Abstract
Background
Cardiac events in long-QT syndrome type-2 (LQT2) patients are predominately associated with sudden arousal. However, exercise-induced events also occur in this population.
Objectives
We hypothesized that risk factors show a trigger-specific association with cardiac events in LQT2 patients.
Methods
The study population comprised 634 genetically-confirmed LQT2 patients from the US portion of the International LQTS Registry. Multivariate Cox proportional hazards regression analysis was used to determine the independent contribution of clinical and genetic risk factors to the first occurrence of trigger-specific cardiac events, categorized as arousal, exercise-induced, and non-arousal/non-exercise, from birth through age 40 years.
Results
Study patients experienced 204 cardiac events during follow-up, of which 44% were associated with arousal-triggers, 13% with exercise activity, and 43% with non-exercise/non-arousal triggers. Risk factors for arousal triggered cardiac events included gender (female:male >13 years: HR=9.10 [p<0.001]), and the presence of pore-loop mutations (HR=2.19 [p=0.009]). In contrast, non pore-loop transmembrane mutations were the predominant risk factor for exercise-triggered events (HR=6.84 [p<0.001]), whereas gender was not a significant risk factor for this end point. Non-exercise/non-arousal events were associated with heterogeneous causes. Risk factors for this end point included gender, mutation-location and type, and a prolonged QTc (≥500 msec) Beta-blocker therapy was associated with a pronounced reduction in the risk of exercise-triggered events (HR=0.29 [p<0.01]), but had a non-significant effect on the risk of arousal- and non-exercise/non-arousal events.
Conclusions
Our findings suggest that management of patients with the LQT2 genotype should employ a trigger-specific approach to risk-assessment and medical therapy.
Keywords: long-QT syndrome, ion channel mutations, sudden cardiac death, risk factors, beta-blockers
The congenital Long-QT Syndrome (LQTS) is an inherited channelopathy in which most affected individuals have prolonged ventricular repolarization manifest on the electrocardiogram (ECG) as QT prolongation.1 To date, more than 500 mutations have been identified in 13 LQTS-susceptibility genes, with the LQT1-3 genotypes comprising more than 95% of genotype positive LQTS and approximately 75% of all LQTS patients.2 Type 1 LQTS (LQT1) results from a mutation in the KCNQ1 gene that encodes the slowly activating delayed rectifier K+ channel, IKs. Mutations in the KCNH2 gene, which codes for the rapidly activating delayed rectifier K+ channel (IKr), result in type 2 LQTS (LQT2). Type 3 LQTS (LQT3) is associated with mutations in the SCN5A gene, which codes for the Na+ voltage-gated channel.2
In each of the most common LQTS genotypes, LQT1-3, cardiac events have been shown to be strongly associated with specific triggers.3,4 In patients with LQT2, the majority of cardiac event stimuli are sudden arousal triggers, whereas a lower proportion of events are associated with exercise activity.3,4 In addition to these phenotype-genotype associations, specific KCNH2 mutation locations, such as the ion conduction pathway (in the pore-loop region of the KCNH2 channel), have been shown to be associated with increased risk for arrhythmic events.5,6 Gender has also been identified as an important independent contributor to event risk in LQT2, as adolescent and adult women were shown to have higher risk for cardiac events than the corresponding men in this population.7–10
Previous studies have focused on the identification of genotype-specific risk factors for cardiac events in LQTS patients.1–10 However, it is possible that risk factors show different associations with triggers for arrhythmic events within each genotype. Specifically, we hypothesized that clinical and genetic risk factors exhibit a trigger-specific association with arousal- and exercise- induced cardiac events in carriers of the LQT2 genotype.
Methods
Study Population
The study population of 634 subjects was derived from 158 proband-identified families with genetically confirmed KCNH2 mutations drawn from the US portion of the International LQTS Registry. The proband in each family had corrected QT (QTc) prolongation not due to a known cause. Patients with evidence of 2 or more LQTS mutations were excluded from the study. All subjects or their guardians provided informed consent for the genetic and clinical studies.
Phenotype characterization
Routine clinical and electrocardiographic (ECG) parameters were acquired at the time of enrollment. Measured parameters on the first recorded ECG included QT and R-R intervals in milliseconds, with QT corrected for heart rate by Bazett’s formula.11 Clinical data were recorded on prospectively designed forms and included patient and family history and demographic, ECG, therapeutic, and cardiac event information. Data regarding triggers for cardiac events were collected for each patient (as reported by the patient [if alive], family members, or primary care physician) after the occurrence of an event through a specific questionnaire, and further corroborated by the study coordinators through the patient’s medical files and oral history from individuals about themselves or about family members. Subsequently, the study specialists categorized each reported trigger using pre-specified codes. Follow-up data regarding beta-blocker therapy included the starting date, type of beta-blocker, and discontinuation date in case it occurred. Among subjects who died, the usage of a beta-blocker before death was determined retrospectively.
