Abstract
In contrast to other cell types, vascular smooth muscle cells modify their phenotype in response to external signals. NADPH oxidase 4 (Nox4) is critical for maintenance of smooth muscle gene expression; however, the underlying mechanisms are incompletely characterized. Using smooth muscle α-actin (SMA) as a prototypical smooth muscle gene and transforming growth factor-β (TGF-β) as a differentiating agent, we examined Nox4-dependent signaling. TGF-β increases Nox4 expression and activity in human aortic smooth muscle cells (HASMC). Transfection of HASMC with siRNA against Nox4 (siNox4) abolishes TGF-β-induced SMA expression and stress fiber formation. siNox4 also significantly inhibits TGF-β-stimulated p38MAPK phosphorylation, as well as that of its substrate, mitogen-activated protein kinase-activated protein kinase-2 (MK-2). Moreover, the p38MAPK inhibitor SB-203580 nearly completely blocks the SMA increase induced by TGF-β. Inhibition of either p38MAPK or NADPH oxidase-derived reactive oxygen species impairs the TGF-β-induced phosphorylation of Ser103 on serum response factor (SRF) and reduces its transcriptional activity. Binding of SRF to myocardin-related transcription factor (MRTF) is also necessary, because downregulation of MRTF by siRNA abolishes TGF-β-induced SMA expression. Taken together, these data suggest that Nox4 regulates SMA expression via activation of a p38MAPK/SRF/MRTF pathway in response to TGF-β.
Keywords: Vascular smooth muscle, smooth muscle α-actin, transforming growth factor-β, NADPH oxidase 4, serum response factor, reactive oxygen species, p38 mitogen activated protein kinase
Vascular smooth muscle cells (VSMC) from adult tissue exhibit a differentiated phenotype, which is defined by the expression of contractile proteins such as smooth muscle alpha actin (SMA), calponin (CNN) and smooth muscle myosin heavy chain (SM-MHC) [1]. In contrast to other cell types, VSMC can modify their phenotype in response to external signals, transforming into a synthetic phenotype that is able to proliferate and migrate and secrete increased amounts of matrix proteins. This modification occurs in different cardiovascular diseases such as atherosclerosis, restenosis or hypertension, contributing to their pathophysiology [1]. Redifferentiation of synthetic VSMC limits lesion formation; however, the mechanisms responsible for re-expression of VSMC contractile genes are incompletely understood.
We previously established that NADPH oxidase 4 (Nox4) is critical for the maintenance of differentiation marker gene expression in VSMC [2]. Other studies have shown that Nox4 regulates smooth muscle-specific gene expression induced by transforming growth factor β (TGF-β) in stem cells [3], fibroblasts [4] and pulmonary artery smooth muscle cells [5, 6]. In addition, Nox4 and its activator Poldip2, appear to regulate stress fiber formation, conceivably by activating RhoA [7]. However, almost nothing is known about the mechanisms by which reactive oxygen species (ROS) derived from Nox4 affect expression of these genes and ultimately drive stress fiber formation. Indeed, very little is known about any downstream effectors of Nox4.
TGF-β is a potent inducer of SMA gene expression in many cell types, including VSMC [8, 9], mesangial cells [10, 11], fibroblasts [4, 6, 12, 13] and stem cells [3]. In addition to Nox4, signaling through multiple pathways has been implicated in TGF-β-induced gene expression, which can be divided in Smad-dependent and Smad-independent pathways (reviewed in [14, 15]). Phosphorylation of Smad3 by TGF-β is important for the induction of SMA [4, 12, 16]. Recently, other non-Smad pathways have also been shown to be important for the full expression of SMA by TGF-β. Thus, Deaton et al. [17] demonstrated that p38 mitogen activated protein kinase (p38MAPK) is important for TGF-β-induced SMA expression in rat pulmonary arterial smooth muscle cells, modulating the activity of the transcription factors MEF2, SRF and GATA. Furthermore, pharmacological inhibition of p38MAPK inhibits the increase of SMA in the epithelial-mesenchymal transition (EMT) induced by TGF-β [16, 18]. Recently, Liu et al. [19] showed that mitogen-activated protein kinase-activated-protein kinase-2 (MK-2), a substrate of p38MAPK, participates in the upregulation of SMA by TGF-β in fibroblasts. Of note, previous work has shown that p38MAPK activation is exquisitely sensitive to ROS [20, 21].
Another non-Smad pathway that has been linked to TGF-β-induced SMA expression is the small GTPase RhoA and its effectors. Indeed, RhoA kinase [5] and mammalian Diaphanous (mDia) [22-24] regulate the release of myocardin-related transcription factor (MRTF) from the G-actin pool by inducing stress fiber formation, and SMA expression via Serum Response Factor (SRF) activation [25, 26]. This is of particular interest because Nox4 has been shown to activate RhoA [7] as well as SRF expression [2].
SRF was originally discovered as a transcription factor implicated in c-fos and c-egr expression induced by serum [27, 28]; however, it soon became apparent that it was also an important regulator of smooth muscle specific genes. Promoters of these genes contain CArG boxes [29-31], which bind to SRF in the presence of the co-activators myocardin and MRTF [32-35]. In addition, SRF can undergo several modifications that alter its function. For example, phosphorylation of Ser162 [36] and Thr159 [37] on SRF has been shown to decrease SRF-dependent SMA expression, whereas phosphorylation of Ser103 enhances its activity [38, 39].
Our previous work in serum-deprived VSMC suggested a link between Nox4, ROS, SRF and differentiation marker gene expression [2, 7], but there are no studies examining the upstream signaling pathways by which Nox4-derived ROS regulate smooth muscle-specific gene expression. Here, using SMA as a prototypical smooth muscle gene and TGF-β as our inducing stimulus, we report that Nox4-mediated activation of p38MAPK leads to the phosphorylation and activation of SRF, increases SRF-dependent transcriptional activity, and increases SMA expression.
MATERIALS AND METHODS
Materials
TGF-β was obtained from R&D Systems (Minneapolis, MN). The following antibodies were used for Western blot: SMA, calponin and α-tubulin (Sigma, St. Louis, MO); phospho-p38MAPK, p38MAPK, phospho-MK2 and phospho-SRF (Cell Signaling Technology, Beverly, MA); SRF (Clone 2-313, Millipore, Billerica, MA); and MRTFA (C-19) (Santa Cruz Biotechnology, Santa Cruz, CA). For immunocytochemistry, AlexaFluor468 Phalloidin was purchased from Invitrogen (Carlsbad, CA), secondary anti-mouse was from Jackson Immunoresearch (West Grove, PA) and DAPI was from Sigma (St. Louis, MO). The inhibitor of p38MAPK (SB-203580) was purchased from Enzo Life Sciences and the MRTF/SRF activity inhibitor (CCG-1423) was from Cayman (Ann Arbor, MN).
Cell culture
Human aortic smooth muscle cells (HASMC) were obtained from Invitrogen (Carlsbad, CA). Cells were cultured as recommended by the manufacturer and used between passages 5-7.
Western blot
HASMC were lysed in Hunter’s buffer (25 mM HEPES, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10 mM Na-pyrophosphate, 10 mM NaF, 0.1 mM Na-orthovanadate, 1% Na deoxycholate, 1% Triton X-100, 0.1% SDS, 10% Glycerol, and protease inhibitors), as described previously (7). Proteins were separated using SDS-PAGE and transferred to nitrocellulose membranes, blocked, and incubated with appropriate primary antibodies. Proteins were detected by ECL (Amersham). Band intensity was quantified by densitometry using ImageJ 1.38 software.
