Abstract
Background
Percutaneous trasluminal renal angioplasty (PTRA) is the most frequent therapeutic approach to resolve renal artery stenosis (RAS). However, renal function recovers in only 30% of the cases. The causes of these poor outcomes are still unknown. We hypothesize that preserving the renal microcirculation distal to RAS will improve the responses to PTRA.
Methods and Results
RAS was induced in 28 pigs. In 14, vascular endothelial growth factor (VEGF)-165 was infused intra-renally (RAS+VEGF, 0.05 µg/kg). Single-kidney function was assessed in all pigs in vivo using ultra-fast CT after 6 weeks. Half of the RAS/RAS+VEGF completed their observation, and the other half underwent PTRA, VEGF was repeated, and CT studies repeated 4 weeks later. Pigs were then euthanized, the stenotic kidney removed, renal microvascular (MV) architecture reconstructed ex-vivo using 3D micro-CT, and renal fibrosis quantified.
Degree of RAS and hypertension were similar in RAS and RAS+VEGF. Renal function and MV density were decreased in RAS but improved in RAS+VEGF. PTRA largely resolved RAS, but the improvements of hypertension and renal function were greater in RAS+VEGF+PTRA than in RAS+PTRA, accompanied by a 34% increase in MV density and decreased fibrosis.
Conclusion
Preservation of the MV architecture and function in the stenotic kidney improved the responses to PTRA, indicating that renal MV integrity plays a role in determining the responses to PTRA. This study indicates that damage and early loss of renal MV is an important determinant of the progression of renal injury in RAS and instigates often irreversible damage.
Keywords: renal artery stenosis, revascularization, microcirculation, imaging, renal function
Introduction
Renal artery stenosis (RAS) is a predictor of major cardiovascular events, adverse renal outcomes, and mortality1, independent of other prevalent cardiovascular risk factors. RAS is present in up to 40% of patients with coronary or peripheral atherosclerotic vascular disease2. This increase in prevalence is paralleled by a growing use of renal artery interventions such as percutaneous transluminal renal angioplasty (PTRA), the most frequent therapeutic approach to treat RAS which has increased dramatically during the past two decades3. The technical advances and increase in success rates (almost 100%4) have been the impetus of the growing use of PTRA to treat RAS. However, the outcomes of this intervention are far from optimal since resolution of hypertension is not obtained and renal function does not recover or even further aggravates in up to 70% of the cases5 despite successful resolution of the stenosis and restoration of renal blood flow. The reasons for these relatively poor outcomes are still unknown.
Functional and structural abnormalities in the microvasculature of the stenotic kidney contribute to the pathophysiology of ischemic renal injury. Microvascular (MV) remodeling, damage and loss have been observed in several experimental models of both acute and chronic ischemic renal injury, underscoring the importance that renal MV disease has for the progression of renal damage6, 7. However, whether a deterioration of the renal microcirculation in the chronically stenotic kidney plays a role in defining the renal outcomes in response to revascularization has never been investigated. We have previously shown in a model of chronic RAS that mimics early chronic human renovascular disease, that the stenotic kidney develops MV rarefaction as the disease evolves, which is paralleled by a progressive deterioration of renal function, extensive renal fibrosis, and a significant decrease in renal bioavailability of the central angiogenic factor vascular endothelial growth factor (VEGF)8, a key player in maintaining the renal MV integrity and promoting MV proliferation and repair. In addition, we have recently shown that deleterious changes were largely attenuated by preserving renal VEGF bioavailability9 in the stenotic kidney. Decreases in VEGF availability and activity have been shown to play a role in triggering renal injury in different physiological and pathophysiological processes such as aging, experimental acute and chronic ischemia, and diabetes10–12, mainly via impairing the maintenance and repair of the renal MV networks.
The development of renal ischemia is considered the main and central cause of the progressive nature of kidney diseases in general, since development of MV damage and loss further promotes nephron loss13, likely reflecting a vicious circle. Since restoration of blood flow is not always sufficient for a complete recovery of renal function, this study was designed to test the hypothesis that preserving the renal microcirculation in the stenotic kidney will not only preserve the renal function during the evolution of RAS, but also more importantly, will improve the responses to renal revascularization. Based on our previous studies showing the concomitant decrease in MV density and renal bioavailability of VEGF, we prevented VEGF decrease by infusing VEGF in the stenotic kidney and later determined the effects of this targeted intervention on the renal responses to PTRA.