Genotype characterization
KCNH2 mutations were identified with the use of standard genetic tests performed in academic molecular genetic research laboratories and/or in commercial laboratories. Genetic alterations of the amino acid sequence were characterized by location in the channel protein and by the type of mutation (missense, splice site, in-frame insertions/deletions, nonsense [stop codon], and frameshift).12,13 The transmembrane (TM) region of the KCNH2 encoded protein was defined as the coding sequence involving amino acid residues from 404 through 659 (pore-loop region: 548–659), with the N-terminus region defined before residue 404 (Per-Arnt-Sim [PAS] region: 41–144), and the C-terminus region after residue 659.
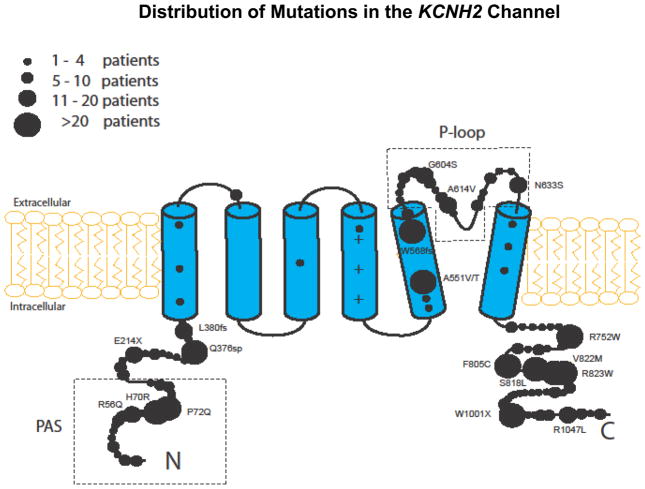
The location of the mutations in the channel protein was categorized in the present study as follows (Fig. 1): 1) TM region which includes 6 membrane-spanning segments (S1–S6) including the linkers, but excluding the pore-loop region, 2) the pore-loop region between S5 and S6; 3) PAS region (categorized separately from the N-terminus region since mutations in this region may result in a more severe clinical course)14–16; and 4) the N-terminus (excluding the PAS region) and C-terminus regions, as a single covariate. Mutation-type was categorized as missense vs. non-missense. The specific mutations included in the present study, by location, type, and number of patients, are detailed in the Supplementary Appendix Table. The distribution of study mutations in the KCNH2 channel, by the relative number of patients, is shown in Figure 1.
Figure 1.
Distribution of mutations in the KCNH2 channel by the relative number of study patients.
End points
The primary end point of the study was the first occurrence of a cardiac event, comprising syncope (defined as transient loss of consciousness that was abrupt in onset and offset), aborted cardiac arrest ([ACA] requiring external defibrillation as part of the resuscitation), or LQTS-related sudden cardiac death ([SCD] death abrupt in onset without evident cause, if witnessed, or death that was not explained by any other cause if it occurred in an non-witnessed setting such as sleep), whichever occurred first, from birth through age 40 years. The composite cardiac event end point was further categorized into 3 subgroups by the trigger reported to be associated with the event, including 1) arousal triggers (defined as loud noise and acute emotional arousal); 2) exercise triggers (defined as vigorous physical activity and swimming or pool activity); and 3) non-arousal/non-exercise triggers (comprising 90 patients with a heterogeneous group of the remaining identified triggers, including bathing or showering [n=10]; surgery or anesthesia [n=4]; fever or illness [n=12]; a missed dose of β-blocker [n=9]; antihistamine use (n=6); other medications [n=18]; extreme heat [n=3]; and sleeping [n=28]).
Statistical Analysis
The clinical characteristics of study patients were compared by the occurrence of trigger-specific cardiac events using the chi-square test for categorical variables, and the t-test and the Mann-Whitney-Wilcoxon test for continuous variables. The Kaplan-Meier estimator was used to assess the time to a first life-threatening event and the cumulative event rates by risk factors, and groups were compared using the log-rank test.
Multivariate Cox proportional hazards regression analysis was carried out to evaluate the independent contribution of clinical and genetic factors to the first occurrence of each the 3 trigger-specific endpoints. Competitive risk models were carried out separately for the first occurrence of each of the 3 endpoints. Prespecified covariates in the multivariate models included gender, mutation location, QTc ≥500 msec, and time-dependent beta-blocker therapy. To avoid violation of the proportional hazards assumption due to gender-risk crossover during adolescence,7–10 we included an age-gender interaction-term in the multivariate models.
The statistical software used for the analyses was SAS version 9.20 (SAS Institute Inc, Cary, NC). A 2-sided 0.05 significance level was used for hypothesis testing.