RNA isolation and Real Time Quantitative PCR (RT-qPCR)
Total RNA was purified from cells using the RNeasy kit, as recommended in the manufacturer’s protocol. First-strand cDNA synthesis was performed using total RNA, random dodecamer primers, and Superscript III reverse transcriptase (Invitrogen), according to the enzyme supplier’s instructions. cDNAs obtained were additionally purified using microbiospin 30 columns (BioRad). Quantification of nox4, 18S rRNA, and SMA was performed by amplification of HASMC cDNA using the LightCycler real-time thermocycler (Roche). Primers and amplification conditions for 18S, and nox4 and SMA were used as previously published [4]. Copy number was calculated by the instrument software from standard curves of genuine templates.
Luciferase assay
HASMC (1×106) were transfected with 2 μg of pGL4.34 SRF.RE Vector (Promega) and R1-Renilla using a Nucleofector (Amaxa Biosystems) set to the U25 program. SRF.RE vector is designed to measure specifically the Serum Response Element that it is present in smooth muscle genes. After transfection, HASMC were allowed to attach in complete media overnight, and were incubated for 24 h in serum free medium before treatment with TGF-β (2 ng/ml) for 24 h. Firefly luciferase (SRF) and Renilla reniformis luciferase activities were determined using a Dual Luciferase Reporter Assay System (Promega) according to the manufacturer’s protocol in a TD20/20 Luminometer (Turner Designs, Sunnyvale,CA). Luciferase activity (SRF) was normalized to Renilla activity.
Immunofluorescence microscopy
HASMC were plated on collagen I-coated glass coverslips (BD) and either serum deprived for 24 h or transiently transfected with siRNA for 72 h. The cells were prepared as described previously [7], followed by incubation with Phalloidin (F-actin), DAPI (nuclei), or primary antibody to SMA for 1 h. For SMA, cells were incubated with a secondary antibody linked to Rhodamine. Vectashield mounting medium (Vector Laboratories, Inc.) was used for all confocal experiments. Images were acquired with a Zeiss LSM 510 META Laser Scanning Confocal Microscope System using a Plan-Apo 420782-9900 63x oil objective lens (numerical aperture: 1.40) and Zeiss ZEN acquisition software. To validate the specificity of the antibodies, controls with no primary antibody were performed and showed no fluorescence. Single label controls were also performed in all multiple labeling experiments (not shown). When comparing cells from different treatment groups, all image threshold settings of the confocal microscope remained constant.
siRNA studies
HASMC were transfected with 25 nM small interference RNA against Nox4 (siNox4) [2] or with the All-Star negative control siRNA (Qiagen) using Oligofectamine (Invitrogen) in OPTI-MEM (Gibco). Cells were then cultured in serum-free media for 72 h prior to treatments. For siRNA against MRTFA, 1×106 cells were nucleotransfected with 200 pmol of siRNA against MRTFA [40] or All Star negative control siRNA (Quiagen) using the U-025 program (Amaxa). After attaching, cells were then cultured in serum-free media for 48 h prior to treatments.
NADPH oxidase activity
Membrane samples from HASMs were prepared as described previously [41]. HASMs were harvested, washed twice with ice-cold 50 mM phosphate buffer (PBS), scraped, centrifuged at 400g (10 min), and resuspended in 1 ml of lysis buffer (50 mM phosphate (treated for 2h with 5g/100ml Chelex-100 and filtered) containing the protease inhibitors aprotinin (10 μg/ml), leupeptin (0.5 μg/ml), pepstatin (0.7 μg/ml), and PMSF (0.5 mM) (pH 7.4)). Cells were sonicated (power: 4 watts, using Microson 2425 from Misonix Inc; Farmingdale, NY, USA) for 10 s on ice and centrifuged at 28,000g for 15 min at 4°C. The membrane pellet was resuspended in 150 μl of lysis buffer and protein concentration was measured using the Bradford microplate method.
Twenty μg of protein were added to 1 mM 1-hydroxy-3-carboxy-pyrrolidine (CPH), 200μM NADPH, and 0.1 mM diethylenetriaminepentaacetic acid (DTPA) in a total volume of 100μL of Chelex-treated PBS. CPH is a nitrone spin trap that provides quantitative measurements of O2•− radicals with high sensitivity. ESR spectroscopy was used for quantitative measurements of O2•− production. In duplicate samples, NADPH was omitted. Superoxide formation was assayed as NADPH-dependent, SOD-inhibitable formation of 3-carboxy-proxyl (CP•). Samples were placed in 50 μl glass capillaries (Corning, New York). The ESR spectra were recorded using an EMX ESR spectrometer (Bruker) and a super-high Q microwave cavity. The ESR instrumental settings were as follows: field sweep, 50 G; microwave frequency, 9.78 GHz; microwave power, 20 mW; modulation amplitude, 2 G; conversion time, 656 ms; time constant, 656 ms; 512 points resolution and receiver gain, 1 × 105. Kinetics were recorded using a 1312ms conversion time and a 5248ms time constant by monitoring the ESR amplitude of the low-field component of the ESR spectrum of CP•. Superoxide dismutase (50 U/ml) added directly to the sample inhibited 95-98% of CP• production.
Statistical analysis
Results are expressed as mean ± S.E.M. from at least three independent experiments. Statistical significance was assessed using analysis of variance (ANOVA), followed by Bonferroni’s Multiple Comparison post-hoc test. A value of p<0.05 was considered significant.
RESULTS
TGF-β induces Nox4 expression and activity in HASMC
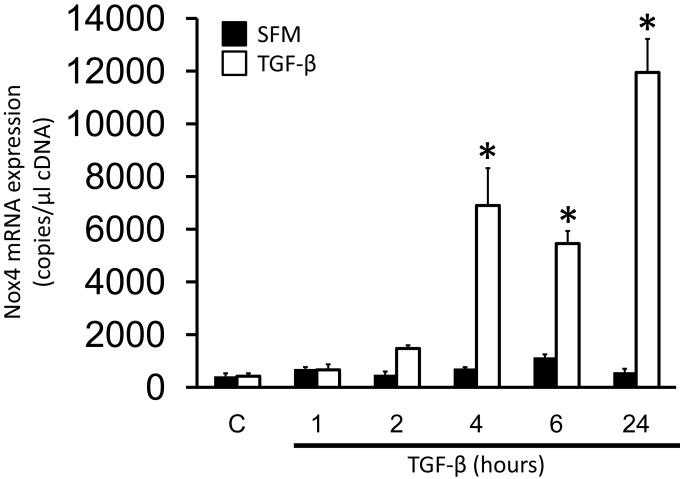
Previous studies have demonstrated an increase in Nox4 mRNA and protein levels after TGF-β treatment in other cell types [3, 6]. To determine if TGF-β upregulates Nox4 in HASMC, cells were treated with TGF-β (2 ng/ml) for different times and Nox4 mRNA expression was measured by RT-qPCR (Fig.1). TGF-β increases Nox4 mRNA levels as early as 4 h, reaching maximum induction at 24 h (20±4-fold increase over control).
Figure 1. TGF-β induces up regulation of Nox4 in HASMC.
HASMC were treated with TGF-β (2 ng/ml) for 1, 2, 4, 6 and 24 h and Nox4 mRNA was analyzed by RT-qPCR. * p < 0.05 vs serum free medium (SFM). All experiments were performed at least three independent times.
