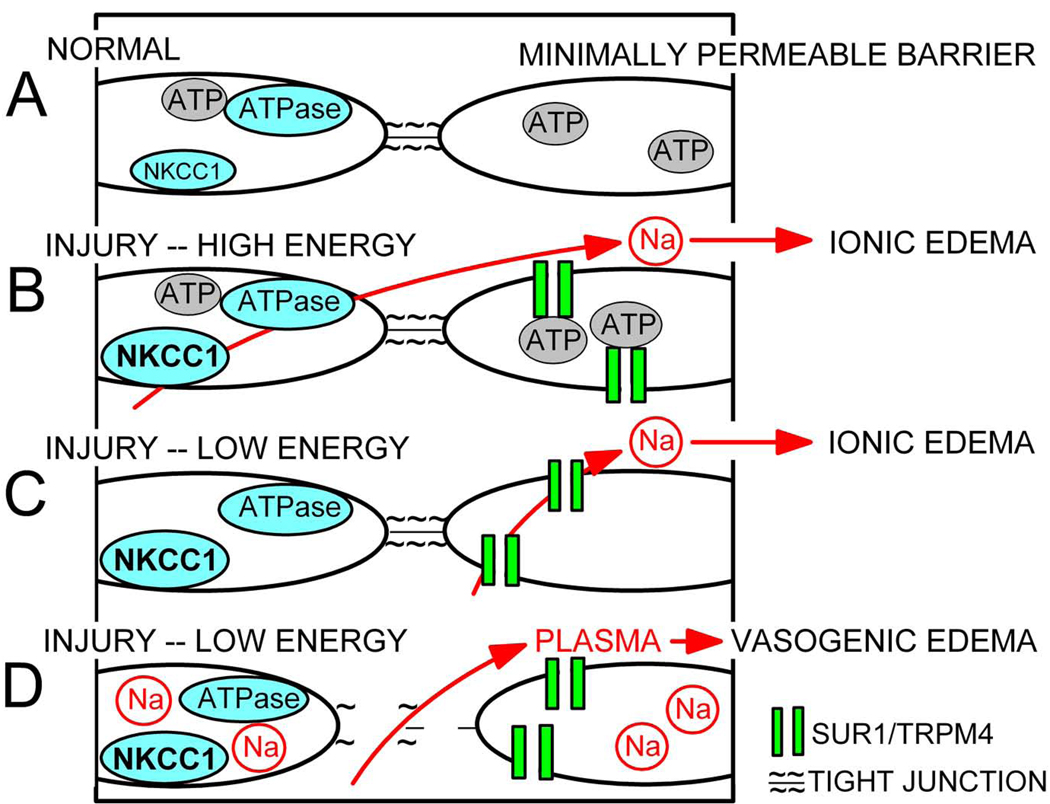
Cerebral edema may be distinguished as “ionic” edema or “vasogenic” edema, depending upon the principal solute that is transported across the capillary barrier (reviewed in Simard et al.39). An excess of sodium-rich, protein-poor fluid is referred to as ionic edema, whereas protein-rich fluid is referred to as vasogenic edema. Formation of ionic edema is believed to be due to transcellular flux of sodium across the endothelial cell barrier, which as mentioned previously, can be due to activity of NKCC1/Na+-K+ ATPase or to SUR1/TRPM4 (Fig. 2).
Figure 2. Molecular mechanisms of edema formation.
A–D: Portions of endothelial blood-brain barrier, depicted as pairs of adjacent endothelial cells joined by tight junctions, under normal conditions, wherein NKCC1 is minimally active and SUR1/TRPM4 is not expressed (A), and following CNS injury (B–D). In B, NKCC1 is activated and SUR1/TRPM4 is newly synthesized due to the injury; because energy levels are relatively high, influx of sodium via NKCC1 on the luminal side is compensated by extrusion of sodium via Na+-K+ ATPase on the abluminal side, leading to transcellular flux of sodium which drives formation of ionic edema; SUR1/TRPM4 is inactive due to block by ATP. In C, because energy levels are low, NKCC1 is inactive and ATP block of SUR1/TRPM4 is relieved, again leading to transcellular flux of sodium and formation of ionic edema. In D, excess accumulation of sodium inside of endothelial cells results in cytoskeletal alterations and compromise of intercellular tight junctions, leading to paracellular flux of plasma, a.k.a. formation of vasogenic edema.