Results
Of the 634 LQT2 patients involved in this study, 204 had a first cardiac event associated with an acute arousal trigger (n=90 [44% of first cardiac events]), exercise trigger (n=24 [12%]), or non-arousal/non-exercise trigger (n=90 [44%]). The clinical characteristics of study subjects without events and by the type of triggered event are presented in Table 1. Compared to the non-event group, all 3 trigger-associated event groups displayed a significantly longer QTc, and a higher frequency of LQTS-related therapies. Notably, patients who experienced cardiac events had slower heart rates, possibly due the higher frequency of beta-blocker usage in this population. Within the cardiac events subgroups, the proportion of women who experienced arousal-triggered events was significantly higher than those who experienced exercise or non-arousal/non-exercise-related events (Table 1).
Table 1.
Baseline Characteristics by Cardiac Event Trigger Type
| Characteristics | Acute Arousal (n=90) | Exercise (n=24) | Non-Arousal/Non-Exercise (n=90) | No Event (n=430) | P Value |
|---|---|---|---|---|---|
| Gender | |||||
| Female, % | 82 | 58 | 67 | 44 | <0.001 |
| ECG at enrollment | |||||
| QTc, ms | 502 ± 52 | 513 ± 58 | 505 ± 64 | 467 ± 45 | <0.001 |
| RR, ms | 934 ± 236 | 970 ± 184 | 877 ± 210 | 787 ± 244 | <0.001 |
| QRS, ms | 80 ± 14 | 82 ± 12 | 81 ± 18 | 81 ± 17 | 0.93 |
| LQTS-therapies | |||||
| β-blockers, % | 81 | 75 | 76 | 42 | <0.001 |
| LCSD, % | 7.8 | 0 | 3 | 0 | <0.001 |
| Pacemaker, % | 26 | 8 | 14 | 1 | <0.001 |
| ICD, % | 39 | 25 | 33 | 4 | <0.001 |
| Type of cardiac event | |||||
| Syncope, % | 93 | 94 | 91 | NA | 0.33 |
| ACA, % | 4 | 2 | 5 | NA | 0.12 |
| Shock, % | 1 | 1 | 0 | NA | 0.84 |
| SCD % | 2 | 3 | 4 | NA | 0.83 |
| Recurrent cardiac events* | |||||
| Subsequent events of the same type, % | 84 | 71 | 54 | NA | <0.001 |
| Subsequent events of a different type, % | 13 | 12 | 17 | NA | 0.82 |
ACA = aborted cardiac arrest; ICD = implantable cardioverter defibrillator; LCSD = left cardiac sympathetic denervation; LQTS = long-QT syndrome.
Denotes the proportion of patient who experienced a subsequent cardiac event at any time during follow-up that had the same/different type of trigger as the first cardiac event.
Recurrent events with the same type of trigger occurred in 84% of the patients who experienced a first arousal-triggered cardiac event, 71% of the patients who experienced a first exercise-triggered cardiac event, and 54% of the patients who experienced a first non-arousal/non-exercise event. The respective rates of subsequent events that were not associated with the same type of trigger were 13%, 12%, and 17% (Table 1).
Gender and the risk of arousal- and exercise- triggered cardiac events in LQT2 patients
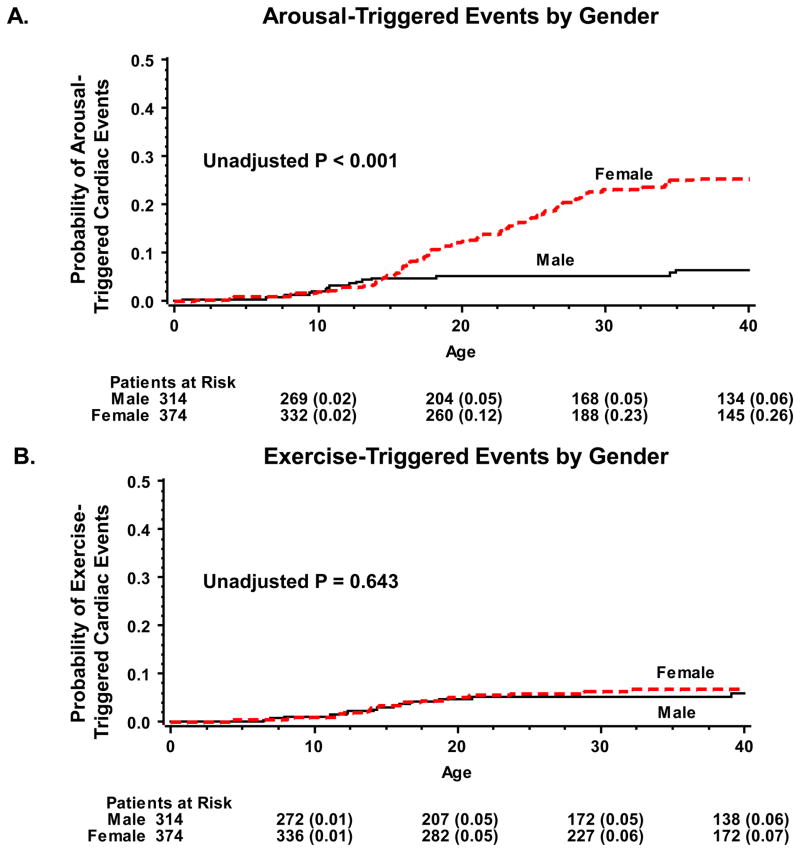
Kaplan-Meier survival analysis showed that the rate of arousal-triggered events was similar in males and females during childhood, whereas after the onset of adolescence the rate of arousal triggered events was significantly higher in women than in men (Fig. 2A). Accordingly, at age 40 years the rate of cardiac event triggered by acute arousal was 26% among women as compared with only 6% among men (log-rank p-value for the overall difference during follow-up <0.001). Consistent with the univariate survival data, multivariate analysis showed that gender was not significantly associated with increased risk for arousal-triggered cardiac events among patients ≤13 years of age, whereas in the older age-groups women had >9-fold (p<0.001) increase in the risk of arousal-triggered cardiac events as compared with men (Table 2; first row).