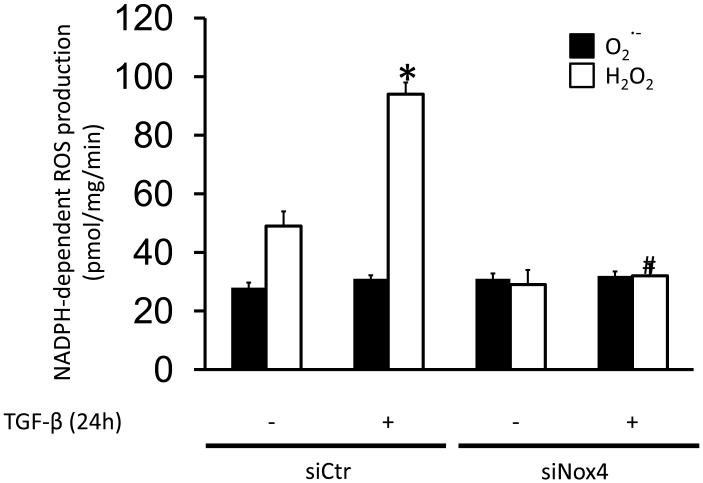
Nox4 activity is in large part regulated by its level of expression [21]; thus, we evaluated if TGF-β also induces its activity. It has been reported that the major detectable ROS generated by Nox4 is H2O2 [42, 43]. We therefore measured NADPH-dependent H2O2 production in membrane fractions of HASMC treated with TGF-β for 24h. We observed a 1.9±0.2-fold increase in H2O2 production, but only a small change in superoxide production in response to TGF-β at 24 hours (Fig. 2). To confirm that this increase is due to Nox4 activity, HASMC were transfected with siRNA against Nox4 and were then treated with TGF-β for 24 h. siNox4 completely blocked TGF-β-induced, NADPH-dependent H2O2 production (Fig. 2), confirming that Nox4 is responsible for the ROS produced in response to TGF-β. In order to determine the specificity of siNox4, we measured its effect on Nox4 and Nox5 mRNA levels, the two most highly expressed homologues in these cells [44]. While Nox4 mRNA was dramatically reduced (siCtr, 3390±380* vs. siNox4, 810±210 copies per μl of cDNA, * p< 0.05, n=3), no changes in Nox5 expression were observed in HAVSMC transfected with siNox4 (siCtr, 6324±583 vs. siNox4, 5908±373 copies per μl of cDNA, n.s, n=3).
Figure 2. TGF-β increases H2O2 generation by Nox4.
HASMC were transfected with control siRNA (siCtrl) or siRNA against Nox4 (siNox4). After 72 h, HASMC were treated with TGF-β (2 ng/ml) for 24 h and H2O2 and O2− production were measured by ESR. * p < 0.05 vs siCtr and # p < 0.05 vs siCtr + TGF-β. All experiments were performed at least three independent times.
Nox4 is required for TGF-β induced SMA expression and stress fiber formation
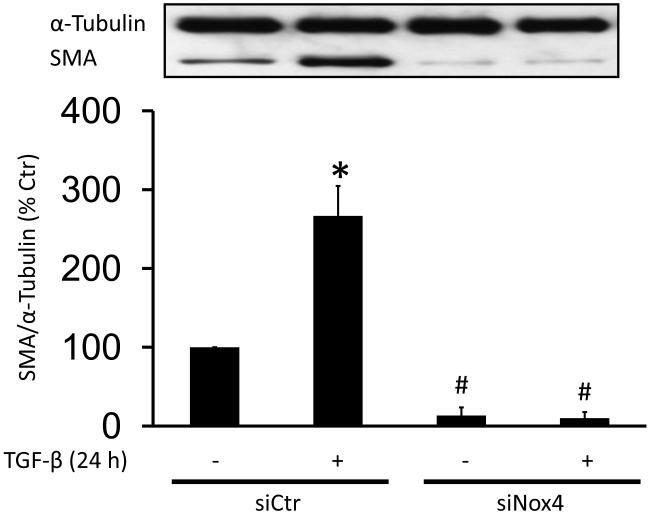
To investigate whether the activation of Nox4 is required for TGF-β induction of SMA, HASMC were incubated with TGF-β for 24 h and SMA expression was measured by western blot. Treatment with TGF-β caused an increase in SMA mRNA and protein expression in HASMC (Fig. S1). Moreover, this increase in SMA expression is Nox4-dependent, since transfection with siNox4 completely blocks TGF-β-induced SMA expression (Fig. 3A). In order to analyze whether the effect of Nox4 is confined to SMA expression, we also measured calponin (CNN) expression and observed similar results (Fig. S2A).To confirm that NADPH oxidase-derived ROS are required for this effect, we pre-incubated HASMC with diphenylene iodonium (DPI, 10 μM), a flavin-containing oxidase inhibitor, N-Acetylcysteine (NAC, 10 mM), an antioxidant or ebselen (10 μM), a glutathione peroxidase mimetic, prior to treatment with TGF-β for 24 h (2 ng/ml). We have previously shown that these concentrations of inhibitors block ROS production in VSMC ([45, 46] and Fig. S3B). All pretreatments inhibit TGF-β-induced SMA expression (Fig. S3A). Together with the siNox4 data, these experiments indicate that Nox4-derived ROS mediate TGF-β-induced SMA expression in HASMC.
Figure 3. Nox4 is critical for the upregulation of SMA via TGF-β.
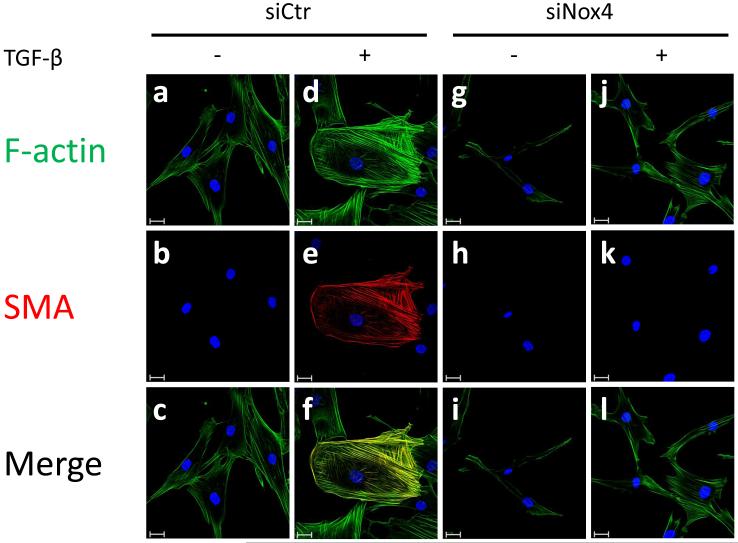
(A) HASMC were transfected with control siRNA (siCtr) or siRNA against Nox4 (siNox4). After 72 h, HASMC were treated with TGF-β (2 ng/ml) for 24 h. Total protein was extracted and SMA was analyzed using a specific SMA antibody. α-tubulin was used as an internal control. * p < 0.05 vs siCtr and # p < 0.05 vs siCtr + TGF-β. All experiments were performed at least three independent times. (B) HASMC were transfected with siCtr (a-f) or siNox4 (g-l). After 72 h, HASMC were incubated with or without TGF-β (2 ng/ml, 24 h) (non stimulated a-c and g-i, stimulated d-f and j-l). Then, HASMC were fixed and stained with phalloidin for F-actin (a, d, g and j), anti-SMA antibody(b, e, h and k) or DAPI for nuclei. Scale Bar=20 μm.