Formation of vasogenic edema is more complex and requires the introduction of additional cellular elements. Dynamic control of the endothelial barrier involves signaling between the endothelial cytoskeleton and the adhesion complexes between neighboring cells.17,51 The actual barrier is made up of the physical elements of tight junction complexes, the major constituents of which include transmembrane (junctional adhesion molecule-1, occludin, and claudins) and cytoplasmic (zonula occludens [ZO] -1 and -2, cingulin, AF-6, and 7H6) proteins linked to the actin cytoskeleton of endothelial cells.14,51 The proper cellular location and sealing of inter-endothelial tight junctions depends on the scaffolding properties of ZO-1 and its relation to the actin cytoskeleton.10,17 Injury or insult causes rearrangement of the cytoskeleton, ZO-1 disruption, cell retraction and formation of intercellular gaps,2,10,55 which allow paracellular flow of protein-rich plasma, a.k.a., formation of vasogenic edema (Fig. 2).
In many cells, an increase in sodium concentration or a perturbation of cell volume leads to reorganization of the actin cytoskeleton and weakening of intercellular tight junctions, leading to an increase in barrier permeability.31 This same phenomenon is believed to occur in brain endothelial cells in which SUR1/TRPM4 channels are activated. Upregulation and activation of SUR1/TRPM4 channels leads to excess sodium influx and cytotoxic edema of endothelial cells,6,7,42 providing a plausible molecular mechanism to account for the cytoskeletal rearrangement that leads to formation of vasogenic edema. We recently reported the redistribution of ZO-1 in the endothelium of the posterior cerebral artery in association with formation of vasogenic edema following subarachnoid hemorrhage, along with concomitant reduction in these abnormalities by SUR1 inhibition with glibenclamide,38 consistent with the hypothesis that vasogenic edema is tied to a significant disruption of the endothelial actin cytoskeleton. This conceptualization provides a coherent picture linking an insult that results in upregulation and opening of SUR1/TRPM4 channels in endothelial cells to sodium influx, endothelial cell swelling and actin cytoskeletal re-arrangement, loss of endothelial tight junction integrity, and formation of vasogenic edema.
SUR1/TRPM4 channel: role in progressive secondary hemorrhage in TBI and ischemia
Apart from its important role in formation of edema, the SUR1/TRPM4 channel is also critically involved in progressive secondary hemorrhage.12,42 When ATP is depleted to critical levels for sustained periods of time, unopposed opening of SUR1/TRPM4 channels results eventually in oncotic death of endothelial cells, leading to capillary fragmentation and secondary hemorrhage, resulting in even greater tissue destruction. This mechanism of secondary hemorrhage was first discovered during study of a rodent model of spinal cord injury (SCI).12,42 SCI results in progressive secondary hemorrhage, characterized by a progressively expansive lesion with fragmentation of capillaries, hemorrhage that doubles in volume over 12 hr, tissue necrosis and severe neurological dysfunction. Necrotic lesions are surrounded by widespread upregulation of SUR1 in capillaries and neurons. Following SCI, block of SUR1 by glibenclamide essentially eliminates capillary fragmentation and progressive secondary hemorrhage, is associated with a 3-fold reduction in lesion volume, and results in marked neurobehavioral functional improvement.
ATP requirements of NKCC1/Na+-K+ ATPase versus the SUR1/TRPM4 channel – synergy in molecular mechanisms
It is particularly noteworthy that the two molecular mechanisms of ionic edema formation involving NKCC1/Na+-K+ ATPase and the SUR1/TRPM4 channel exhibit opposite dependencies on the cellular concentration of ATP (Fig. 2).