Figure 2.
Probability of (A) arousal triggered events; and (B) exercise-triggered events, by gender.
Table 2.
Multivariate Cox Regression Analysis: Cardiac Event Risk Factors by Trigger Type*
| END POINT | ||||||
|---|---|---|---|---|---|---|
| Acute Arousal-triggered cardiac events | Exercise-triggered cardiac events | Non-Arousal/Non-Exercise- triggered cardiac events | ||||
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P -value | |
| Gender/Age | ||||||
| Age ≤13 yrs: Males vs. females | 1.51 (0.61–3.73) | 0.37 | 1.16 (0.38–3.54) | 0.79 | 1.52 (0.84–2.74) | 0.16 |
| Age >13 yrs: Females vs. male | 9.10 (3.61–20.7) | <0.001 | 1.27 (0.53–3.04) | 0.60 | 4.69 (2.89–7.62) | <0.001 |
| Mutation location | ||||||
| Pore-loop | 2.18 (1.21–3.93) | 0.009 | 2.93 (1.00–8.61) | 0.05 | 2.64 (1.77–3.93) | <0.001 |
| Non pore-loop TM | 1.76 (0.90–3.45) | 0.10 | 6.84 (2.89–16.18) | <0.001 | 1.94 (1.24–3.05) | 0.003 |
| N-term-PAS | 1.37 (0.77–2.43) | 0.28 | 1.18 0.35–3.98 |
0.79 | 1.53 (1.03–2.27) | 0.03 |
| N-/C-term (non-PAS) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Mutation type | ||||||
| Missense vs. nonmissense | 1.20 (0.70–2.04) | 0.51 | 0.54 (0.27–1.10) | 0.09 | 2.25 (1.49–3.41) | <0.001 |
| QTc | ||||||
| QTc ≥ 500 ms | 1.41 (0.89–2.26) | 0.15 | 1.49 (0.74–2.98) | 0.26 | 1.83 (1.34–2.50) | <0.001 |
Results obtained from multivariate Cox proportional hazards regression models that were carried out separately for each triggered event end point; findings are further adjusted for time-dependent medical therapy with beta-blockers.
TM = transmembrane; PAS = Per-Arnt-Sim region of the KCNH2 channel.
In contrast to the significant association of female gender with arousal-triggered cardiac events, Kaplan-Meier survival analysis showed that the rate of exercise-triggered cardiac events among men and women was virtually the same throughout follow-up (Fig. 2B). Similarly, multivariate analysis did not demonstrate a statistically significant difference in the risk of exercise-triggered cardiac events between men and women in either age-group (Table 2; first row).
Mutation location and the risk of arousal- and exercise- triggered cardiac events in LQT2 patients
Kaplan-Meier survival analysis showed that the rates of arousal-triggered cardiac events from birth through age 40 years were highest among patients who harbored mutations in the pore-loop and TM regions; intermediate among patients who harbored mutations in the PAS region; and lowest among patients with mutations in the C-term and N-term (non-PAS) region (log-rank p-value for the overall difference = 0.026; Fig. 3A). Consistently, multivariate analysis showed that patients with mutations in the pore-loop region exhibited a >2-fold (p=0.009) increase in the risk for arousal induced triggers as compared with patients with C-term/N-term (non-PAS) mutations (Table 2; second row).
Figure 3.
Probability of (A) arousal triggered events; and (B) exercise-triggered events, by mutation-location.
In contrast, when the rates of exercise-triggered events were assessed in the study population, event rates at age 40 years were highest among patients with mutations in the non pore-loop TM region (26%); intermediate among patients with mutations in the pore-loop region (6%); and lowest among patients in the C-term/N-term (including PAS) regions (4%; log-rank p-value for the overall difference <0.001). Similarly, multivariate analysis showed that patients with mutations in the non pore-loop TM region exhibited nearly a 7-fold (p<0.001) increase in the risk of exercise-triggered cardiac event, and patients with mutations in the pore-loop region exhibited an intermediate risk (HR=2.93 [p=0.05] as compared with patients with mutations in the C-term/N-term (non-PAS) regions [Table 2; third row]).