To examine the subcellular fate of increased SMA, we used immunocytochemistry. As expected, TGF-β treatment increases the number of SMA-containing stress fibers, an effect that is completely blocked by siNox4 (Fig. 3B).
p38MAPK mediates TGF-β-induced SMA expression and stress fiber formation
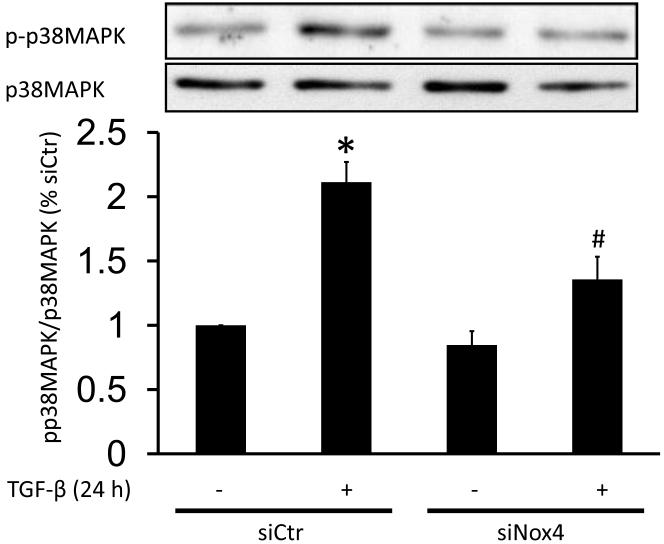
In some cell types, p38MAPK is activated by TGF-β with slow kinetics [16]. Our group and others have shown that p38MAPK is a target of ROS [20, 47, 48]. Thus, we investigated whether Nox4 modulates p38MAPK activation by TGF-β. Our results show that a 24 h treatment with TGF-β increases p38MAPK activation (213±15% vs siCtr), and that this activation is partially blunted in siNox4-transfected HASMC (130±19% vs siCtr) (Fig.4). This inhibition is also reflected in a reduction in activation of MK-2, a substrate of p38MAPK (Fig. S4A).
Figure 4. Nox4 regulates p38MAPK activation by TGF-β.
HASMC were transfected with control siRNA (siCtr) or siRNA against Nox4 (siNox4). After 72 h, HASMC were treated with TGF-β (2 ng/ml) for 24 h. Total protein was extracted and active p38MAPK was analyzed using a specific against phospho-p38MAPK. Total p38MAPK was used as a loading control. * p < 0.05 vs siCtr and # p < 0.05 vs siCtr + TGF-β. All experiments were performed at least three independent times.
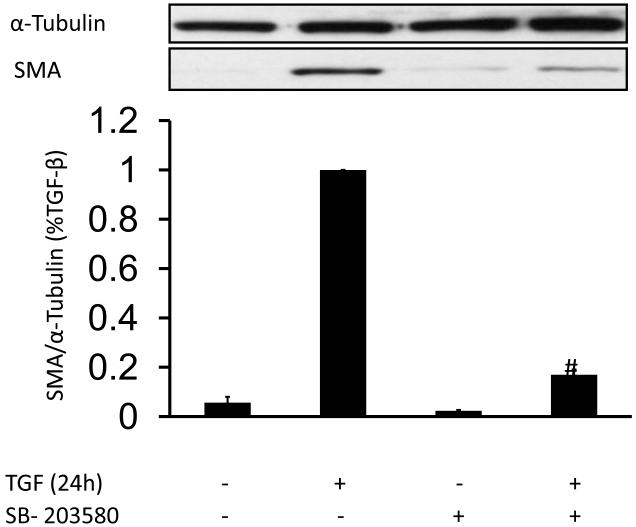
Next, we tested the hypothesis that p38MAPK activation is required for TGF-β-induced SMA expression. HASMC were preincubated with SB-203580 (10 μM), a specific p38MAPK inhibitor that we have previously shown to prevent p38MAPK activation in VSMC [20], and then treated with 2 ng/ml TGF-β for 24 h. Inhibition of p38MAPK blocks the induction of SMA expression by TGF-β (Fig. 5A). Similar results were obtained for CNN expression (Fig. S2B). Together, these data suggest that p38MAPK is a downstream effector of Nox4-mediated SMA and expression in this system.
Figure 5. p38MAPK is critical for SMA expression induced by TGF-β.
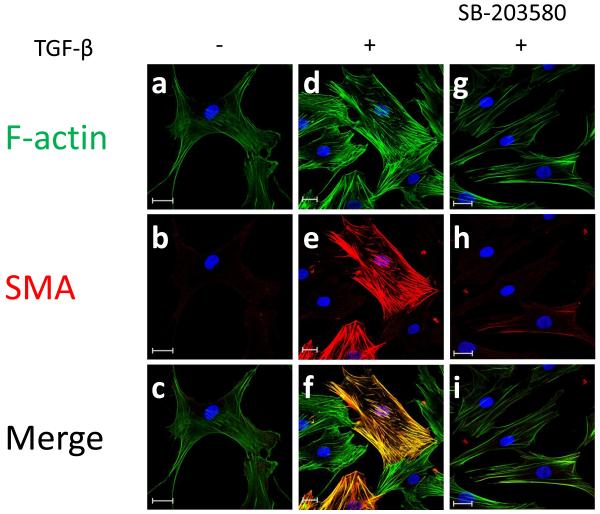
(A) HASMC were preincubated SB-203580 (10 μM), a specific p38MAPK inhibitor for 90 min. Then, HASMC were stimulated with TGF-β (2 ng/ml) for 24 h. Total protein was extracted and SMA was analyzed using a specific SMA antibody. α-tubulin was used as an internal control. # p < 0.05 vs TGF-β alone. All experiments were performed at least five independent times. (B) HASMC were preincubated with SB-203580 (10 μM) for 90 minutes. Then, HASMC were stimulated with or without TGF-β (2 ng/ml) (non stimulated a-c, TGF-β alone d-f, SB-203580+TGF-β g-i) for 24 h. At the end of the incubation period, cells were fixed and stained with phalloidin for F-actin (a, d and g), anti-SMA antibody (b, e and h) or DAPI for nuclei. Scale Bar=20μm.
To confirm the role of p38MAPK in SMA incorporation into stress fibers after TGF-β treatment, we examined stress fiber distribution using immunocytochemistry in cells treated with inhibitors of these pathways. Fig. 5B shows that pretreatment with SB-203580 blocks TGF-β-induced SMA-containing stress fiber formation. These results suggest that p38MAPK is primarily important in regulating SMA expression.