NKCC1, expressed on the luminal side of the endothelium, loads sodium into endothelial cells,4,11,18,28,29 which then expel the sodium into the brain’s extracellular space by the activity of abluminal Na+-K+ ATPase. Because of the obligatory requirement for ATP for functional activation of Na+-K+ ATPase, the contribution of NKCC1/Na+-K+ ATPase to ionic edema is important, both in the early stages of ischemia as well as during reperfusion, at times when endothelial cells have adequate levels of ATP to drive the activity of the Na+-K+ ATPase.
Conversely, the SUR1/TRPM4 channel, which is expressed on both the luminal and abluminal membranes, is inactive when ATP levels are adequate. The channel opens only when ATP levels are significantly decreased (EC50, ~1 µM at neutral pH), and when opened on both luminal and abluminal sides, it facilitates transcapillary flux of sodium from blood to the brain’s extracellular space, which in turn drives the formation of ionic edema.39
This opposite dependence on cellular ATP suggests that one or the other mechanism will activate/deactivate as ATP concentrations rise and fall during periods of ischemia/reperfusion, thereby resulting in continuous formation of ionic edema, regardless of energy status. When ATP levels are adequate, NKCC1/Na+-K+ ATPase will be active and SUR1/TRPM4 will be blocked; when ATP levels are low, NKCC1/Na+-K+ ATPase will be inactive and SUR1/TRPM4 will be become active (Fig. 2). Given this framework, inhibiting one and not the other will yield incomplete inhibition of edema formation (Fig. 1), whereas inhibiting both simultaneously is expected to yield strong synergy in combating formation of edema.
Bumetanide and glibenclamide – synergistic combination therapy
Given their prominent role in the microvascular failure that largely underlies the cerebral edema and progressive secondary hemorrhage resulting from traumatic and ischemic brain injury, a novel therapeutic combination of drugs targeting NKCC1 and the SUR1/TRPM4 channel would be expected to yield critical synergy in preventing capillary failure, optimizing microvascular circulation, maximizing tissue preservation, and thereby improving functional outcome. Bumetanide and glibenclamide are two FDA-approved drugs with strong neuroprotective potential that inhibit NKCC1 and the SUR1/TRPM4 channel, respectively.
Bumetanide is a loop diuretic of the sulfamyl category used to treat heart failure. Experience with its use in humans over three decades indicates that it is safe, effective and well tolerated when taken chronically,3,49 suggesting that short-term use post-injury would also be safe and well tolerated. Bumetanide is a relatively specific inhibitor of NKCC1 at low concentrations (~2–10 µM). No published works have studied bumetanide accumulation in the CNS after systemic administration, but the drug’s favorable lipid:water partition coefficient, along with its documented effects on cerebral edema and seizures in vivo in animals and humans, suggest that bumetanide can cross the blood–brain barrier sufficiently to inhibit NKCC1 in the brain. Pilot studies are now underway studying the efficacy of bumetanide, administered with phenobarbital, for the treatment of another neurological condition, neonatal seizures (FDA IND #101690; see http://www.cureepilepsy.org/research/current.asp).15
Glibenclamide (a.k.a. glyburide), a sulfonylurea used to treat type II diabetes, is one of two oral antidiabetics in the World Health Organization Model List of Essential Medicines. Experience with its use in humans over four decades indicates that it is safe, effective and well tolerated when taken chronically,26,33 suggesting that short-term use post-injury would also be safe and well tolerated. A recent analysis was carried out of patients with diabetes mellitus hospitalized within 24 hr of onset of acute ischemic stroke to determine whether concurrent use of sulfonylureas might affect stroke outcome.16 The study compared 33 patients taking a sulfonylurea (e.g., glibenclamide) at admission through discharge (treatment group) and 28 patients not on a sulfonylurea (control group). The primary outcome, a decrease in National Institutes of Health Stroke Scale (NIHSS) of 4 points or more from admission to discharge or a discharge NIHSS score = 0 (“major neurological improvement”), was reached by 36% of patients in the treatment group and 7% in the control group (P=0.007), consistent with significant neuroprotection.
