Abstract
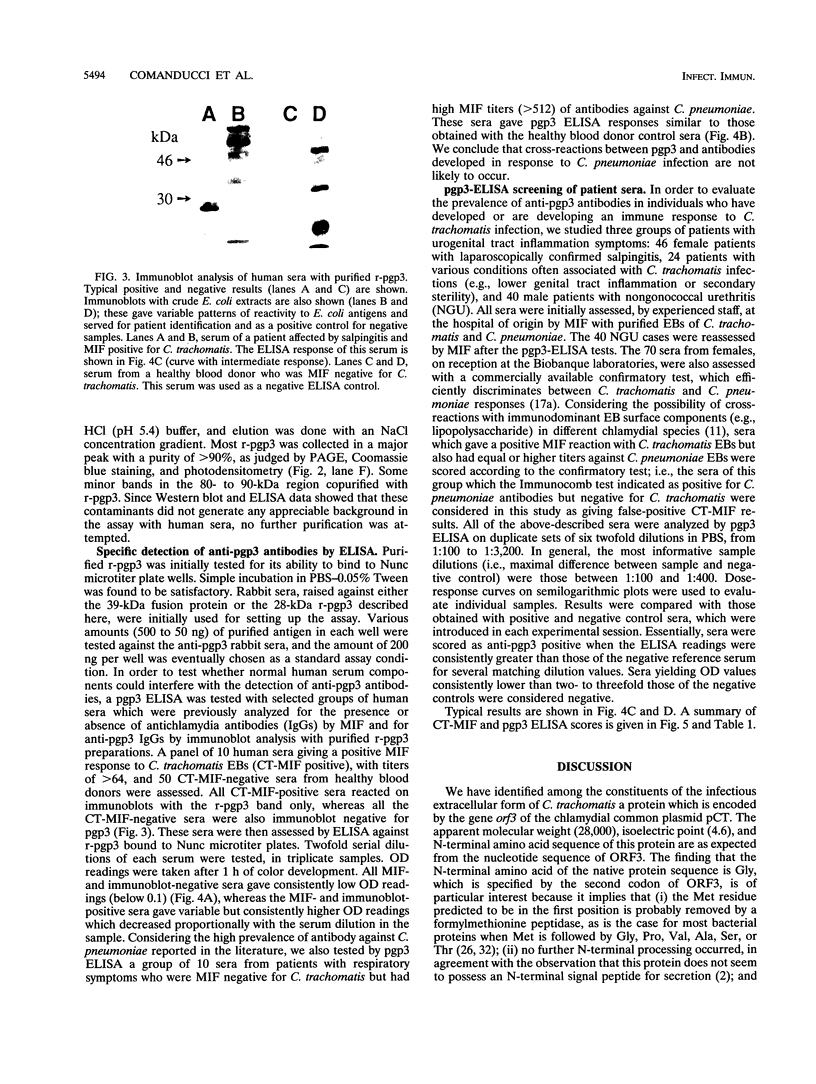
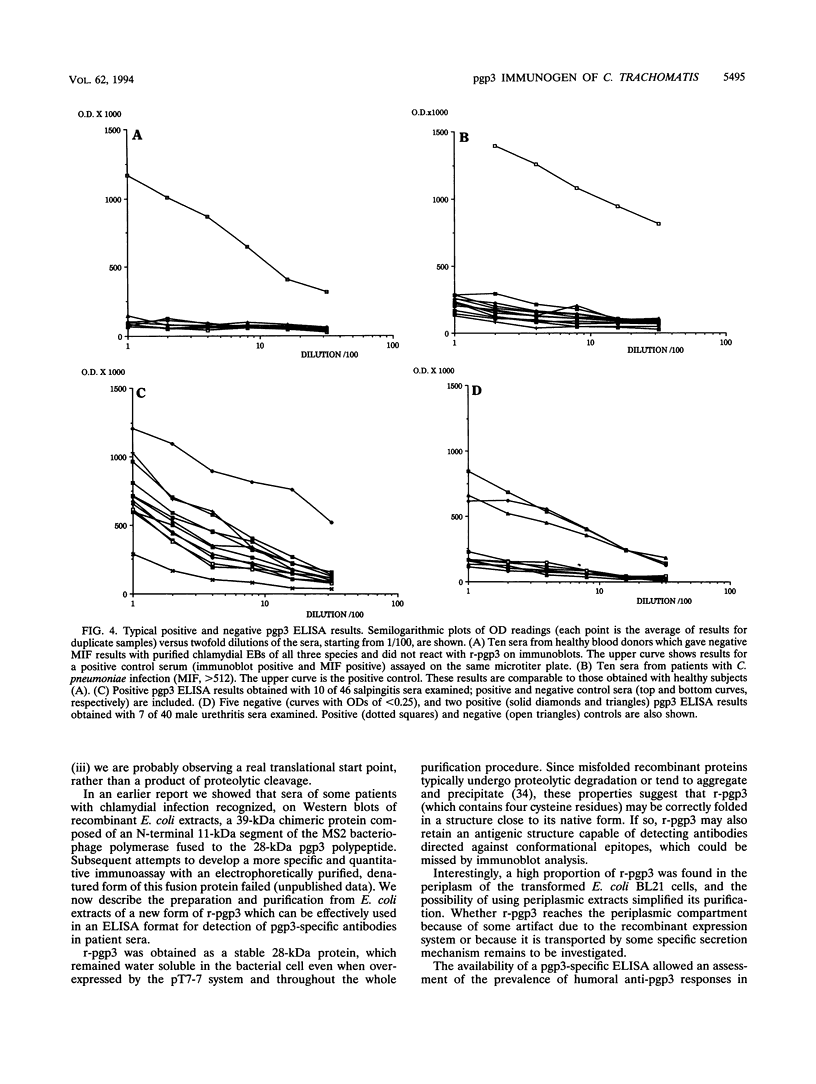
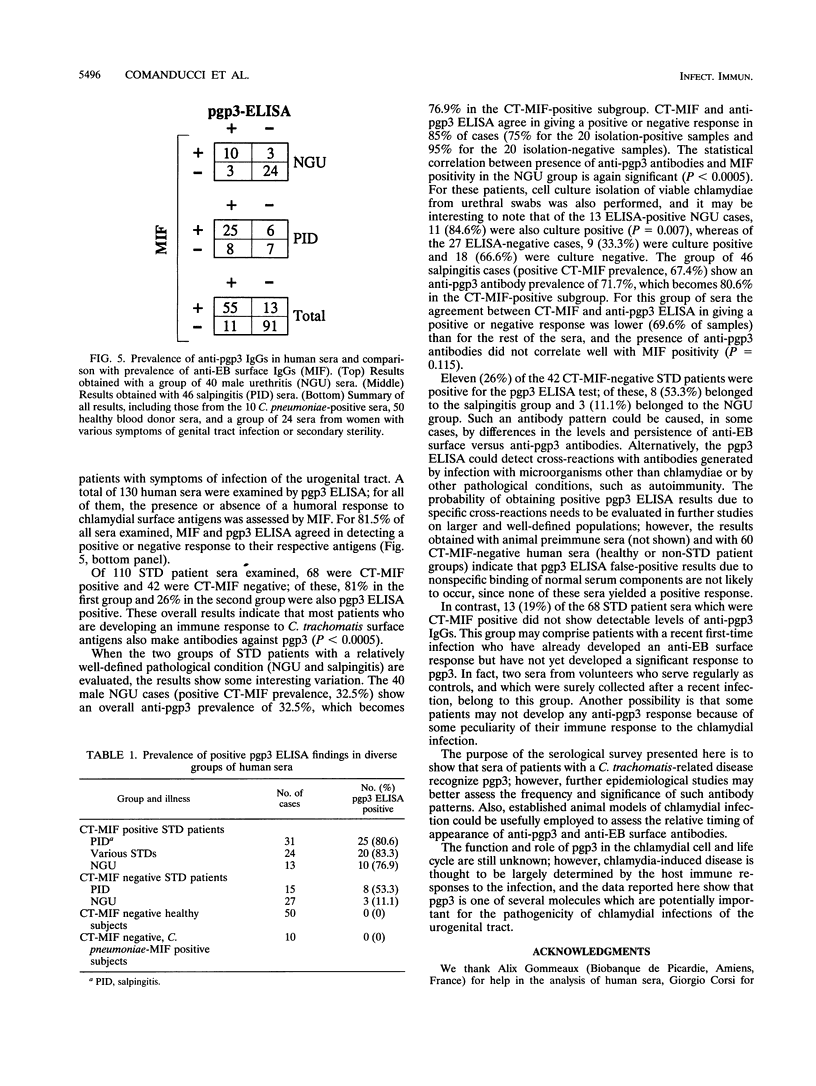
We identified, by two-dimensional electrophoretic analysis and microsequencing, a protein of Chlamydia trachomatis elementary bodies which corresponds to the polypeptide (pgp3) encoded by open reading frame 3 (ORF3). Amino acid analysis showed that the first residue (Gly) found in the native protein is the one encoded by the second ORF3 codon, implying a typical bacterial removal of the first Met residue. Relatively large amounts of recombinant pgp3 (r-pgp3) in a stable, water-soluble form were obtained by overexpressing ORF3 in Escherichia coli and purifying the product from periplasmic extracts under nondenaturing conditions. These r-pgp3 preparations allowed specific detection of anti-pgp3 antibodies by enzyme-linked immunosorbent assay. Analysis of a group of 170 sera from healthy blood donors and from patients who were seropositive or -negative for C. trachomatis and Chlamydia pneumoniae showed that an immune response to pgp3 occurs in the majority (ca. 81%) of patients with sexually transmitted diseases who are seropositive for C. trachomatis and generally correlates with the response to cell surface antigens. No reaction between r-pgp3 and C. pneumoniae-positive sera was detected.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comanducci M., Cevenini R., Moroni A., Giuliani M. M., Ricci S., Scarlato V., Ratti G. Expression of a plasmid gene of Chlamydia trachomatis encoding a novel 28 kDa antigen. J Gen Microbiol. 1993 May;139(5):1083–1092. doi: 10.1099/00221287-139-5-1083. [DOI] [PubMed] [Google Scholar]
- Comanducci M., Ricci S., Cevenini R., Ratti G. Diversity of the Chlamydia trachomatis common plasmid in biovars with different pathogenicity. Plasmid. 1990 Mar;23(2):149–154. doi: 10.1016/0147-619x(90)90034-a. [DOI] [PubMed] [Google Scholar]