Methods
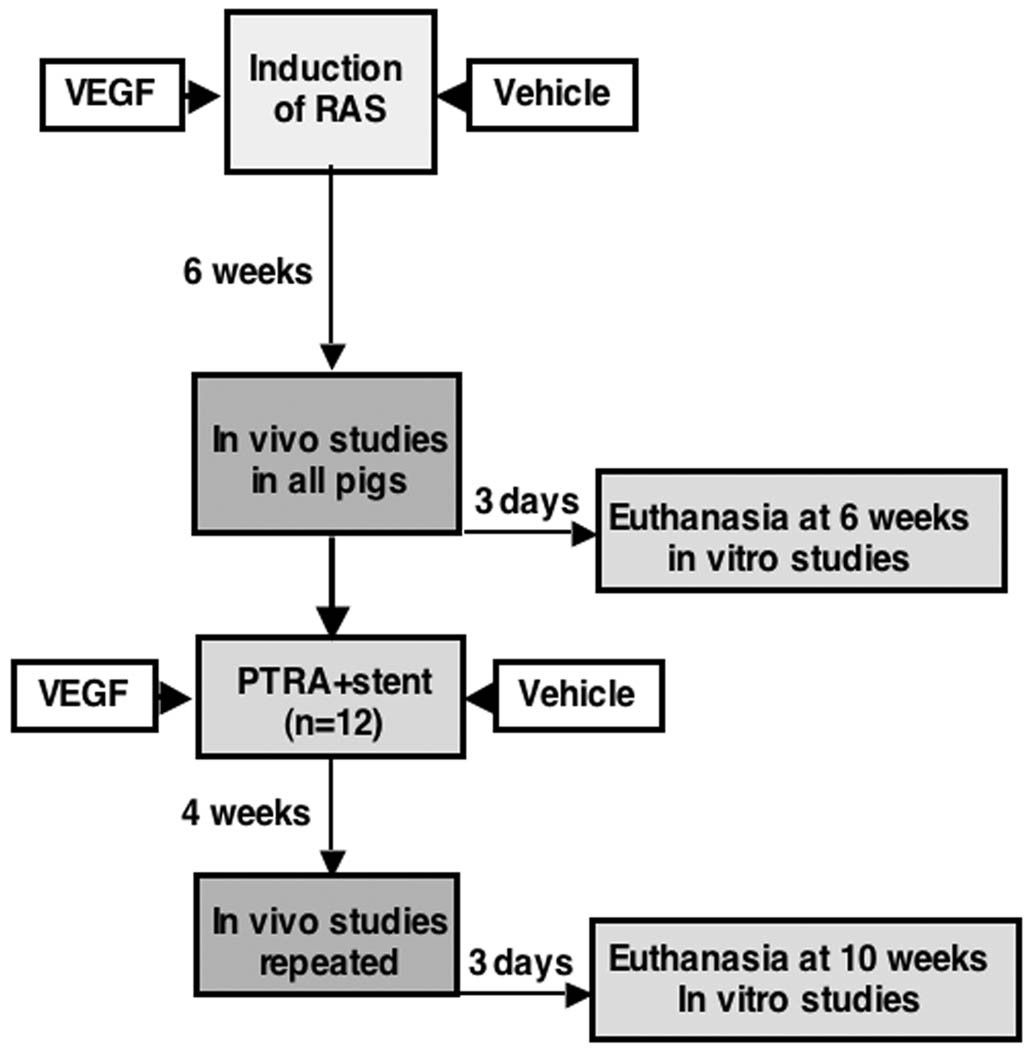
The Institutional Animal Care and Use Committee at the University of Mississippi Medical Center approved all the procedures. Forty-two pre-juvenile domestic pigs (50–55kg) were studied after 6 and then 10 weeks of observation as described in Figure 1. In 28 pigs, unilateral RAS was induced at baseline by placing a local-irritant coil inside the main renal artery, which induced gradual development of RAS, as previously described14, 15. The pigs were then randomized into two groups: those that were not further treated (RAS, n=14) or those treated with an intra-renal infusion of VEGF (0.05 µg/kg, RAS+VEGF, n=14) at the time of insertion of the coil and onset of the stenosis, as we have recently shown9. Administration of VEGF was performed intra-renally through a 5F balloon catheter (beyond where the coil was placed to induce RAS). It was administered as a slow bolus over 10 minutes and did not have any immediate effect on blood pressure. This dose of VEGF was used in our recent study9, and was selected based on a previous clinical study demonstrating that it was well tolerated and had a sustained effect to increase the collateral MV density and perfusion of the ischemic myocardium16, 17. Throughout the 6 weeks following the induction of RAS, blood pressure was continuously monitored using a telemetry system (PhysioTel, Data Sciences International) implanted at baseline in the right femoral artery. Mean arterial pressure (MAP) was recorded at 5-minute intervals and averaged for each 24-hour period14, 15. Other animals were used as normal controls (normal, n=14).
Figure 1.
Representative flow-chart summarizing the interventions and time-points.
At six weeks after induction of RAS, all the pigs underwent renal angiography to quantify the degree of RAS. The pigs were anesthetized with intra-muscular telazol (5 mg/kg) and xylazine (2 mg/kg), intubated, and mechanically ventilated on room air. Anesthesia was maintained with a mixture of ketamine (0.2 mg/kg/min) and xylazine (0.03 mg/kg/min) in normal saline, administered via an ear vein cannula (0.05 mL/kg/min). Under sterile conditions and fluoroscopic guidance, an 8F arterial catheter was advanced to the renal artery, proximal to the stenosis and renal angiography was performed, as previously described14, 15, 18. Extent of the stenosis was assessed as the decrease in luminal diameter of the renal artery at the most stenotic point compared to a proximal stenosis-free segment. After angiography, the catheter was positioned in the superior vena cava, and in vivo helical multi-detector computer tomography (MDCT) flow studies were performed. Briefly, sequential acquisition of 160 consecutive scans were obtained after a central venous injection of iopamidol (0.5 mL/kg/2 sec), for assessment of single-kidney renal blood flow (RBF, ml/min), perfusion (ml/minute/g tissue), and glomerular filtration rate (GFR, mL/min), as previously detailed and validated14, 19, 20. Studies were repeated during supra-renal infusion of the prototypical endothelium-dependent vasodilator acetylcholine (Ach, 5 µg/kg/min), to test intra-renal endothelial function. Renal vascular resistance was calculated by dividing the mean arterial pressure (at the moment of the in vivo studies) and MDCT-derived RBF.
Half of the animals were euthanized after completion of the in vivo studies at 6 weeks. Upon completion of the MDCT studies and still under anesthesia, all of the remaining RAS and RAS+VEGF animals underwent PTRA under fluoroscopic guidance using a balloon catheter+tantalum stent deployment (to optimize vascular patency for revascularization). Briefly, a 7mm × 1cm PTCA balloon catheter (OptaPro, Cordis Corp, FL) was engaged in the stenotic renal artery and inflated for 30 seconds at 10 atm, and a few minutes later again at 14 atm, to fully dilate the stenosis. Then, a standard tantalum stent, matched to the size of the renal artery and length of stenosis (usually a few mm) was implanted in the renal artery following balloon dilatation. Administration of intra-renal VEGF (0.05 µg/kg) was repeated in those RAS pigs that received VEGF at the induction of RAS. Blood pressure was continuously monitored by telemetry and all the pigs were observed for 4 additional weeks and then underwent renal angiography to determine the effects of PTRA on the renal artery, followed by in vivo basal and stimulated MDCT in vivo studies as performed at 6 weeks to determine the effects of PTRA on renal hemodynamics and function.