Nox4/p38MAPK regulates SRF phosphorylation and SRF/MRTF-dependent activation of the SMA promoter
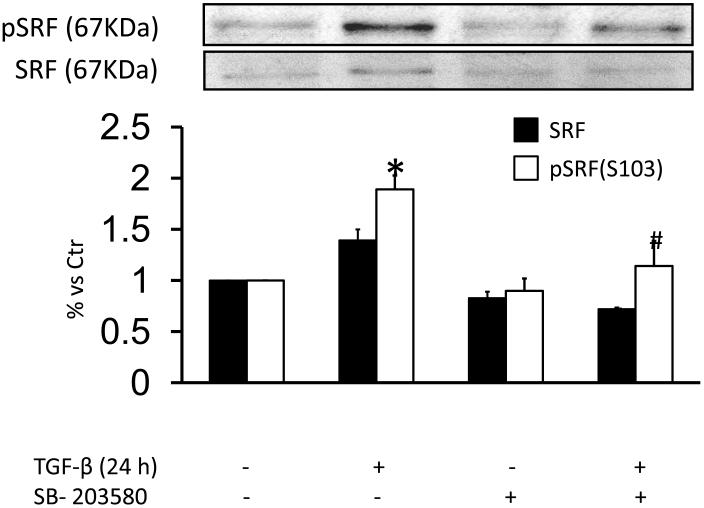
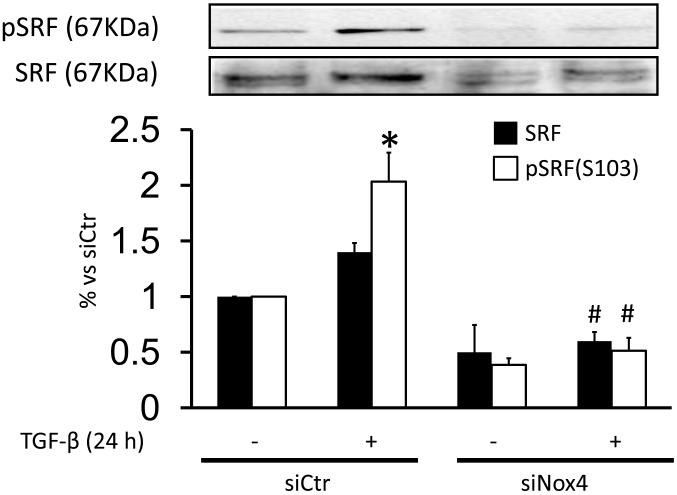
Although transcriptional control of SMA is complex, SRF binding to CArG box elements in its promoter has been shown to be required for SMA induction [8]. Phosphorylation of Ser103 (pSRF-S103) by the p38MAPK downstream effector MK-2 [38] is one mechanism by which SRF activity is regulated. Because TGF-β induction of SMA in HASMC is dependent on p38MAPK, we investigated the ability of TGF-β to both activate MK-2 and phosphorylate SRF. As shown in Fig. Fig. 6A and S4B, a 24 h treatment with TGF-β increased phosphorylation of both of these proteins, an effect that was inhibited by SB-203580. Additionally, we observed a moderate increase in total SRF expression in TGF-β-stimulated cells (140±10% vs Ctr), which was completely blocked by preincubation with SB-203580 (Fig. 6A). To determine the role of Nox4 in these responses, we studied whether siNox4 could modulate SRF phosphorylation or expression. Consistent with the ability of Nox4 to activate p38MAPK, TGF-β-treated HASMC transfected with siNox4 showed a decrease in pSRF-S103 compared with the control. We also observed a decrease in SRF expression, as reported previously [2] (Fig. 6B).
Figure 6. Nox4/p38MAPK activity regulates TGF-β-induced phosphorylation of SRF on Serine 103.
(A) HASMC were preincubated with SB-203580 (10 μM) for 90 min. Then, HASMC were stimulated with TGF-β (2 ng/ml) for 24 h. Total protein was extracted and levels of pS103-SRF and SRF were analyzed using specific antibodies. * p< 0.05 vs Ctr and # p < 0.05 vs TGF-β alone. All experiments were performed at least three independent times. (B) HASMC were transfected with control siRNA (siCtr) or siRNA against Nox4 (siNox4). After 72 h, HASMC were treated with TGF-β (2 ng/ml) for 24 h. Total protein was extracted and pS103-SRF and SRF were analyzed using specific antibodies. * p < 0.05 vs siCtr and # p < 0.05 vs siCtr + TGF-β. All experiments were performed at least three independent times.
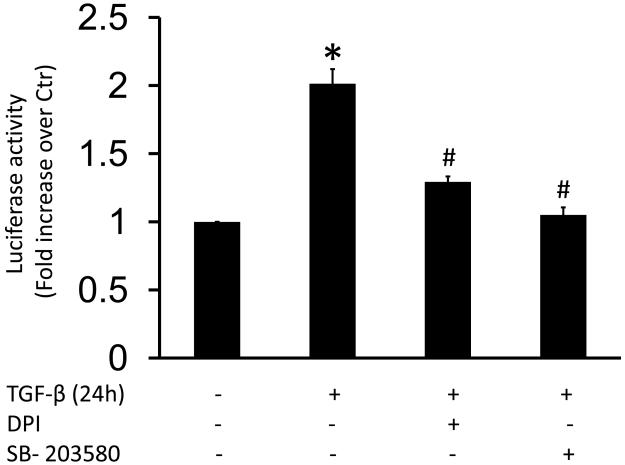
To determine whether ROS production and/or phosphorylation of SRF by p38MAPK/MK-2 are important for SRF activity, we used a luciferase assay. HASMC were transfected with SRE luciferase reporter plasmid and then stimulated with TGF-β. We observed a 2-fold increase in SRF activity in response to TGF-β, which was completely blocked by preincubation with DPI and SB-203580 (Fig. 7). No changes in basal SRF activity were found after treatment with these inhibitors (data not shown).
Figure 7. TGF-β-induced p38MAPK activation and ROS production increases SRF activity.
HASMC (1×106) were transfected with Serum Response Element Luciferase (2 μg, SRF.RE) and Renilla plasmids. Then, HASMC were preincubated with SB-203580 (10 μM) or DPI (10 μM) for 90 min and subsequently treated with TGF-β (2 ng/ml) for 24 h. SRF activity was measured as a ratio between luciferase and Renilla (internal control) activities. Data are shown as the average of three independent experiments performed in duplicate. * p < 0.05 vs Ctr and # p < 0.05 vs TGF-β alone.
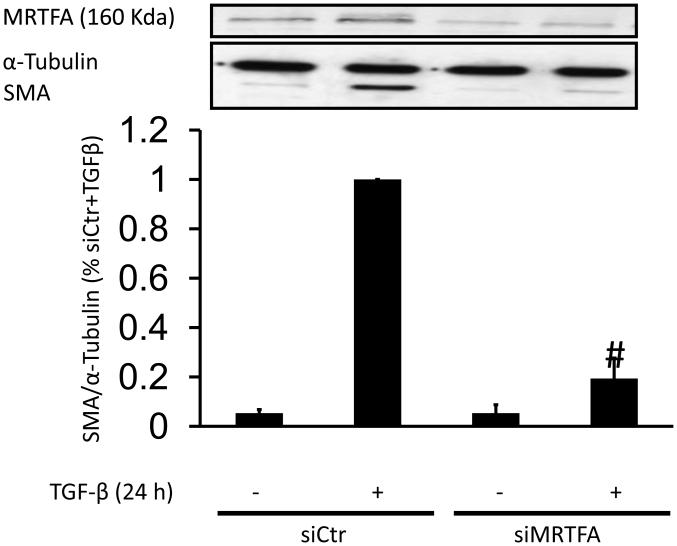
Finally, because MRTF is a coactivator of SRF that induces SMA expression [32, 49], we investigated the potential participation of MRTF in TGF-β-induced SRF-mediated induction of SMA and calponin expression in HASMC using siRNA against MRTFA, . HASMC were transfected with siRNA MRTFA and then treated with TGF-β for 24 h. siMRTFA nearly abolished the effect of TGF-β on SMA (Fig. 8) and CNN (Fig. S2C) expression. Additionally, we preincubated HAVSMC with a pharmacological inhibitor of MRTF/SRF activity, CCG-1432 (0.1-1 μM) [50] and then treated them with TGF-β for 24 h. Similar to the results obtained with siRNA against MRTFA, CCG-1432 inhibits SMA expression induced by TGF-β in a dose-dependent manner, reaching a maximum effect at 1 μM (Fig S5A).
Figure 8. MRTF is required for TGF-β induced SMA expression.
HASMC were transfected with control siRNA (siCtr) or siRNA against MRTFA (siMRTFA). After 48 h, HASMC were treated with TGF-β (2 ng/ml) for 24 h. Total protein was extracted and levels of SMA and MRTFA were analyzed using a specific antibodies. α-tubulin was used as an internal control. # p < 0.05 vs TGF-β alone.
DISCUSSION
The cytokine TGF-β has many biological effects, both physiological and pathophysiological. Due to its ability to induce smooth muscle genes, it has been associated with the progression of different pathologies such as pulmonary fibrosis [6] and diabetic nephropathy [51]. However, TGF-β is also required to maintain a normal, contractile phenotype in SMC. Among of all smooth muscle genes, SMA is the most abundant in SMC and the best characterized [52]. Here, we dissect a mechanism by which TGF-β utilizes ROS derived from Nox4 to upregulate SMA and calponin, and identify p38MAPK and SRF as two key downstream targets of Nox4 (Fig. 7).
