Abstract
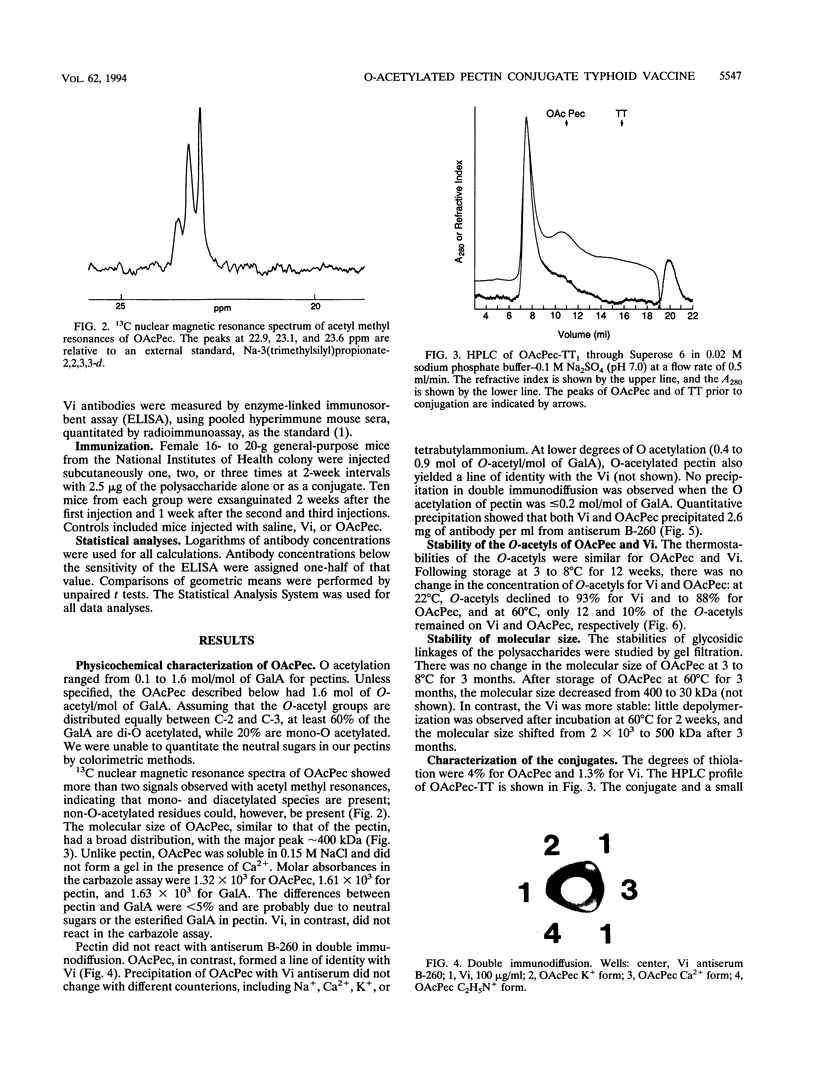
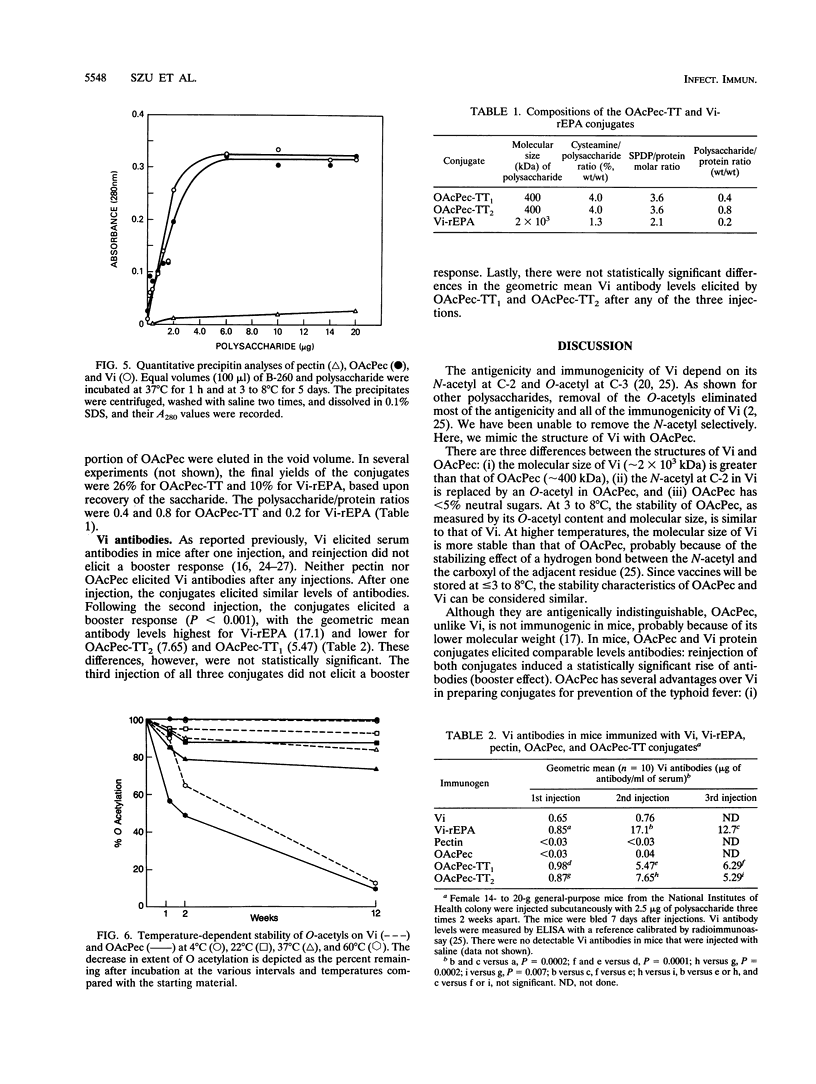
Pectin, a plant polysaccharide, is mostly a linear homopolymer of poly(1-->4)-alpha-D-GalpA with < 5% neutral sugars: its molecular size has a broad distribution around 400 kDa, and the degree of esterification is < 5%. The structure of the capsular polysaccharide of Salmonella typhi (Vi) differs from pectin in that it is N acetylated at C-2 and O acetylated at C-3, and has a molecular size of approximately 2 x 10(3) kDa. There is no serological cross-reaction between pectin and Vi. Pectin, when O acetylated at C-2 and C-3, is antigenically identical to Vi in double immunodiffusion. Unlike Vi, O-acetylated pectin (OAcPec) is not immunogenic in mice, probably because of its comparatively low molecular weight. After storage at 3 to 8 degrees C for 3 months, there was no change in the O-acetyl content or the M(r) of OAcPec. At 60 degrees C, the M(r) of OAcPec declined more rapidly than that of Vi. OAcPec conjugated to tetanus toxoid elicited Vi antibodies in mice, and reinjection elicited a booster response. The levels of Vi antibodies elicited by OAcPec-tetanus toxoid conjugates were lower than those elicited by Vi conjugates, but these differences were not statistically significant. OAcPec has some advantages because it can be measured by standardized colorimetric assays and because it forms more soluble conjugates with proteins than does Vi. One disadvantage is that its glycosidic bond is not as stable as that of Vi. The use of a plant polysaccharide, pectin, as an immunogen for prevention of a systemic infection caused by a capsulated pathogen (S. typhi) provides a novel approach to improve the preparation and immunogenicity of polysaccharide-based vaccines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya I. L., Lowe C. U., Thapa R., Gurubacharya V. L., Shrestha M. B., Cadoz M., Schulz D., Armand J., Bryla D. A., Trollfors B. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med. 1987 Oct 29;317(18):1101–1104. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bystricky S., Szu S. C. O-acetylation affects the binding properties of the carboxyl groups on the Vi bacterial polysaccharide. Biophys Chem. 1994 Jul;51(1):1–7. doi: 10.1016/0301-4622(94)00002-6. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi S. J., Robbins J. B., Schneerson R. Antibodies to poly[(2----8)-alpha-N-acetylneuraminic acid] and poly[(2----9)-alpha-N-acetylneuraminic acid] are elicited by immunization of mice with Escherichia coli K92 conjugates: potential vaccines for groups B and C meningococci and E. coli K1. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7175–7179. doi: 10.1073/pnas.88.16.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R., van de Walle M., Shiloach A., Joslyn A., Kaufman J., Shiloach J. Use of high density cultures of Escherichia coli for high level production of recombinant Pseudomonas aeruginosa exotoxin A. Appl Microbiol Biotechnol. 1991 Oct;36(1):65–69. doi: 10.1007/BF00164700. [DOI] [PubMed] [Google Scholar]
- Fattom A., Lue C., Szu S. C., Mestecky J., Schiffman G., Bryla D., Vann W. F., Watson D., Kimzey L. M., Robbins J. B. Serum antibody response in adult volunteers elicited by injection of Streptococcus pneumoniae type 12F polysaccharide alone or conjugated to diphtheria toxoid. Infect Immun. 1990 Jul;58(7):2309–2312. doi: 10.1128/iai.58.7.2309-2312.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIDELBERGER M., REBERS P. A. Immunochemistry of the pneumococcal types II. V. and VI. I. The relation of Type VI to Type II and other correlations between chemical constitution and precipitation in antisera to type VI. J Bacteriol. 1960 Aug;80:145–153. doi: 10.1128/jb.80.2.145-153.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugman K. P., Gilbertson I. T., Koornhof H. J., Robbins J. B., Schneerson R., Schulz D., Cadoz M., Armand J. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet. 1987 Nov 21;2(8569):1165–1169. doi: 10.1016/s0140-6736(87)91316-x. [DOI] [PubMed] [Google Scholar]
- LANDY M. Studies on Vi antigen. VI. Immunization of human beings with purified Vi antigen. Am J Hyg. 1954 Jul;60(1):52–62. doi: 10.1093/oxfordjournals.aje.a119703. [DOI] [PubMed] [Google Scholar]
- LANDY M. Studies on Vi antigen. VII. Characteristics of the immune response in the mouse. Am J Hyg. 1957 Jan;65(1):81–93. doi: 10.1093/oxfordjournals.aje.a119858. [DOI] [PubMed] [Google Scholar]