After completion of all the in vivo studies (at 6 and at 10 weeks, Figure 1), the pigs were allowed to recover for 2 days to allow for contrast media washout, and were then euthanized with a lethal intravenous injection of sodium pentobarbital (100mg/kg). Kidneys were removed using a retroperitoneal incision and immersed in heparinized saline (10 units/mL). A lobe of tissue was used for micro-CT reconstruction, while another lobe of tissue was removed from one end of the kidney, snap-frozen in liquid nitrogen and stored at −80° C to quantify mRNA expression of VEGF receptors Flt-1 and Flk-1 by RT-PCR9, or preserved in 10% formalin to later perform immunohistochemistry against CD319 and investigate renal morphology in mid-hilar renal cross-sections stained with trichrome14.
MDCT analysis
Manually-traced regions of interest were selected in MDCT images in the aorta, renal cortex, medulla, and papilla, and their densities sampled. Time-density curves were generated and fitted with extended gamma-variate curve-fits, and the area enclosed under each segment of the curve and its first moment calculated using the curve-fitting parameters. These were used to calculate single-kidney RBF (ml/min), GFR (mL/min), and renal perfusion (ml/minute/g tissue), using previously-validated methods14, 19, 20.
Micro-CT
The stenotic kidney was perfused under physiological perfusion pressure (Syringe Infusion Pump 22, Harvard Apparatus, Holliston, MA) with an intravascular contrast agent, (Microfil MV122, Flow Tech, Inc., Carver, MA). The kidney samples were scanned at 0.3° increments using a micro-CT scanner and reconstructed at 9 µm resolution for subsequent analysis, as previously described8, 9, 21. Images were analyzed with the Analyze® software package (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). The cortex was tomographically divided into 12 levels (starting at the juxtamedullary cortex), obtained at equal intervals, and the spatial density and distribution of microvessels (diameters 10–500µm) were calculated9.
Renal VEGF
Renal protein concentration of VEGF in the stenotic kidney was measured in tissue homogenates using an enzyme immunoassay (ELISA, R&D, Minneapolis, MN)9.
Immunohistochemistry
Because of their size, capillaries (microvessels under 10 µm) cannot be identified by the micro-CT technique. Therefore, peritubular and glomerular capillaries were quantified in 5 µm paraffin-embeded mid-hilar renal cross-sections to assess the expression of CD31 (Santa Cruz, CA, 1:80). The secondary antibody, IgG Envision Plus (Dako, Carpinteria, CA), was followed by staining with the Vector NovaRED substrate kit (Vector Laboratories, Burlingame, CA), following vendor’s instructions.
Histology
Mid-hilar 5 µm cross sections of each kidney (1 per animal) were examined using a computer-aided image-analysis program (NIS Element 3.0, Nikon Instruments, Melville, NY). In each representative slide, trichrome staining was semi-automatically quantified in 15–20 fields by the computer program, expressed as percentage of staining of total surface area, and the results from all fields averaged14. Glomerular score (percentage of sclerotic glomeruli) was assessed by recording the number of sclerotic glomeruli out of 100 counted glomeruli as previously described14. In addition, to quantify CD31 immunoreactivity, randomly selected 15–20 visual fields from each sample (1 slide per animal) were analyzed at ×40 magnification. Capillaries were then identified as vessels at approximately 8–10 µm in diameter constituted of a single layer of endothelial cells and quantified as the number of capillaries per visual field, as recently described9.
Statistical Analysis
Results are expressed as mean ± SEM. Comparisons within groups were performed using paired student’s t-test, and among groups using one-way ANOVA, with Fisher’s LSD post-hoc tests for correction for multiple comparisons. Statistical significance was accepted for p≤0.05. For data measured over time (blood pressure) a two-way repeated measures ANOVA was used, and statistical significance was accepted for p≤0.05.