Missense mutations were not associated with a statistically significant increase in the risk for either arousal- or exercise-triggered cardiac events (Table 2; third row).
Non-arousal/non-exercise triggers for cardiac events in LQT2 patients
A heterogeneous group of triggers (see Methods section for definition) were evident in patients with non-arousal/non-exercise cardiac end points. Patients who experienced non-arousal/no-exercise triggered events did not exhibit significant differences in the clinical characteristics, with the exception of a somewhat faster heart rate, from the other 2 triggered groups. The frequency of cardiac events during follow-up among the 3 groups was similar (Table 1). Risk factors for non-arousal/no-exercise triggered events included males ≤ 13 years age-group, females >13 years age-group, mutation-location and type, and a prolonged QTc (≥500 msec) (Table 2).
Beta-blocker therapy
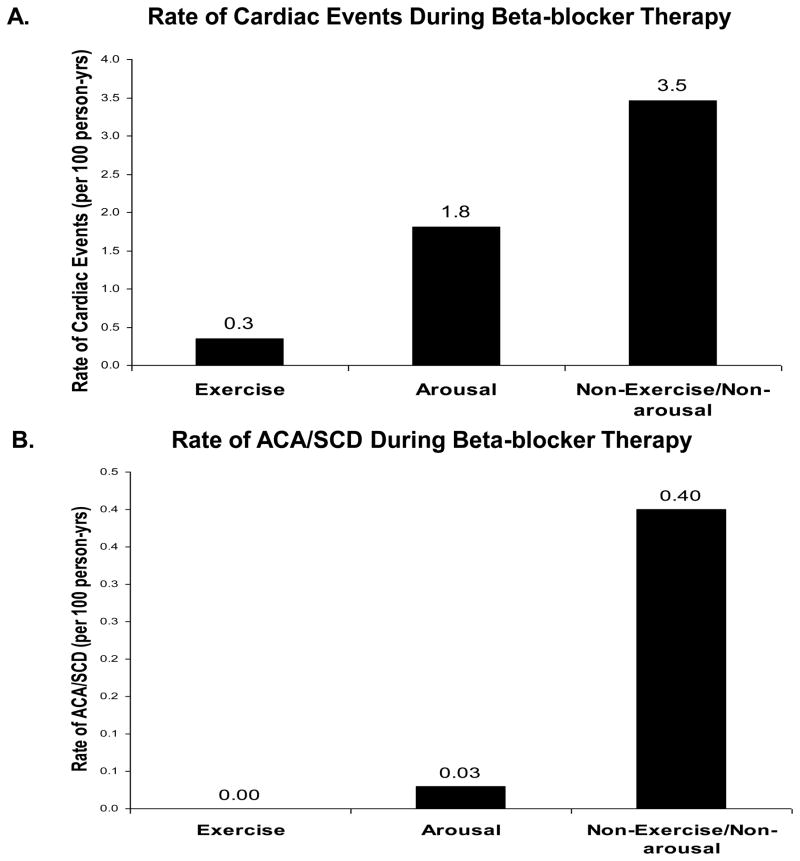
Multivariate analysis showed that time-dependent beta-blocker therapy was associated with a 21% (p=0.04) reduction in the risk of a first cardiac event in the total study population (Table 3). Risk-reduction associated with beta-blocker therapy was pronounced for cardiac events associated with exercise triggers (71% risk-reduction [p=0.01]) and statistically non-significant for arousal- and non-arousal/non-exercise triggered events (Table 3). Consistent with these findings, the rate of cardiac events during medical therapy with beta-blockers was lowest for those associated with exercise triggers; intermediate for those associated with arousal triggers; and highest for non-arousal/non-exercise-triggered cardiac events (Fig. 4A). Notably, when the rate of trigger-specific life-threatening events (comprising ACA or SCD) during beta-blocker therapy was assessed (Fig. 4B), exercise- and arousal- triggered life-threatening events were shown to occur at a very low rate during medical therapy (0 and 0.03 events per 100 patient-years, respectively), whereas non-exercise/non-arousal-triggered life-threatening events during beta-blocker therapy occurred at higher rate (0.40 per 100 patient-years).
Table 3.
β-blocker effect in LQT2 patients by the type of trigger.*
| HIGH-RISK GROUP | BETA-BLOCKER THERAPY VS. NO BETA-BLOCKER THERAPY | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| All Cardiac Events† | 0.79 | 0.41–0.98 | 0.04 |
| By trigger-type | |||
| Arousal-triggered events | 0.80 | 0.41–1.53 | 0.49 |
| Exercise-triggered events | 0.29 | 0.11–0.76 | 0.01 |
| Non-arousal/non-exercise triggered events | 0.83 | 0.51–1.37 | 0.47 |
Beta-blocker therapy was assessed as a time-dependent covariate in the multivariate models; results obtained from multivariate Cox proportional hazards regression models were further adjusted for the covariates listed in Table 2.