Although TGF-β was originally linked to the activation of the Smad pathway [53, 54], recent studies have shown that TGF-β controls a number of Smad-independent signaling pathways as well [14]. We observed that in HASMC, p38MAPK activity was increased after 24 h of TGF-β treatment and that this was mediated by Nox4. While the exact mechanism by which Nox4 activates p38MAPK is unknown, we speculate that ROS produced by Nox4 may reduce the activity of the associated phosphatase, as has been shown for many other phosphatases (reviewed in [55]). Of particular relevance to p38MAPK, Hou et al. [56] recently showed that ROS production induces the oxidation of mitogen kinase phosphatase-1 (MKP-1), a phosphatase with activity against MAPKs. Similarly, Kim et al. [57] have shown that ROS production inhibits protein phosphatases 1 and 2A (PP1/2A) and MKP3, leading to an induction of phosphorylation of ERK1/2. Another possibility it is that the induction of Nox4 leads to a decrease in the expression, or an increase in the degradation, of the phosphatase implicated in the dephosphorylation of p38MAPK. Recently, we have demonstrated that H2O2 induces IP3 receptor degradation through proteasome activation in rat aortic VSMCs [58], raising the possibility that this is a universal mechanism by which H2O2 can affect signaling. Finally, it is possible that the upstream kinase of p38MAPK, TAK-1 [16, 59, 60], is a direct or indirect target of Nox4. Future experiments will be required to distinguish between these possibilities.
Several studies have shown that the level of SMA protein expression is largely dependent on the regulation of SMA mRNA expression [4, 17]. SMA gene transcription is controlled by multiple transcriptional factors, both positive such as Smad3 [4], myocardin [61], and MRTF [32, 62], and negative such as KLF4 [9, 63] and YB-1 [64]. SRF is considered a dual function transcription factor: depending on which co-activator it binds, SRF can stimulate differentiation or proliferation. Thus, Wang et al. [61] showed that TGF-β induces the binding of SRF with myocardin, which stimulates the expression of smooth muscle genes, whereas PDGF stimulates binding of SRF with Ternary Complex Factor (TCF), which induces the expression of proliferative genes and inhibits differentiation marker genes. Our results showed that MRTF is critical for TGF-β-induced SMA expression, since transfection with siMRTFA almost completely prevents the increase of SMA by TGF-β in HAVSMC. This result agrees with previous articles reporting that a decrease of MRTF by siRNA inhibits the expression of SMA induced by sphingosine 1-phosphate [32, 65], TGF-β [32] or FBS [40]. Additionally, a pharmacological inhibitor of SRF activity, CCG-1432, almost completely abolished TGF-β-induced SMA expression. Interestingly, preincubation of CCG-1432 induced a reduction of Nox4 mRNA expression, even in TGF-β-stimulated cells (Fig. S5B). Whether this effect is specific for MRTF inhibition, or is due to other potential targets of CCG-1432 such as myocardin or RhoA [50] requires further investigation. Here, we have described for the first time that p38MAPK activity is likely an important upstream activator of MRTF/SRF by TGF-β, since SRF activity is completely blocked by a p38MAPK inhibitor.
Besides binding to different cofactors, SRF can undergo several modifications that alter its affinity for DNA. Thus, phosphorylation by PKA or PKCα on Ser162 or Thr159 decreases the affinity of SRF [36, 37], whereas the phosphorylation by p38MAPK on Ser103 increases it [38]. The role of SRF-Ser103 phosphorylation in the gene expression profile is controversial. Originally this phosphorylation was discovered in response to serum [39, 66-68] and found to promote the binding of SRF to TCF, which decreases smooth muscle gene expression [61]. In contrast, Heidenreich et al. [38] observed that p38MAPK kinase induces SRF phosphorylation at Ser 103 and promotes smooth muscle gene expression [61]. These contradictory results raise the possibility that phosphorylation at Ser103 increases SRF activity, but that its effects on gene expression are determined by its cofactor. Our own data demonstrate that SRF activity is increased by TGF-β via a ROS-sensitive mechanism that involves p38MAPK-dependent phosphorylation of SRF on Ser103 and apparently increases its interaction with MRTF. This results in an increase in expression of smooth muscle-specific genes, including SMA and calponin. However, based on our results we cannot rule out the possibility that p38MAPK alters the activity of other transcription factors as well. Thus, Deaton et al. [17] showed that p38MAPK is required for the activation of GATA and MEF2 by TGF-β. Moreover, Kamaraju and Roberts [69] demonstrated that the activation of p38MAPK by TGF-β is necessary for the proper activation of Smad3. However, our results show that Smad2/3 phosphorylation induced by TGF receptor type II kinase is unmodified for siNox4 transfection or preincubation with p38MAPK inhibitor (unpublished observations), indicating that Smad activation is unlikely a target of p38MAPK and Nox4 in our model. Futhermore, Masszi et al. [70] recently showed that Smad3 acts a negative modulator of MRTFA activity in fibroblast. .Alternatively, p38MAPK might induce the degradation of SMA repressors such as KLF4 [63]. More work is necessary to investigate these additional pathways.
Our results show a slight increase in SRF expression after TGF-β stimulation as has been previously described [71]. Of note, in our experiment we used 24 hours of TGF-β incubation, which probably is not long enough to observe a large increase in SRF expression. However, when we compare the levels of SRF in TGF-β-treated with cells transfected with siNox4 or preincubated with p38MAPK inhibitor, we observe a reduction in SRF expression. Previous reports have shown that SRF regulates its own expression [49, 72]. Because Nox4 and p38MPAK are necessary for the increase in TGF-β-induced SRF activity, we suggest that longer term treatment with TGF-β is likely to induce SRF expression. We propose that Nox4 and p38MAPK are also upstream of the increase of SRF expression induced by TGF-β, suggesting a positive feedback loop.
Using immunocytochemistry, we observed that SMA localizes in actin fibers in TGF-β-treated cells (Fig. 3B). A previous article showed that the reorganization of actin in stress fibers precedes the formation of SMA-containing fibers during myofibroblast spreading [73]. Stress fiber formation is regulated by RhoA activity and focal adhesion turnover (reviewed in [74]). RhoA, in turn, controls stress fiber formation by ROCK and mDia1 [22, 75]. It is worth noting that siNox4 reduced basal RhoA activity and nearly abolished stress fibers (unpublished observations), and that we have previously observed a loss of focal adhesions in cells treated with siNox4 [7]. Recently Goffin et al. [76] showed that an increase of focal adhesion size allows the cell to generate SMA-stress fibers, which increases its contractile capacity. Our result suggests that Nox4 could be active at two levels: it may regulate SMA gene expression and it may regulate focal adhesion (FA) formation/maturation, thus influencing stress fiber assembly.