Results
Pre-PTRA
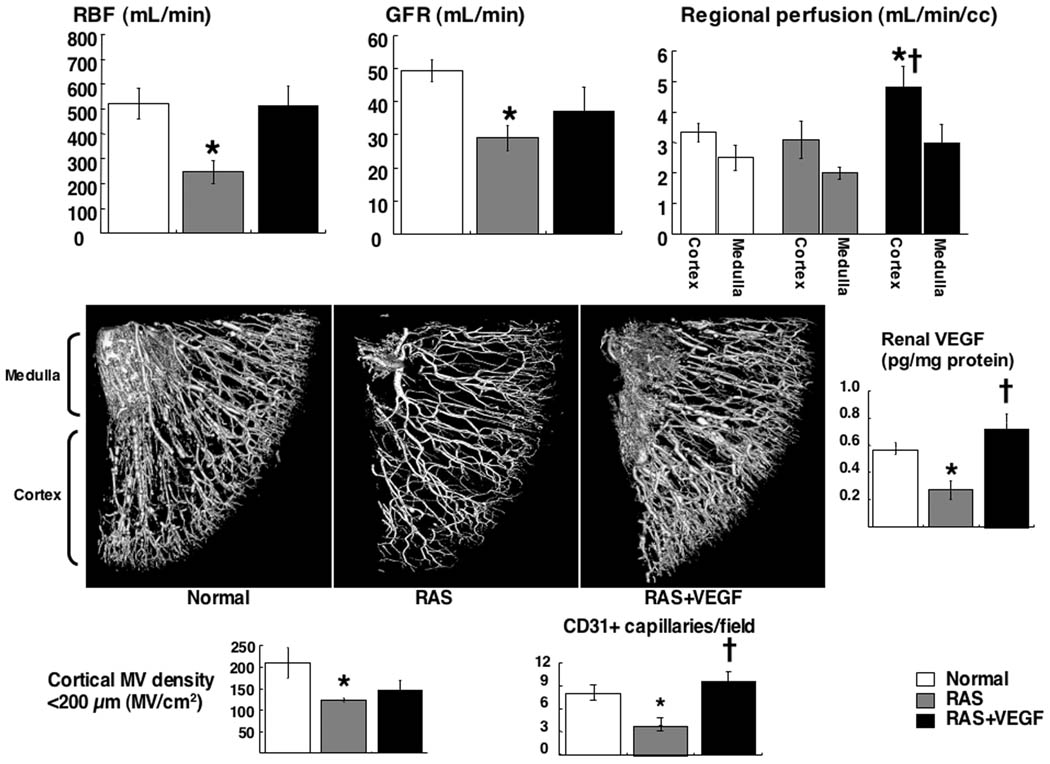
The degree of stenosis and hypertension were similar in RAS and RAS+VEGF animals after 6 weeks (73.2±5.8 and 71.6±7.7 %, and 139.5±3.0 and 147.4±6.0 mm/Hg, respectively, p<0.05 vs. Normal), before PTRA. Renal blood flow, perfusion, glomerular filtration rate (Figure 2-top), cortical MV and capillary density, and renal VEGF (Figure 2-middle and bottom) were significantly decreased in RAS but improved in RAS+VEGF, accompanied by a significant attenuation in renal vascular resistance, glomerulosclerosis and tubulo-interstitial fibrosis, as we have previously shown9.
Figure 2.
Top: Renal blood flow, glomerular filtration rate, and regional perfusion. Middle: Representative 3D tomographic images of the kidney and renal levels of VEGF. Bottom: Quantification of renal cortical microvascular, and capillary density in normal, renal artery stenosis (RAS), and RAS treated with intra-renal VEGF (RAS+VEGF) kidneys after 6 weeks. Intra-renal VEGF improved VEGF bioavailability and the cortical microvasculature in the stenotic kidney, and distinctly preserved the hemodynamics and function. *p<0.05 vs. Normal, †p<0.05 vs. RAS.
Post-PTRA