Multivariate Cox proportional hazards regression models that were carried out separately for each triggered event end point.
Figure 4.
Rate (per-100 patient-years of (A) cardiac events of any type; and (B) ACA or SCD during beta-blocker therapy.
Event rates were analyzed in patients who were on β-blocker therapy at any time during follow-up. Event rates per 100 person-years were calculated by dividing the number of events during β-blocker therapy by the total follow-up time in years on β-blocker therapy, and multiplying the result by 100.
ACA = aborted cardiac arrest; SCD = sudden cardiac death.
Discussion
The present study provides several important implications relating to the importance of trigger-specific risk-assessment and management of LQTS patients (summarized in Fig. 5). We have shown in the LQT2 population, women after the onset of adolescence and patients with mutations in the pore-loop region of the KCNH2 channel experience a significant increase in the risk for arousal-triggered cardiac events. In contrast, non pore-loop TM mutations were the predominant risk factor for exercise-triggered events, whereas gender was not a significant risk factor for this end point. Furthermore, our finding suggest that there is a trigger-specific response to medical therapy with beta-blockers, which were shown to be associated with a pronounced reduction in the risk of events associated with exercise-triggers, but had a non-significant effect on the risk of arousal- and non-exercise/non-arousal- triggered events. These findings suggest that risk assessment in LQT2 patients should include trigger-specific recommendations for lifestyle modifications and medical therapy that relate to the age, gender, and the location of the mutation of an affected patient.
Figure 5.
Schematic presentation of the main findings of the study regarding trigger-specific risk factors and response to therapy in LQT2.
+-signs are approximate representation of the risk/response based on the hazard ratios and associated p-values from the multivariate models.
BB = beta blockers; TM = transmembrane.
Gender-specific risk for cardiac events in LQT2 patients
The association between female gender and arousal-stimulated cardiac events in LQTS has been previously described.4 However, due to sample size limitations prior studies did not assess a genotype-specific association of gender and age with triggers for cardiac events in LQTS patients. The present study demonstrates a powerful independent association between gender and arousal-triggered events among carriers of the LQT2 genotype. We have shown that after the onset of adolescence LQT2 women experience >9-fold increase in the risk for arousal-triggered cardiac events as compared with men in the same age-group. In contrast, gender was not a significant risk factor for exercise-triggered events among carriers of the same genotype. These findings suggest a possible effect of sex hormones on the ion-channel mechanisms that predispose to arousal events in LQT2 women. Observations made through animal models showed that the female myocardium has decreased IKr current density compared to males, thus resulting in slower repolarization times and longer baseline QTc intervals.17 Estrogen was shown to directly inhibit IKr currents, thus further prolonging QT interval in females,18 and possibly contributing to susceptibility of LQT2 women to cardiac events. Furthermore, it has been suggested that arousal stimuli, particularly sudden arousal from sleep, produce a sympathetic surge in the midst of high vagal tone, which is more pronounced among women than men.19 The abruptness of the sympathetic surge prevents appropriate vagal withdrawal, and thus impairs necessary QT shortening. Thus, the increased risk for arousal triggered events among post-adolescence LQT2 women may be influenced by the effect of estrogen on ion-channel function as well as gender differences in baseline autonomic tone.
Mutation-specific risk for cardiac events in LQT2 patients
Recent studies have shown that subjects harboring pore mutations, defined as mutations between S5 and S6, including the pore-loop of the KCNH2 channel exhibit a more severe clinical course as compared with carriers of other mutations in the same channel.5,6 The present study extends these observations and shows that non pore-loop TM mutations (S1 to S5 and S6 membrane spanning domains) are the most powerful risk factor for exercise-triggered cardiac events in LQT2 patients. Mutations in the outer pore helix (S5) domain have been recently shown to affect channel activation, steady state inactivation and interaction with the voltage sensor domain (S1–S4),20 suggesting that these mutations may have a stronger contribution to phase 2 repolarization of the action potential. IKr current contribution to phase 2 repolarization is generally thought to be small, but our results suggest these mutations may contribute to an increase of adrenergic induced early afterdepolarization (EADs) during exercise.
Mutations in the N terminus PAS region were shown in the present study to be associated with a significant increase in the risk of non-exercise/non-arousal-triggered events (that comprised 44% of events among study patients) and with an intermediate risk for arousal-triggered events. PAS domain mutations have been shown to accelerate the inactivation rate of the channel, thereby reducing the outward current during the phase 3 repolarization phase, but generally show a milder effect in the overall channel current than mutations in the pore-loop region, and therefore are not expected to affect phase 2 repolarization.16,21 Such disturbances in the PAS function may underlie our finding that patients with mutations within this region are significantly predisposed to an increased risk for cardiac events that are not related to exercise activity.