Summary
New understanding of the fundamental molecular mechanisms responsible for microvascular failure in CNS ischemia and trauma provides novel insights into genuinely new therapeutic approaches. Given the mechanistic and temporal differences by which NKCC1 and the SUR1/TRPM4 channel contribute to the pathophysiology of secondary injury, it is likely that combination therapy with agents such as bumetanide plus glibenclamide will yield critical synergy in preventing capillary failure – thereby optimizing microvascular circulation, maximizing tissue preservation, and improving functional outcome after CNS ischemia and trauma.
Acknowledgement
This work was supported by grants to JMS from the National Heart, Lung and Blood Institute (HL082517), the National Institute of Neurological Disorders and Stroke (NS061808, NS060801), the Department of Veterans Affairs (Baltimore, MD), and the Christopher and Dana Reeve Foundation; and to VG from the National Institute of Neurological Disorders and Stroke (NS061934).
Reference List
- 1.Beck J, Lenart B, Kintner DB, et al. Na-K-Cl cotransporter contributes to glutamate-mediated excitotoxicity. J Neurosci. 2003;23:5061–5068. doi: 10.1523/JNEUROSCI.23-12-05061.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blum MS, Toninelli E, Anderson JM, et al. Cytoskeletal rearrangement mediates human microvascular endothelial tight junction modulation by cytokines. Am J Physiol. 1997;273:H286–H294. doi: 10.1152/ajpheart.1997.273.1.H286. [DOI] [PubMed] [Google Scholar]
- 3.Brater DC. Clinical pharmacology of loop diuretics in health and disease. Eur Heart J. 1992;13 Suppl G:10–14. doi: 10.1093/eurheartj/13.suppl_g.10. [DOI] [PubMed] [Google Scholar]
- 4.Brillault J, Lam TI, Rutkowsky JM, et al. Hypoxia effects on cell volume and ion uptake of cerebral microvascular endothelial cells. Am J Physiol Cell Physiol. 2008;294:C88–C96. doi: 10.1152/ajpcell.00148.2007. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Luo J, Kintner DB, et al. Na(+)-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:54–66. doi: 10.1038/sj.jcbfm.9600006. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Dong Y, Simard JM. Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J Neurosci. 2003;23:8568–8577. doi: 10.1523/JNEUROSCI.23-24-08568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Simard JM. Cell swelling and a nonselective cation channel regulated by internal Ca2+ and ATP in native reactive astrocytes from adult rat brain. J Neurosci. 2001;21:6512–6521. doi: 10.1523/JNEUROSCI.21-17-06512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortez SC, McIntosh TK, Noble LJ. Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res. 1989;482:271–282. doi: 10.1016/0006-8993(89)91190-6. [DOI] [PubMed] [Google Scholar]
- 10.Fanning AS, Little BP, Rahner C, et al. The unique-5 and -6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol Biol Cell. 2007;18:721–731. doi: 10.1091/mbc.E06-08-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foroutan S, Brillault J, Forbush B, et al. Moderate-to-severe ischemic conditions increase activity and phosphorylation of the cerebral microvascular endothelial cell Na+-K+-Cl- cotransporter. Am J Physiol Cell Physiol. 2005;289:C1492–C1501. doi: 10.1152/ajpcell.00257.2005. [DOI] [PubMed] [Google Scholar]
- 12.Gerzanich V, Woo SK, Vennekens R, et al. De novo expression of TRPM4 initiates secondary hemorrhage in spinal cord injury. Nat Medicine. 2009 doi: 10.1038/nm.1899. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwag BJ, Koh JY, DeMaro JA, et al. Slowly triggered excitotoxicity occurs by necrosis in cortical cultures. Neuroscience. 1997;77:393–401. doi: 10.1016/s0306-4522(96)00473-3. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 15.Kahle KT, Staley KJ. The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures. Neurosurg Focus. 2008;25:E22. doi: 10.3171/FOC/2008/25/9/E22. [DOI] [PubMed] [Google Scholar]