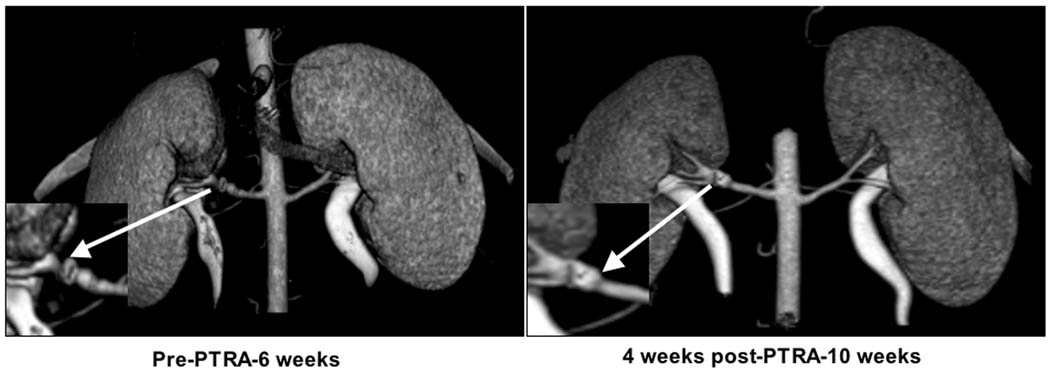
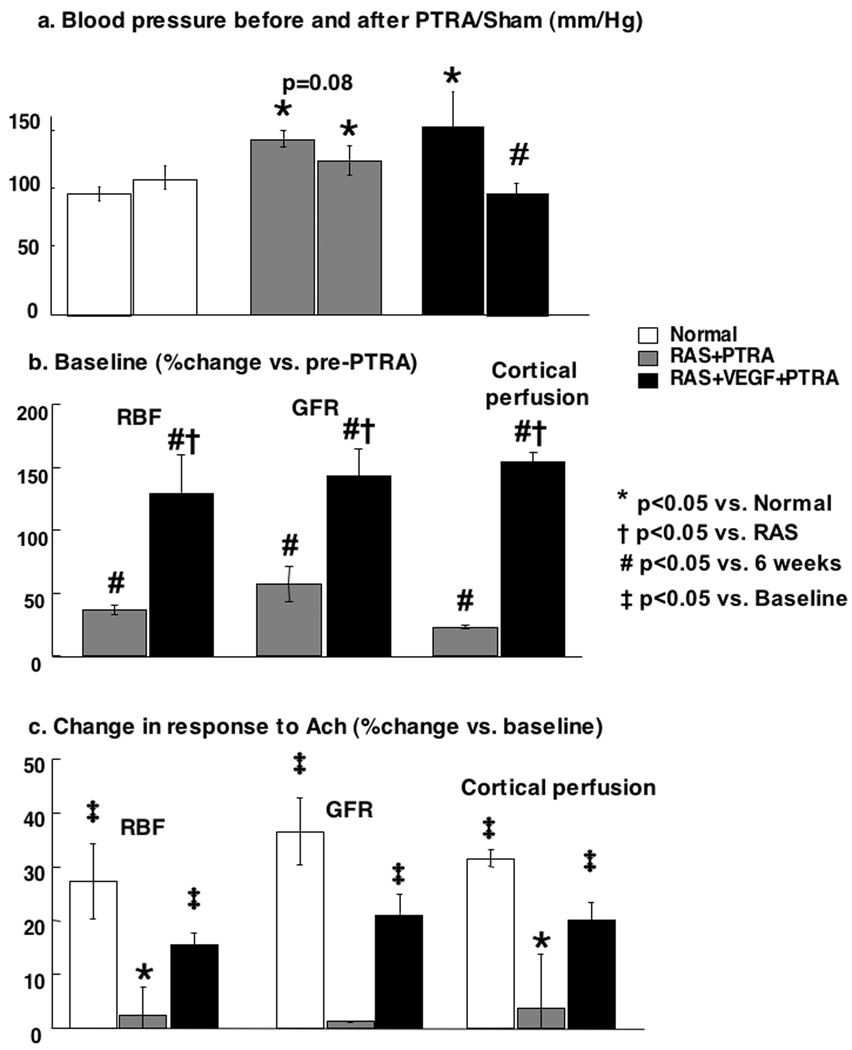
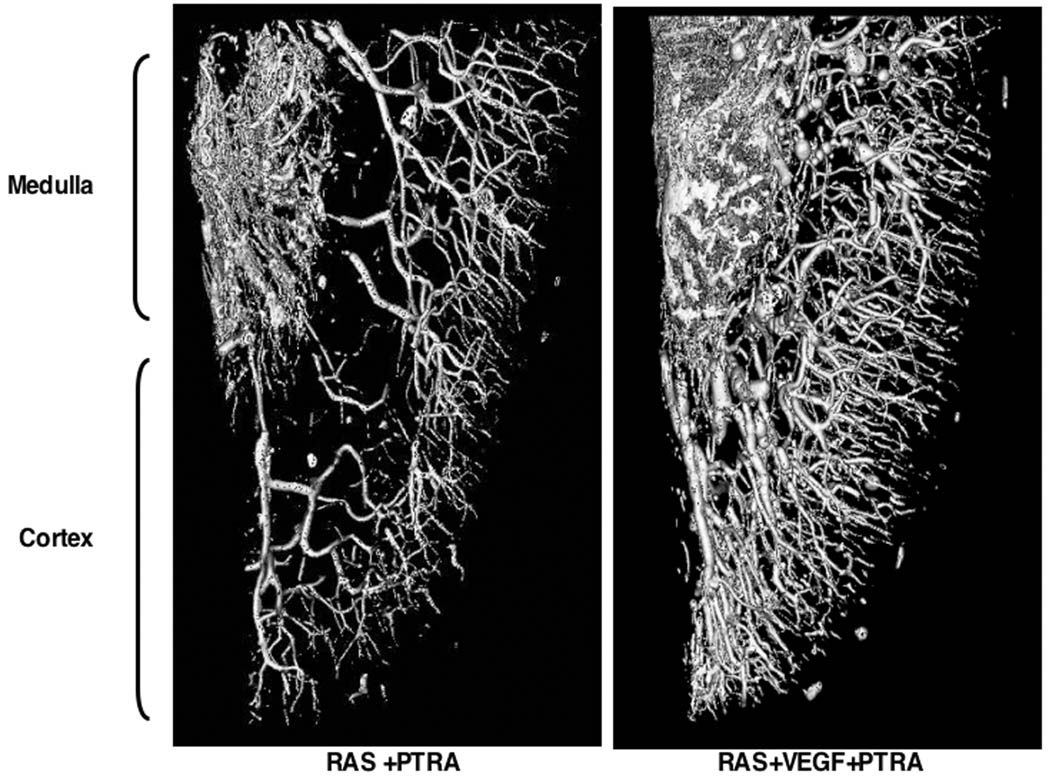
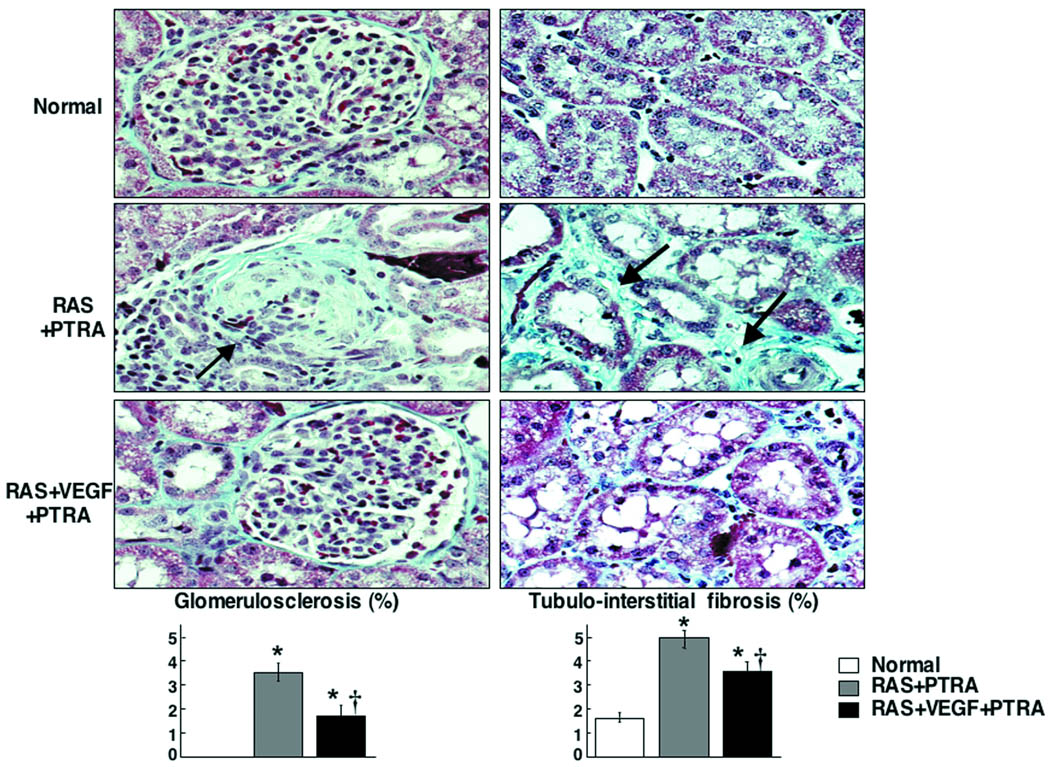
PTRA largely resolved the stenosis in all animals, and no significant residual stenosis was observed 4 weeks after PTRA in any of the RAS or RAS+VEGF pigs (Figure 3). However, a modest decrease in blood pressure was observed in RAS+PTRA, unlike RAS+VEGF+PTRA pigs, in which blood pressure decreased to a larger extent and almost returned to basal levels (Figure 4a). This was accompanied by a more accentuated decreased in renal vascular resistance in RAS+VEGF+PTRA (0.21±0.02 mmHg/mL/min, p=0.4 vs. Normal controls) compared to RAS+PTRA (0.25±0.01 mmHg/mL/min, p=0.07 vs. normal controls). Furthermore, PTRA combined with VEGF administration dramatically improved RBF, GFR, and regional perfusion (Figure 4b), and restored MV endothelial function (Figure 4c, ANOVA<0.05 for all). These effects were accompanied by a 3-fold increase in the mRNA expression of the VEGF receptor Flk-1 and a 34% increase in MV density in RAS+VEGF compared to RAS (Figure 5), and decreased glomerulosclerosis and tubulo-interstitial fibrosis (Figure 6).