In contrast, mutations that are located in the pore-loop region were specifically associated with the highest risk for arousal-triggered cardiac events but also shown to have a high risk of exercise triggered events. Pore-loop mutations have been shown to affect current density and channel inactivation, in addition to having potent dominant negative effects.22–25 These mutations are therefore expected to contribute to an overall decrease in IKr function both in phase 3 and phase 2 repolarization, consistent with an increase in the risk for events associated with both abrupt increase in heart rates and exercise.
Trigger-specific response to beta-blocker therapy
Beta-blockers are considered first-line prophylactic therapy in LQTS.26 Their mechanism of action is probably related to the attenuation of adrenergic-mediated triggers in this disorder, especially in individuals with the LQT1 and LQT2 genotypes.3 Despite this, general recommendation for empiric beta-blocker therapy in LQTS, the protective effect of this mode of medical therapy in the LQTS population is not uniform. Priori et al.27 have shown that LQT2 and LQT3 patients experience a significantly higher rate of cardiac events during beta-blocker treatment than LQT1 patients. Our findings suggest that within the LQT2 population there is a trigger-specific response to beta-blocker therapy. We have shown that LQT2 patients experience a very low rate of exercise-triggered events during beta-blocker therapy, but have a higher rate of arousal- and non-exercise/non-arousal- triggered cardiac events despite medical suggest therapy. These finding suggest that medical therapy with beta-blockers may not fully protect against arousal-triggered arrhythmic events (that are associated with a more abrupt change in heart rate than exercise triggered events) or from non-arousal/non-exercise events (that are not associated with sympathetic activation). Thus, the relatively high rate of LQT2-related cardiac events during beta-blocker therapy, shown by Priori et al.27 may be due to the fact that the events in this population are predominantly associated with non-exercise triggers. It should be noted, however, that 13% and 17% of the patients who experienced a first arousal- and non-exercise/non-arousal- triggered event, respectively, experienced a subsequent triggered event that was not of the same type. Thus, beta-blockers should also be administered to patients who experience a cardiac event that is not associated with exercise activity since subsequent exercise-triggered events may still occur in this population.
Study limitations
Data regarding triggers for cardiac events were obtained through pre-specified questionnaires from patients, family members, or primary care physicians. Despite the fact this information was further corroborated by the study coordinators through oral interviews, it remains subjective and possibly inaccurate in some cases.
It should also be noted that cardiac events that were categorized in the present study as being associated with non-exercise/non-arousal triggers comprised a heterogeneous group, relating to multiple triggers (detailed in the Methods section). Thus, the results relating to risk factors for this end point may reflect an “average” effect of the multiple different triggers, but may not be directly related to one specific trigger that was included in this end point (such as sleep, fever, or menses).
Conclusions and clinical implications
Current management of LQT2 patients relies mostly on general recommendations for lifestyle modifications that include removal of startling, loud noise stimuli in the home and at work with elimination alarm clocks, door bells, and telephone and cell-phone ringing. These recommendations are appropriate for all patients with LQT2, but especially for post-adolescent women with pore mutations. Both men and women who harbor TM mutations should be specifically advised to avoid vigorous physical activity. Beta-blockers are indicated in all LQT2 patients.25 However, our findings suggest that LQT2 patients with clinical and genetic risk factors for non-exercise events are not fully protected with medical therapy. Notably, 84% of the patients who experienced a first cardiac event that was triggered by acute arousal had a subsequent event of the same type. Thus, careful follow-up is warranted in this population, with consideration of more aggressive management if symptoms occur despite medical therapy.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by research grants HL-33843 and HL-51618 from the National Institutes of Health, Bethesda, Md.