- Comanducci M., Ricci S., Ratti G. The structure of a plasmid of Chlamydia trachomatis believed to be required for growth within mammalian cells. Mol Microbiol. 1988 Jul;2(4):531–538. doi: 10.1111/j.1365-2958.1988.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Fahr M. J., Sriprakash K. S., Hatch T. P. Convergent and overlapping transcripts of the Chlamydia trachomatis 7.5-kb plasmid. Plasmid. 1992 Nov;28(3):247–257. doi: 10.1016/0147-619x(92)90056-g. [DOI] [PubMed] [Google Scholar]
- Gormley E. P., Cantwell B. A., Barker P. J., Gilmour R. S., McConnell D. J. Secretion and processing of the Bacillus subtilis endo-beta-1,3-1,4-glucanase in Escherichia coli. Mol Microbiol. 1988 Nov;2(6):813–819. doi: 10.1111/j.1365-2958.1988.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hatt C., Ward M. E., Clarke I. N. Analysis of the entire nucleotide sequence of the cryptic plasmid of Chlamydia trachomatis serovar L1. Evidence for involvement in DNA replication. Nucleic Acids Res. 1988 May 11;16(9):4053–4067. doi: 10.1093/nar/16.9.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser D. F., Harrington M. G., Hochstrasser A. C., Miller M. J., Merril C. R. Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal Biochem. 1988 Sep;173(2):424–435. doi: 10.1016/0003-2697(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Hughes G. J., Frutiger S., Paquet N., Ravier F., Pasquali C., Sanchez J. C., James R., Tissot J. D., Bjellqvist B., Hochstrasser D. F. Plasma protein map: an update by microsequencing. Electrophoresis. 1992 Sep-Oct;13(9-10):707–714. doi: 10.1002/elps.11501301150. [DOI] [PubMed] [Google Scholar]
- Kern D. G., Neill M. A., Schachter J. A seroepidemiologic study of Chlamydia pneumoniae in Rhode Island. Evidence of serologic cross-reactivity. Chest. 1993 Jul;104(1):208–213. doi: 10.1378/chest.104.1.208. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Moulder J. W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991 Mar;55(1):143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pizza M., Bugnoli M., Manetti R., Covacci A., Rappuoli R. The subunit S1 is important for pertussis toxin secretion. J Biol Chem. 1990 Oct 15;265(29):17759–17763. [PubMed] [Google Scholar]