- 16.Kunte H, Schmidt S, Eliasziw M, et al. Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke. 2007;38:2526–2530. doi: 10.1161/STROKEAHA.107.482216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai CH, Kuo KH, Leo JM. Critical role of actin in modulating BBB permeability. Brain Res Brain Res Rev. 2005;50:7–13. doi: 10.1016/j.brainresrev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Lam TI, Anderson SE, Glaser N, et al. Bumetanide reduces cerebral edema formation in rats with diabetic ketoacidosis. Diabetes. 2005;54:510–516. doi: 10.2337/diabetes.54.2.510. [DOI] [PubMed] [Google Scholar]
- 19.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Lu KT, Cheng NC, Wu CY, et al. NKCC1-mediated traumatic brain injury-induced brain edema and neuron death via Raf/MEK/MAPK cascade. Crit Care Med. 2008;36:917–922. doi: 10.1097/CCM.0B013E31816590C4. [DOI] [PubMed] [Google Scholar]
- 21.Lu KT, Wu CY, Cheng NC, et al. Inhibition of the Na+ -K+ -2Cl- -cotransporter in choroid plexus attenuates traumatic brain injury-induced brain edema and neuronal damage. Eur J Pharmacol. 2006;548:99–105. doi: 10.1016/j.ejphar.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 22.Lu KT, Wu CY, Yen HH, et al. Bumetanide administration attenuated traumatic brain injury through IL-1 overexpression. Neurol Res. 2007;29:404–409. doi: 10.1179/016164107X204738. [DOI] [PubMed] [Google Scholar]
- 23.MacVicar BA, Feighan D, Brown A, et al. Intrinsic optical signals in the rat optic nerve: role for K(+) uptake via NKCC1 and swelling of astrocytes. Glia. 2002;37:114–123. doi: 10.1002/glia.10023. [DOI] [PubMed] [Google Scholar]
- 24.Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007;22:E1. doi: 10.3171/foc.2007.22.5.2. [DOI] [PubMed] [Google Scholar]
- 25.Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9 Suppl 1:S287–S292. [PubMed] [Google Scholar]
- 26.Moretti ME, Rezvani M, Koren G. Safety of glyburide for gestational diabetes: a meta-analysis of pregnancy outcomes. Ann Pharmacother. 2008;42:483–490. doi: 10.1345/aph.1K577. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell ME, Lam TI, Tran L, et al. The role of the blood-brain barrier Na-K-2Cl cotransporter in stroke. Adv Exp Med Biol. 2004;559:67–75. doi: 10.1007/0-387-23752-6_6. [DOI] [PubMed] [Google Scholar]
- 28.O'Donnell ME, Lam TI, Tran LQ, et al. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2006;26:1234–1249. doi: 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- 29.O'Donnell ME, Tran L, Lam TI, et al. Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab. 2004;24:1046–1056. doi: 10.1097/01.WCB.0000130867.32663.90. [DOI] [PubMed] [Google Scholar]
- 30.Oertel M, Kelly DF, McArthur D, et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg. 2002;96:109–116. doi: 10.3171/jns.2002.96.1.0109. [DOI] [PubMed] [Google Scholar]
- 31.Rajasekaran SA, Beyenbach KW, Rajasekaran AK. Interactions of tight junctions with membrane channels and transporters. Biochim Biophys Acta. 2008;1778:757–769. doi: 10.1016/j.bbamem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Regan RF, Guo Y. Toxic effect of hemoglobin on spinal cord neurons in culture. J Neurotrauma. 1998;15:645–653. doi: 10.1089/neu.1998.15.645. [DOI] [PubMed] [Google Scholar]
- 33.Rendell M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs. 2004;64:1339–1358. doi: 10.2165/00003495-200464120-00006. [DOI] [PubMed] [Google Scholar]
- 34.Rothman SM. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985;5:1483–1489. doi: 10.1523/JNEUROSCI.05-06-01483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schomberg SL, Su G, Haworth RA, et al. Stimulation of Na-K-2Cl cotransporter in neurons by activation of Non-NMDA ionotropic receptor and group-I mGluRs. J Neurophysiol. 2001;85:2563–2575. doi: 10.1152/jn.2001.85.6.2563. [DOI] [PubMed] [Google Scholar]