Figure 3.
Representative CT-angiography showing RAS at 6 weeks and 4 weeks after percutaneous trasluminal renal angioplasty (PTRA). PTRA largely resolved RAS in all groups.
Figure 4.
Top and middle: Paired comparisons of blood pressure (a) and basal (b) renal blood flow, glomerular filtration rate, and cortical perfusion before (6 weeks) and after PTRA (10 weeks). Bottom: Responses to endothelium dependent challenge using acetylcholine after PTRA, at 10 weeks (c) in normal, renal artery stenosis (RAS)+PTRA, and RAS+VEGF+PTRA. Combined VEGF+PTRA resulted in a larger decrease in blood pressure and restoration of renal hemodynamics and function. *p<0.05 vs. Normal, †p<0.05 vs. RAS, #p<0.05 vs. 6 weeks-prePTRA, ‡p<0.05 vs. Baseline.
Figure 5.
Representative 3D micro-CT reconstruction of the renal MV architecture after PTRA. RAS+VEGF +PTRA showed a 34% increase in MV density compared to RAS+PTRA
Figure 6.
Renal fibrosis and quantification in normal, renal artery stenosis (RAS)+PTRA, and RAS+VEGF+PTRA kidneys. Individual representative glomeruli and tubulo-interstitial region are shown as examples to illustrate the quantitative information. VEGF+PTRA decreased the damage of the stenotic kidney compared to PTRA alone. *p<0.05 vs. Normal, † p<0.05 vs. RAS
Discussion
The current study extends our previous findings9 and highlights the central role of renal MV disease not only for the progression of renal injury, but also for the responses to renal revascularization. The stenotic kidney shows significant MV rarefaction accompanied by a marked deterioration of renal function and increased fibrosis. Preservation of the renal MV architecture and function largely improved the function of the stenotic kidney. Furthermore, this study shows for the first time that targeting the renal microcirculation in the stenotic kidney dramatically improves the renal functional responses to PTRA, indicating that renal MV rarefaction (and consequently the extent of renal damage) plays a critical role in determining the outcomes of revascularization. Moreover, this study indicates that the damage and early loss of the renal microvessels (as observed after 6 weeks of RAS) and the deterioration of the renal angiogenic response (as suggested by decreased VEGF and downstream mediators9) is an important determinant for the progression of renal injury and likely demarcates the point of often irreversible damage in the stenotic kidney.
Catheter-based therapy for hemodynamically significant RAS is the preferred method of revascularization. The use of this intervention has been consistently growing for the past 20 years, with tremendous progress in successfully restoring the blood flow through a previously stenotic renal artery (over 95% of the cases)22. However, it is disconcerting that resolution of hypertension and mainly, improvements in renal function is still at best modest, with improvement rates of around 30% of the cases in most reports23. The reasons of this discordance between the success rate and outcomes are still unknown, and have been the topic of numerous analyses and discussions but without a definitive answer5, 24. White a et al22 have recently published an extensive in depth review where they postulate that the reasons for these relatively poor outcomes may be the result of a combined poor selection of the patients, poor discrimination of the severity of the lesions, and, most importantly, insufficient assessment of the injury of the renal parenchyma distal to the stenosis. While assessment of the severity of RAS could be technically resolved with the use of the clinically-available high resolution imaging techniques (e.g.: CT angiography), the assessment and quantification of renal parenchymal damage distal to the stenosis is, on the other hand, the most difficult problem to sort in clinical practice. An optimal evaluation of renal damage would consequently result in better selection of candidates that would benefit from revascularization. Hence, the key seems to reside mainly in the severity and extent of damage in the stenotic renal parenchyma. We have previously shown that the stenotic kidney has significant inflammation, fibrosis, and reduction of MV density8, 14, 21, changes that are mainly evident at the cortical level, which controls almost 80% of the total RBF. Therefore, this decrease in MV density is likely a central event determining the functional and structural deterioration of the stenotic kidney. Nevertheless, whether that is the reason that may partly explain the poor responses of the stenotic kidney to revascularization have never been investigated nor established.