- Ratti G., Moroni A., Cevenini R. Detection of Chlamydia trachomatis DNA in patients with non-gonococcal urethritis using the polymerase chain reaction. J Clin Pathol. 1991 Jul;44(7):564–568. doi: 10.1136/jcp.44.7.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci S., Cevenini R., Cosco E., Comanducci M., Ratti G., Scarlato V. Transcriptional analysis of the Chlamydia trachomatis plasmid pCT identifies temporally regulated transcripts, anti-sense RNA and sigma 70-selected promoters. Mol Gen Genet. 1993 Mar;237(3):318–326. doi: 10.1007/BF00279434. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanchez J. C., Ravier F., Pasquali C., Frutiger S., Paquet N., Bjellqvist B., Hochstrasser D. F., Hughes G. J. Improving the detection of proteins after transfer to polyvinylidene difluoride membranes. Electrophoresis. 1992 Sep-Oct;13(9-10):715–717. doi: 10.1002/elps.11501301151. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarimo S. S., Pine M. J. Taxonomic comparison of the amino termini of microbial cell proteins. J Bacteriol. 1969 May;98(2):368–374. doi: 10.1128/jb.98.2.368-374.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriprakash K. S., Macavoy E. S. Characterization and sequence of a plasmid from the trachoma biovar of Chlamydia trachomatis. Plasmid. 1987 Nov;18(3):205–214. doi: 10.1016/0147-619x(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tam J. E., Davis C. H., Thresher R. J., Wyrick P. B. Location of the origin of replication for the 7.5-kb Chlamydia trachomatis plasmid. Plasmid. 1992 May;27(3):231–236. doi: 10.1016/0147-619x(92)90025-6. [DOI] [PubMed] [Google Scholar]
- WALLER J. P. THE NH2-TERMINAL RESIDUES OF THE PROTEINS FROM CELL-FREE EXTRACTS OF E. COLI. J Mol Biol. 1963 Nov;7:483–496. doi: 10.1016/s0022-2836(63)80096-0. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970 Sep;70(3):367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]
- Wülfing C., Plückthun A. Protein folding in the periplasm of Escherichia coli. Mol Microbiol. 1994 Jun;12(5):685–692. doi: 10.1111/j.1365-2958.1994.tb01056.x. [DOI] [PubMed] [Google Scholar]