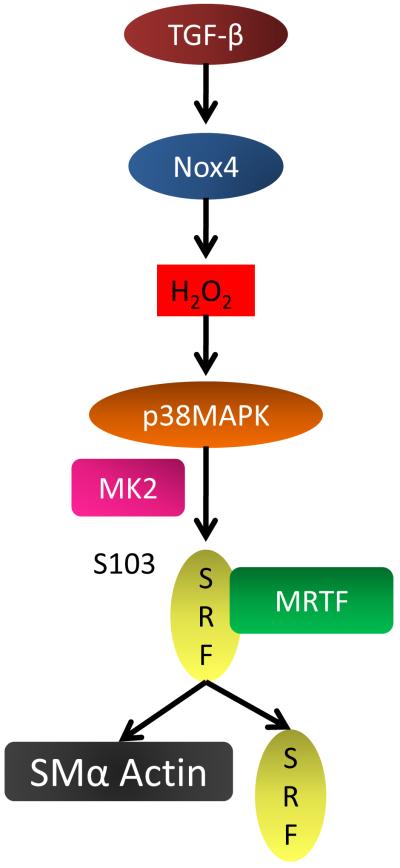
In summary, we found that (i) Nox4 is critical for TGF-β-mediated SMA expression; [56] p38MAPK activation by TGF-β plays an important role in SMA expression; (iii) Nox4 controls p38MAPK activity; and (iv) the increase in SRF activity by TGF-β is mediated by ROS production and p38MAPK activity. Moreover, we observed that Serine 103 phosphorylation of SRF by p38MAPK/MK2 can play an important role in SMA expression by TGF-β (Fig.9). Since previous reports have shown that SMA is essential for the contractile stress fibers, our data suggest that p38MAPK activation by Nox4 might provide a mechanism by which the cells regulate their phenotype and therefore their responsiveness to contractile or proliferative factors.
Figure 9. Proposed model of TGF-β induced SMA expression in HASMC.
Stimulation of HASMC with TGF-β upregulates Nox4, increasing H2O2 production. H2O2 stimulates the activity of p38MAPK/MK2, which phosphorylates SRF at Serine 103 (S103). SRF and MRTF, as a cofactor, upregulate SMA and SRF expression.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants HL38206 and HL095070.
ABBREVIATIONS
- DPI
Diphenylene iodonium
- HASMC
Human aortic vascular smooth muscle cells
- MK-2
Mitogen-activated protein kinase-activated-protein kinase-2
- MRTF
Myocardin-related transcription factor
- NAC
N-acetylcysteine
- Nox4
NADPH oxidase 4
- p38MAPK
p38 mitogen activated protein kinase
- ROS
Reactive oxygen species
- SRF
Serum response factor
- SMA
Smooth muscle α-actin
- TGF-β
Transforming growth factor-β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- [2].Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xiao Q, Luo Z, Pepe AE, Margariti A, Zeng L, Xu Q. Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am J Physiol Cell Physiol. 2009;296:C711–723. doi: 10.1152/ajpcell.00442.2008. [DOI] [PubMed] [Google Scholar]
- [4].Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- [5].Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- [6].Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hautmann MB, Madsen CS, Owens GK. A transforming growth factor beta (TGFbeta) control element drives TGFbeta-induced stimulation of smooth muscle alpha-actin gene expression in concert with two CArG elements. J Biol Chem. 1997;272:10948–10956. doi: 10.1074/jbc.272.16.10948. [DOI] [PubMed] [Google Scholar]
- [9].Liu Y, Sinha S, Owens G. A transforming growth factor-beta control element required for SM alpha-actin expression in vivo also partially mediates GKLF-dependent transcriptional repression. J Biol Chem. 2003;278:48004–48011. doi: 10.1074/jbc.M301902200. [DOI] [PubMed] [Google Scholar]
- [10].Stephenson LA, Haney LB, Hussaini IM, Karns LR, Glass WF., 2nd Regulation of smooth muscle alpha-actin expression and hypertrophy in cultured mesangial cells. Kidney Int. 1998;54:1175–1187. doi: 10.1046/j.1523-1755.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- [11].Sugenoya Y, Yoshimura A, Yamamura H, Inui K, Morita H, Yamabe H, Ueki N, Ideura T, Takahashi K. Smooth-muscle calponin in mesangial cells: regulation of expression and a role in suppressing glomerulonephritis. J Am Soc Nephrol. 2002;13:322–331. doi: 10.1681/ASN.V132322. [DOI] [PubMed] [Google Scholar]
- [12].Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol. 2003;29:397–404. doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- [13].Liu X, Kelm RJ, Jr., Strauch AR. Transforming growth factor beta1-mediated activation of the smooth muscle alpha-actin gene in human pulmonary myofibroblasts is inhibited by tumor necrosis factor-alpha via mitogen-activated protein kinase kinase 1-dependent induction of the Egr-1 transcriptional repressor. Mol Biol Cell. 2009;20:2174–2185. doi: 10.1091/mbc.E08-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wrighton KH, Lin X, Feng XH. Phospho-control of TGF-beta superfamily signaling. Cell Res. 2009;19:8–20. doi: 10.1038/cr.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Deaton RA, Su C, Valencia TG, Grant SR. Transforming growth factor-beta1-induced expression of smooth muscle marker genes involves activation of PKN and p38 MAPK. J Biol Chem. 2005;280:31172–31181. doi: 10.1074/jbc.M504774200. [DOI] [PubMed] [Google Scholar]
- [18].Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, Lee HB. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- [19].Liu T, Warburton RR, Guevara OE, Hill NS, Fanburg BL, Gaestel M, Kayyali US. Lack of MK2 inhibits myofibroblast formation and exacerbates pulmonary fibrosis. Am J Respir Cell Mol Biol. 2007;37:507–517. doi: 10.1165/rcmb.2007-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- [21].Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Copeland JW, Treisman R. The diaphanous-related formin mDia1 controls serum response factor activity through its effects on actin polymerization. Mol Biol Cell. 2002;13:4088–4099. doi: 10.1091/mbc.02-06-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- [24].Geneste O, Copeland JW, Treisman R. LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics. J Cell Biol. 2002;157:831–838. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Staus DP, Blaker AL, Taylor JM, Mack CP. Diaphanous 1 and 2 regulate smooth muscle cell differentiation by activating the myocardin-related transcription factors. Arterioscler Thromb Vasc Biol. 2007;27:478–486. doi: 10.1161/01.ATV.0000255559.77687.c1. [DOI] [PubMed] [Google Scholar]
- [26].Lagna G, Ku MM, Nguyen PH, Neuman NA, Davis BN, Hata A. Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. J Biol Chem. 2007;282:37244–37255. doi: 10.1074/jbc.M708137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dalton S, Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992;68:597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- [28].Shaw PE, Schroter H, Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989;56:563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- [29].Shimizu RT, Blank RS, Jervis R, Lawrenz-Smith SC, Owens GK. The smooth muscle alpha-actin gene promoter is differentially regulated in smooth muscle versus non-smooth muscle cells. J Biol Chem. 1995;270:7631–7643. doi: 10.1074/jbc.270.13.7631. [DOI] [PubMed] [Google Scholar]
- [30].Hendrix JA, Wamhoff BR, McDonald OG, Sinha S, Yoshida T, Owens GK. 5′ CArG degeneracy in smooth muscle alpha-actin is required for injury-induced gene suppression in vivo. J Clin Invest. 2005;115:418–427. doi: 10.1172/JCI22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- [32].Hinson JS, Medlin MD, Lockman K, Taylor JM, Mack CP. Smooth muscle cell-specific transcription is regulated by nuclear localization of the myocardin-related transcription factors. Am J Physiol Heart Circ Physiol. 2007;292:H1170–1180. doi: 10.1152/ajpheart.00864.2006. [DOI] [PubMed] [Google Scholar]
- [33].Sebe A, Masszi A, Zulys M, Yeung T, Speight P, Rotstein OD, Nakano H, Mucsi I, Szaszi K, Kapus A. Rac, PAK and p38 regulate cell contact-dependent nuclear translocation of myocardin-related transcription factor. FEBS Lett. 2008;582:291–298. doi: 10.1016/j.febslet.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Du KL, Chen M, Li J, Lepore JJ, Mericko P, Parmacek MS. Megakaryoblastic leukemia factor-1 transduces cytoskeletal signals and induces smooth muscle cell differentiation from undifferentiated embryonic stem cells. J Biol Chem. 2004;279:17578–17586. doi: 10.1074/jbc.M400961200. [DOI] [PubMed] [Google Scholar]
- [35].Selvaraj A, Prywes R. Megakaryoblastic leukemia-1/2, a transcriptional co-activator of serum response factor, is required for skeletal myogenic differentiation. J Biol Chem. 2003;278:41977–41987. doi: 10.1074/jbc.M305679200. [DOI] [PubMed] [Google Scholar]