The current study attempted to elucidate the role of MV disease by testing the hypothesis that changes in the renal MV architecture and function are crucial for the progression of renal injury and, mainly, for the renal responses to revascularization. We use a well established model of RAS, a surrogate of early chronic renovascular disease that results in the development of a hemodynamically significant stenosis, leading to hypertension and significant renal functional and structural injury as early as after 4–6 weeks. This model resembles human disease in several ways since RAS gradually develops and involves progressive vascular wall injury as occurs in human RAS, constituting a clinically relevant model of renovascular disease. In addition, being a large animal model, it offers a unique opportunity to perform surgical and/or pharmacological interventions in a manner that could be potentially applicable to humans, as well as it allow to determining the effects of the disease and responses to interventions accurately We have shown that the renal functional deterioration in our model is accompanied by reduced renal VEGF and MV density9. By infusing VEGF intra-renally at the onset of the stenosis, we have recently shown this intervention improved MV density and function and decreased fibrosis, without modifying the degree of RAS or hypertension, suggesting a targeted effect into the stenotic renal parenchyma. Previous studies have shown sustained long-term effects of VEGF in improving tissue perfusion and development of new vessels16, 17. We observed sustained beneficial effects of a single intra-renal infusion of VEGF, which were reflected not only by the improvements in renal function and preservation of the cortical and medullary MV architecture, but also by the restoration of downstream mediators of VEGF such as angiopoietins and eNOS9. Also, the augmented in VEGF bioavailability in the RAS+VEGF treated kidneys facing an enhanced expression of the VEGF receptor Flk-19 may have also contributed to a more organized vascular proliferative response25 and MV function by this approach. Furthermore, it also possible that a reduction in renal fibrosis may have in turn resulted in preservation of the sources of VEGF26, further contributing to maintain the angiogenic cascade in the stenotic kidney in response to an ischemic insult. In addition to mediating renal MV proliferation and repair, VEGF can also exert renoprotection by stimulating proliferation and survival of renal epithelial cells which in turn can further attract endothelial cells to promote vasculogenesis in a complex autocrine/paracrine mechanism27.
Since catheter-based revascularization is the most frequent therapeutic approach to treat RAS in humans, all RAS and RAS+VEGF pigs underwent PTRA and a stent was deployed to assure vascular patency. Interestingly, despite complete restoration of blood flow and resolution of the hemodynamically significant stenosis, blood pressure and renal vascular resistance decreased less in the RAS pigs compared to RAS+VEGF, where both decreased to a level that was not different compared to normal-time controls. The more modest decrease of these parameters in RAS post-PTRA argues against a pure “renovascular” origin of hypertension in this model, which likely mimics what occurs in humans. Indeed, clinical data show that resolution of hypertension after successful revascularization is observed in less than 10% of the patients28, which possibly weighs on the degree of renal damage as an important contributor to hypertension. In addition, another study29 suggests that the GFR pre-PTRA may anticipate whether the outcomes of revascularization will impact on renal function enhancement and/or hypertension control. The significant preservation of renal function and attenuation of fibrosis and MV damage and loss pre-PTRA in RAS+VEGF animals (despite similar degree of RAS) likely played a role for the significant improvement in RBF, GFR, and regional perfusion, and in restoring MV endothelial function post-PTRA, unlike untreated RAS where renal function remained attenuated. Importantly, and supporting our hypothesis, these beneficial effects were accompanied by a 34% increase in MV density in VEGF+PTRA compared to PTRA alone and decreased fibrosis. Moreover, the significant improvement in the MV responses to endothelium-dependent challenge with acetylcholine in VEGF-treated pigs indicates this intervention resulted in not only “more”, but also in “better”, functional new vessels.
A limitation of this study is that most of these effects were largely preventive since VEGF was administered at the induction of RAS. Hence, further studies testing this or similar30 targeted interventions on renal microvessels after established renal injury (e.g.: administering VEGF after RAS and renal injury develop), in combination with renoprotective drugs frequently used in patients with renovascular disease (e.g. angiotensin-receptor blockers, statins), and at a later stage of the disease will cement the role that protection of renal MV integrity plays in defining the fate of the stenotic kidney and its outcomes after catheter-based interventions. Furthermore, we cannot rule out the possibility of VEGF achieving beneficial effects on the stenotic kidney independently of MV improvements by directly down-regulating other injurious mechanisms for the stenotic kidney such as fibrosis12 or apoptosis31, 32. We are aware that another limitation is that the study design is not yet directly clinically applicable. However, the results of our studies challenge current therapies in human renovascular disease and strongly imply a link between renal MV architecture and function and progression of renal injury, since the degree of RAS and hypertension was similar in all RAS animals but prevention of MV rarefaction preserved the hemodynamics, function, and improved the responses to PTRA. Our findings support a novel concept and may constitute the first step to unravel the complex mechanisms that determine the recovery of the stenotic kidney in humans. By refining the timing of PTRA, the most frequent and established therapeutic approach to treat patients with renal artery stenosis, and determining whether combining PTRA with a targeted intervention deemed to protect the renal microcirculation is feasible, our studies could potentially open new avenues to improve the treatment of patients with chronic renovascular disease.
Acknowledgements
We gratefully acknowledge Mr. James E. Bailey and Mr. Fredrick S. Fails for their technical assistance during the MDCT in vivo studies.
Sources of funding
Supported by grant 0830100N (Scientist Development Grant) from the American Heart Association and HL095638 from the National Institute of Health.
Footnotes
Subject Codes: [130] Animal models of human disease; [129] Angiogenesis; [57] Angiography; [30] CT and MRI; [27] Other Treatment.
Disclosures: None. There are no conflicts of interest to disclose by any of the authors.