Abbreviations
- ACA
aborted cardiac arrest
- ECG
electrocardiogram
- LQTS
long QT syndrome
- LQT1,2,3
long QT syndrome types 1,2, and 3
- QTc
corrected QT
- SCD
sudden cardiac death
Footnotes
Disclosures:
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol. 2008;51:2291–2300. doi: 10.1016/j.jacc.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Kass RS. Long QT Syndrome: from channels to cardiac arrhythmias. J Clin Invest. 2005;115:2018–2024. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;3:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Robinson JL, Gessman L, et al. Comparison of clinical and genetic variables of cardiac events associated with loud noise versus swimming among subjects with the long QT syndrome. Am J Cardiol. 1999;84:876–879. doi: 10.1016/s0002-9149(99)00458-0. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Kaufman ES, et al. Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circulation. 2002;105:794–799. doi: 10.1161/hc0702.105124. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu W, Moss AJ, Wilde AA, et al. Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol. 2009;54:2052–2062. doi: 10.1016/j.jacc.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locati EH, Zareba W, Moss AJ, et al. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome. Findings from the International LQTS Registry. Circulation. 1998;97:2237–2244. doi: 10.1161/01.cir.97.22.2237. [DOI] [PubMed] [Google Scholar]
- 8.Zareba W, Moss AJ, Locati EH, et al. International Long QT Syndrome Registry: Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol. 2003;42:103–109. doi: 10.1016/s0735-1097(03)00554-0. [DOI] [PubMed] [Google Scholar]
- 9.Sauer AJ, Moss AJ, McNitt S, et al. Long-QT syndrome in adults. J Am Coll Cardiol. 2007;49:329–337. doi: 10.1016/j.jacc.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg I, Bradley J, Moss A, et al. on behalf of the International LQTS Registry Investigators. Beta-blocker efficacy in high-risk patients with the congenital long-QT syndrome types 1 and 2: Implications for patient management. J Cardiovasc Electrophysiol. 2010 Mar 5; doi: 10.1111/j.1540-8167.2010.01737.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazett HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–367. [Google Scholar]
- 12.Splawski I, Shen J, Timothy KW, et al. Spectrum of mutations in long-QT syndrome genes: KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 13.Cabral JHM, Lee A, Cohen SL, et al. Crystal Structure and Functional Analysis of the HERG Potassium Channel N Terminus: A Eukaryotic PAS Domain. Cell. 1998;95:649–655. doi: 10.1016/s0092-8674(00)81635-9. [DOI] [PubMed] [Google Scholar]
- 14.Rossenbacker T, Mubagwa K, Jongbloed RJ, et al. Novel mutation in the Per-Arnt-Sim domain of KCNH2 causes a malignant form of long-QT syndrome. Circulation. 2005;111:961–968. doi: 10.1161/01.CIR.0000156327.35255.D8. [DOI] [PubMed] [Google Scholar]
- 15.Paulussen A, Raes A, Matthijs G, Snyders DJ, Cohen N, Aerssens J. A novel mutation (T65P) in the PAS domain of the human potassium channel HERG results in the long QT syndrome by trafficking deficiency. J Biol Chem. 2002;277:48610–48616. doi: 10.1074/jbc.M206569200. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Zou A, Splawski I, et al. Long QT syndrome-associated mutations in the Per-Arnt-Sim (PAS) domain of HERG potassium channels accelerate channel deactivation. J Biol Chem. 1999;274:10113–10118. doi: 10.1074/jbc.274.15.10113. [DOI] [PubMed] [Google Scholar]
- 17.Liu XK, Katchman A, Drici MD, et al. Gender difference in the cycle length-dependent QT and potassium currents in rabbits. J Pharmacol Exp Ther. 1998;285:672–679. [PubMed] [Google Scholar]
- 18.Kurokawa J, Tamagawa M, Harada N, et al. Acute effects of oestrogen on the guinea pig and human IKr channels and drug-induced prolongation of cardiac repolarization. J Physiol. 2008;586:2961–2973. doi: 10.1113/jphysiol.2007.150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valladares EM, Eljammal SM, Motivala S, Ehlers CL, Irwin MR. Sex differences in cardiac sympathovagal balance and vagal tone during nocturnal sleep. Sleep Med. 2008;9:310–316. doi: 10.1016/j.sleep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Ju P, Pages G, Riek RP, Chen PC, et al. The pore domain outer helix contributes to both activation and inactivation of the HERG K+ channel. J Biol Chem. 2009;284:1000–1008. doi: 10.1074/jbc.M806400200. [DOI] [PubMed] [Google Scholar]
- 21.Morais Cabral JH, Lee A, Cohen SL, Chait BT, Li M, Mackinnon R. Crystal structure and functional analysis of the HERG potassium channel N terminus: a eukaryotic PAS domain. Cell. 1998;95:649–655. doi: 10.1016/s0092-8674(00)81635-9. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Zhang M, Jiang M, Tseng GN. Structural and functional role of the extracellular s5-p linker in the HERG potassium channel. J Gen Physiol. 2002;120:723–737. doi: 10.1085/jgp.20028687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huo J, Zhang Y, Huang N, Liu P, Huang C, Guo X, Jiang W, Zhou N, Grace A, Huang CL, Ma A. The G604S-hERG mutation alters the biophysical properties and exerts a dominant-negative effect on expression of hERG channels in HEK293 cells. Pflugers Arch. 2008;456:917–928. doi: 10.1007/s00424-008-0454-0. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Furukawa T, Tanaka T, et al. Novel mechanism of HERG current suppression in LQT2: shift in voltage dependence of HERG inactivation. Circ Res. 1998;83:415–422. doi: 10.1161/01.res.83.4.415. [DOI] [PubMed] [Google Scholar]
- 25.Huang FD, Chen J, Lin M, Keating MT, Sanguinetti MC. Long-QT syndrome-associated missense mutations in the pore helix of the HERG potassium channel. Circulation. 2001;104:1071–1075. doi: 10.1161/hc3501.093815. [DOI] [PubMed] [Google Scholar]
- 26.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 Guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2006;114:e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 27.Priori SG, Napolitano C, Schwartz PJ, et al. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–4. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.