- [36].Iyer D, Chang D, Marx J, Wei L, Olson EN, Parmacek MS, Balasubramanyam A, Schwartz RJ. Serum response factor MADS box serine-162 phosphorylation switches proliferation and myogenic gene programs. Proc Natl Acad Sci U S A. 2006;103:4516–4521. doi: 10.1073/pnas.0505338103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Blaker AL, Taylor JM, Mack CP. PKA-Dependent Phosphorylation of Serum Response Factor Inhibits Smooth Muscle-Specific Gene Expression. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.109.197285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Heidenreich O, Neininger A, Schratt G, Zinck R, Cahill MA, Engel K, Kotlyarov A, Kraft R, Kostka S, Gaestel M, Nordheim A. MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J Biol Chem. 1999;274:14434–14443. doi: 10.1074/jbc.274.20.14434. [DOI] [PubMed] [Google Scholar]
- [39].Rivera VM, Miranti CK, Misra RP, Ginty DD, Chen RH, Blenis J, Greenberg ME. A growth factor-induced kinase phosphorylates the serum response factor at a site that regulates its DNA-binding activity. Mol Cell Biol. 1993;13:6260–6273. doi: 10.1128/mcb.13.10.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Medjkane S, Perez-Sanchez C, Gaggioli C, Sahai E, Treisman R. Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat Cell Biol. 2009;11:257–268. doi: 10.1038/ncb1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hanna IR, Hilenski LL, Dikalova A, Taniyama Y, Dikalov S, Lyle A, Quinn MT, Lassegue B, Griendling KK. Functional association of nox1 with p22phox in vascular smooth muscle cells. Free Radic Biol Med. 2004;37:1542–1549. doi: 10.1016/j.freeradbiomed.2004.08.011. [DOI] [PubMed] [Google Scholar]
- [42].Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med. 2008;45:329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- [46].Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–1226. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- [47].Touyz RM, Yao G, Quinn MT, Pagano PJ, Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:512–518. doi: 10.1161/01.ATV.0000154141.66879.98. [DOI] [PubMed] [Google Scholar]
- [48].Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, Kawata S. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology. 2005;41:1272–1281. doi: 10.1002/hep.20719. [DOI] [PubMed] [Google Scholar]
- [49].Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- [50].Evelyn CR, Wade SM, Wang Q, Wu M, Iniguez-Lluhi JA, Merajver SD, Neubig RR. CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol Cancer Ther. 2007;6:2249–2260. doi: 10.1158/1535-7163.MCT-06-0782. [DOI] [PubMed] [Google Scholar]
- [51].Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11:881–889. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Owens GK, Thompson MM. Developmental changes in isoactin expression in rat aortic smooth muscle cells in vivo. Relationship between growth and cytodifferentiation. J Biol Chem. 1986;261:13373–13380. [PubMed] [Google Scholar]
- [53].Raftery LA, Twombly V, Wharton K, Gelbart WM. Genetic screens to identify elements of the decapentaplegic signaling pathway in Drosophila. Genetics. 1995;139:241–254. doi: 10.1093/genetics/139.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM. Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics. 1995;139:1347–1358. doi: 10.1093/genetics/139.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- [56].Hou N, Torii S, Saito N, Hosaka M, Takeuchi T. Reactive oxygen species-mediated pancreatic beta-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology. 2008;149:1654–1665. doi: 10.1210/en.2007-0988. [DOI] [PubMed] [Google Scholar]
- [57].Kim HS, Song MC, Kwak IH, Park TJ, Lim IK. Constitutive induction of p-Erk1/2 accompanied by reduced activities of protein phosphatases 1 and 2A and MKP3 due to reactive oxygen species during cellular senescence. J Biol Chem. 2003;278:37497–37510. doi: 10.1074/jbc.M211739200. [DOI] [PubMed] [Google Scholar]
- [58].Martin-Garrido A, Boyano-Adanez MC, Alique M, Calleros L, Serrano I, Griera M, Rodriguez-Puyol D, Griendling KK, Rodriguez-Puyol M. Hydrogen peroxide down-regulates inositol 1,4,5-trisphosphate receptor content through proteasome activation. Free Radic Biol Med. 2009;47:1362–1370. doi: 10.1016/j.freeradbiomed.2009.07.006. [DOI] [PubMed] [Google Scholar]
- [59].Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- [60].Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- [61].Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- [62].Lockman K, Taylor JM, Mack CP. The histone demethylase, Jmjd1a, interacts with the myocardin factors to regulate SMC differentiation marker gene expression. Circ Res. 2007;101:e115–123. doi: 10.1161/CIRCRESAHA.107.164178. [DOI] [PubMed] [Google Scholar]
- [63].Kawai-Kowase K, Ohshima T, Matsui H, Tanaka T, Shimizu T, Iso T, Arai M, Owens GK, Kurabayashi M. PIAS1 mediates TGFbeta-induced SM alpha-actin gene expression through inhibition of KLF4 function-expression by protein sumoylation. Arterioscler Thromb Vasc Biol. 2009;29:99–106. doi: 10.1161/ATVBAHA.108.172700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang A, Liu X, Cogan JG, Fuerst MD, Polikandriotis JA, Kelm RJ, Jr., Strauch AR. YB-1 coordinates vascular smooth muscle alpha-actin gene activation by transforming growth factor beta1 and thrombin during differentiation of human pulmonary myofibroblasts. Mol Biol Cell. 2005;16:4931–4940. doi: 10.1091/mbc.E05-03-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- [66].Marais RM, Hsuan JJ, McGuigan C, Wynne J, Treisman R. Casein kinase II phosphorylation increases the rate of serum response factor-binding site exchange. EMBO J. 1992;11:97–105. doi: 10.1002/j.1460-2075.1992.tb05032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Janknecht R, Hipskind RA, Houthaeve T, Nordheim A, Stunnenberg HG. Identification of multiple SRF N-terminal phosphorylation sites affecting DNA binding properties. EMBO J. 1992;11:1045–1054. doi: 10.1002/j.1460-2075.1992.tb05143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Misra RP, Rivera VM, Wang JM, Fan PD, Greenberg ME. The serum response factor is extensively modified by phosphorylation following its synthesis in serum-stimulated fibroblasts. Mol Cell Biol. 1991;11:4545–4554. doi: 10.1128/mcb.11.9.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kamaraju AK, Roberts AB. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-beta-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J Biol Chem. 2005;280:1024–1036. doi: 10.1074/jbc.M403960200. [DOI] [PubMed] [Google Scholar]
- [70].Masszi A, Speight P, Charbonney E, Lodyga M, Nakano H, Szaszi K, Kapus A. Fate-determining mechanisms in epithelial-myofibroblast transition: major inhibitory role for Smad3. J Cell Biol. 2010;188:383–399. doi: 10.1083/jcb.200906155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hirschi KK, Lai L, Belaguli NS, Dean DA, Schwartz RJ, Zimmer WE. Transforming growth factor-beta induction of smooth muscle cell phenotpye requires transcriptional and post-transcriptional control of serum response factor. J Biol Chem. 2002;277:6287–6295. doi: 10.1074/jbc.M106649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:C70–81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- [73].Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell. 2003;14:2508–2519. doi: 10.1091/mbc.E02-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ridley AJ. Rho: theme and variations. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- [75].Sahai E, Alberts AS, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172:259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.