References
- 1.Amighi J, Schlager O, Haumer M, Dick P, Mlekusch W, Loewe C, Bohmig G, Koppensteiner R, Minar E, Schillinger M. Renal artery stenosis predicts adverse cardiovascular and renal outcome in patients with peripheral artery disease. Eur J Clin Invest. 2009;39:784–792. doi: 10.1111/j.1365-2362.2009.02180.x. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17:1383–1397. doi: 10.1097/01.RVI.0000240426.53079.46. quiz 1398. [DOI] [PubMed] [Google Scholar]
- 3.Textor SC, Lerman L, McKusick M. The uncertain value of renal artery interventions: where are we now? JACC Cardiovasc Interv. 2009;2:175–182. doi: 10.1016/j.jcin.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White CJ. Catheter-based therapy for atherosclerotic renal artery stenosis. Circulation. 2006;113:1464–1473. doi: 10.1161/CIRCULATIONAHA.105.540039. [DOI] [PubMed] [Google Scholar]
- 5.Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol. 2004;15:1974–1982. doi: 10.1097/01.ASN.0000133699.97353.24. [DOI] [PubMed] [Google Scholar]
- 6.Horbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, Sutton TA. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2007;293:F688–F695. doi: 10.1152/ajprenal.00452.2006. [DOI] [PubMed] [Google Scholar]
- 7.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13:1–7. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1854–1859. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 9.Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR. Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant. 2010;25:1079–1087. doi: 10.1093/ndt/gfp605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohenstein B, Hausknecht B, Boehmer K, Riess R, Brekken RA, Hugo CP. Local VEGF activity but not VEGF expression is tightly regulated during diabetic nephropathy in man. Kidney Int. 2006;69:1654–1661. doi: 10.1038/sj.ki.5000294. [DOI] [PubMed] [Google Scholar]
- 11.Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, Kerjaschki D, Schreiner GF, Johnson RJ. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis. 2001;37:601–611. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448–1457. doi: 10.1681/ASN.V1271448. [DOI] [PubMed] [Google Scholar]
- 13.Rabelink TJ, Wijewickrama DC, de Koning EJ. Peritubular endothelium: the Achilles heel of the kidney? Kidney Int. 2007;72:926–930. doi: 10.1038/sj.ki.5002414. [DOI] [PubMed] [Google Scholar]
- 14.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002;106:1165–1171. doi: 10.1161/01.cir.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 15.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol. 1999;10:1455–1465. doi: 10.1681/ASN.V1071455. [DOI] [PubMed] [Google Scholar]
- 16.Hendel RC, Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Bonow RO. Effect of intracoronary recombinant human vascular endothelial growth factor on myocardial perfusion: evidence for a dose-dependent effect. Circulation. 2000;101:118–121. doi: 10.1161/01.cir.101.2.118. [DOI] [PubMed] [Google Scholar]
- 17.Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Losordo DW, Hendel RC, Bonow RO, Eppler SM, Zioncheck TF, Holmgren EB, McCluskey ER. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J. 2001;142:872–880. doi: 10.1067/mhj.2001.118471. [DOI] [PubMed] [Google Scholar]
- 18.Chade AR, Krier JD, Rodriguez-Porcel M, Breen JF, McKusick MA, Lerman A, Lerman LO. Comparison of acute and chronic antioxidant interventions in experimental renovascular disease. Am J Physiol Renal Physiol. 2004;286:F1079–F1086. doi: 10.1152/ajprenal.00385.2003. [DOI] [PubMed] [Google Scholar]
- 19.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology. 2007;243:405–412. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 20.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol. 2001;281:F630–F638. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 21.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. Faseb J. 2006;20:1706–1708. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- 22.White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med. 2009;6:176–190. doi: 10.1038/ncpcardio1448. [DOI] [PubMed] [Google Scholar]
- 23.Textor SC, Wilcox CS. Renal artery stenosis: a common, treatable cause of renal failure? Annu Rev Med. 2001;52:421–442. doi: 10.1146/annurev.med.52.1.421. [DOI] [PubMed] [Google Scholar]
- 24.Levin A, Linas S, Luft FC, Chapman AB, Textor S. Controversies in renal artery stenosis: a review by the American Society of Nephrology Advisory Group on Hypertension. Am J Nephrol. 2007;27:212–220. doi: 10.1159/000101000. [DOI] [PubMed] [Google Scholar]
- 25.Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol. 2001;159:993–1008. doi: 10.1016/S0002-9440(10)61775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF--a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol. 2007;106:32–37. doi: 10.1159/000101798. [DOI] [PubMed] [Google Scholar]
- 27.Villegas G, Lange-Sperandio B, Tufro A. Autocrine and paracrine functions of vascular endothelial growth factor (VEGF) in renal tubular epithelial cells. Kidney Int. 2005;67:449–457. doi: 10.1111/j.1523-1755.2005.67101.x. [DOI] [PubMed] [Google Scholar]
- 28.Safian RD, Madder RD. Refining the approach to renal artery revascularization. JACC Cardiovasc Interv. 2009;2:161–174. doi: 10.1016/j.jcin.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Ramos F, Kotliar C, Alvarez D, Baglivo H, Rafaelle P, Londero H, Sanchez R, Wilcox CS. Renal function and outcome of PTRA and stenting for atherosclerotic renal artery stenosis. Kidney Int. 2003;63:276–282. doi: 10.1046/j.1523-1755.2003.00734.x. [DOI] [PubMed] [Google Scholar]
- 30.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, Law PY, Hebbel RP. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999;247:495–504. doi: 10.1006/excr.1998.4359. [DOI] [PubMed] [Google Scholar]
- 32.Ye L, Haider H, Guo C, Sim EK. Cell-based VEGF delivery prevents donor cell apoptosis after transplantation. Ann Thorac Surg. 2007;83:1233–1234. doi: 10.1016/j.athoracsur.2006.04.008. author reply 1234. [DOI] [PubMed] [Google Scholar]