- 36.Simard JM, Castellani RJ, Ivanova S, et al. Sulfonylurea receptor 1 in the germinal matrix of premature infants. Pediatr Res. 2008;64:648–652. doi: 10.1203/PDR.0b013e318186e5a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simard JM, Geng Z, Kyoon WS, et al. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2008 doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simard JM, Kent TA, Chen M, et al. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simard JM, Norenberg M, Landsman D, et al. SUR1 is upregulated in human and rat spinal cord injury -- role of SUR1 in acute-phase lesion evolution; Christopher and Dana Reeve Foundation Bi-Annual Meeting; Atlanta, GA. 2008. [Google Scholar]
- 41.Simard JM, Tarasov KV, Gerzanich V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim Biophys Acta. 2007;1772:947–957. doi: 10.1016/j.bbadis.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simard JM, Tsymbalyuk O, Ivanov A, et al. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117:2105–2113. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simard JM, Woo SK, Bhatta S, et al. Drugs acting on SUR1 to treat CNS ischemia and trauma. Curr Opin Pharmacol. 2008;8:42–49. doi: 10.1016/j.coph.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simard JM, Yurovsky V, Tsymbalyuk N, et al. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2008 doi: 10.1161/STROKEAHA.108.522409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith JS, Chang EF, Rosenthal G, et al. The role of early follow-up computed tomography imaging in the management of traumatic brain injury patients with intracranial hemorrhage. J Trauma. 2007;63:75–82. doi: 10.1097/01.ta.0000245991.42871.87. [DOI] [PubMed] [Google Scholar]
- 46.Staub F, Stoffel M, Berger S, et al. Treatment of vasogenic brain edema with the novel Cl- transport inhibitor torasemide. J Neurotrauma. 1994;11:679–690. doi: 10.1089/neu.1994.11.679. [DOI] [PubMed] [Google Scholar]
- 47.Su G, Kintner DB, Flagella M, et al. Astrocytes from Na(+)-K(+)-Cl(−) cotransporter-null mice exhibit absence of swelling and decrease in EAA release. Am J Physiol Cell Physiol. 2002;282:C1147–C1160. doi: 10.1152/ajpcell.00538.2001. [DOI] [PubMed] [Google Scholar]
- 48.Su G, Kintner DB, Sun D. Contribution of Na(+)-K(+)-Cl(−) cotransporter to high-[K(+)](o)- induced swelling and EAA release in astrocytes. Am J Physiol Cell Physiol. 2002;282:C1136–C1146. doi: 10.1152/ajpcell.00478.2001. [DOI] [PubMed] [Google Scholar]
- 49.Supuran CT. Diuretics: from classical carbonic anhydrase inhibitors to novel applications of the sulfonamides. Curr Pharm Des. 2008;14:641–648. doi: 10.2174/138161208783877947. [DOI] [PubMed] [Google Scholar]
- 50.Thurman DJ, Alverson C, Dunn KA, et al. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Ueno M. Molecular anatomy of the brain endothelial barrier: an overview of the distributional features. Curr Med Chem. 2007;14:1199–1206. doi: 10.2174/092986707780597943. [DOI] [PubMed] [Google Scholar]
- 52.Unterberg AW, Stover J, Kress B, et al. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 53.Vajtr D, Benada O, Kukacka J, et al. Correlation of ultrastructural changes of endothelial cells and astrocytes occuring during blood brain barrier damage after traumatic brain injury with biochemical markers of BBB leakage and inflammatory response. Physiol Res. 2008 doi: 10.33549/physiolres.931253. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Mori T, Sumii T, et al. Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke. 2002;33:1882–1888. doi: 10.1161/01.str.0000020121.41527.5d. [DOI] [PubMed] [Google Scholar]
- 55.Wojciak-Stothard B, Entwistle A, Garg R, et al. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 56.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 57.Yan Y, Dempsey RJ, Flemmer A, et al. Inhibition of Na(+)-K(+)-Cl(−) cotransporter during focal cerebral ischemia decreases edema and neuronal damage. Brain Res. 2003;961:22–31. doi: 10.1016/s0006-8993(02)03832-5. [DOI] [PubMed] [Google Scholar]