- Martin D. G., Jarvis F. G., Milner K. C. Physicochemical and biological properties of sonically treated Vi antigen. J Bacteriol. 1967 Nov;94(5):1411–1416. doi: 10.1128/jb.94.5.1411-1416.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters C. C., Tenbergen-Meekes A. M., Evenberg D. E., Poolman J. T., Zegers B. J., Rijkers G. T. A comparative study of the immunogenicity of pneumococcal type 4 polysaccharide and oligosaccharide tetanus toxoid conjugates in adult mice. J Immunol. 1991 Jun 15;146(12):4308–4314. [PubMed] [Google Scholar]
- Robbins J. D., Robbins J. B. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis. 1984 Sep;150(3):436–449. doi: 10.1093/infdis/150.3.436. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stone A. L., Szu S. C. Application of optical properties of the Vi capsular polysaccharide for quantitation of the Vi antigen in vaccines for typhoid fever. J Clin Microbiol. 1988 Apr;26(4):719–725. doi: 10.1128/jcm.26.4.719-725.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B., Taylor A. Immunochemical properties of Vi antigen from Salmonella typhi Ty2: presence of two antigenic determinants. Infect Immun. 1980 Aug;29(2):539–544. doi: 10.1128/iai.29.2.539-544.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Li X. R., Schneerson R., Vickers J. H., Bryla D., Robbins J. B. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun. 1989 Dec;57(12):3823–3827. doi: 10.1128/iai.57.12.3823-3827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Li X. R., Stone A. L., Robbins J. B. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect Immun. 1991 Dec;59(12):4555–4561. doi: 10.1128/iai.59.12.4555-4561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Stone A. L., Robbins J. D., Schneerson R., Robbins J. B. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987 Nov 1;166(5):1510–1524. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Taylor D. N., Trofa A. C., Clements J. D., Shiloach J., Sadoff J. C., Bryla D. A., Robbins J. B. Laboratory and preliminary clinical characterization of Vi capsular polysaccharide-protein conjugate vaccines. Infect Immun. 1994 Oct;62(10):4440–4444. doi: 10.1128/iai.62.10.4440-4444.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. A., Buchanan-Smith J. G. A colorimetric method for the quantitation of uronic acids and a specific assay for galacturonic acid. Anal Biochem. 1992 Feb 14;201(1):190–196. doi: 10.1016/0003-2697(92)90194-c. [DOI] [PubMed] [Google Scholar]




