Abstract
Antivascular endothelial growth factor (anti-VEGF) therapies represent the standard of care for most patients presenting with neovascular (wet) age-related macular degeneration (neovascular AMD). Anti-VEGF drugs require repeated injections and impose a considerable burden of care, and not all patients respond. Radiation targets the proliferating cells that cause neovascular AMD, including fibroblastic, inflammatory, and endothelial cells. Two new neovascular AMD radiation treatments are being investigated: epimacular brachytherapy and stereotactic radiosurgery. Epimacular brachytherapy uses beta radiation, delivered to the lesion via a pars plana vitrectomy. Stereotactic radiosurgery uses low voltage X-rays in overlapping beams, directed onto the lesion. Feasibility data for epimacular brachytherapy show a greatly reduced need for anti-VEGF therapy, with a mean vision gain of 8.9 ETDRS letters at 12 months. Pivotal trials are underway (MERLOT, CABERNET). Preliminary stereotactic radiosurgery data suggest a mean vision gain of 8 to 10 ETDRS letters at 12 months. A large randomized sham controlled stereotactic radiosurgery feasibility study is underway (CLH002), with pivotal trials to follow. While it is too early to conclude on the safety and efficacy of epimacular brachytherapy and stereotactic radiosurgery, preliminary results are positive, and these suggest that radiation offers a more durable therapeutic effect than intraocular injections.
Keywords: wet age-related macular degeneration, neovascular, radiation therapy, epimacular brachytherapy, stereotactic radiosurgery, anti-VEGF
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness affecting adults over 50 years old in developed nations. In the UK, it accounts for almost 50% of those registered as blind or partially sighted, that is, between 182,000 and 300,000 people.1 AMD occurs in 2 distinct groups: 90% is the ‘dry’ atrophic form and 10% is the ‘wet’ neovascular form. Although there are more incident cases of dry AMD than wet AMD, the latter accounts for 90% of patients with severe vision loss. The Royal National Institute for the Blind and the National Institute for Health and Clinical Excellence (NICE) have estimated that 26,000 new cases of neovascular AMD will be eligible for treatment per year in the UK alone.2
The characteristic feature of most cases of wet AMD is subfoveal choroidal neovascularization (CNV). CNV is a process in which the vessels from the choriocapillaris perforate Bruch’s membrane and enter the subretinal pigment epithelial and subretinal spaces. When these new CNV lesions leak or rupture, the accumulation of fluid and blood, together with the subsequent scarring, seriously impairs the photoreceptor layer.3
Different techniques to treat or prevent neovascular AMD have been tried over the past few years with varying results. Until the recent introduction of antivascular endothelial growth factor (anti-VEGF) medications, the management of AMD was based largely on reducing the decline in visual function. However, ranibizumab (Lucentis®; Novartis, Basel, Switzerland) has been shown to produce a significant visual gain in approximately a third of patients, with a mean gain of 7.2 letters in the group as a whole.4,5 These large studies required patients to have monthly intraocular injections, however, this represents an expensive and burdensome course of treatment. Furthermore, when treatment is reduced to an as-required regimen, as advised by NICE, the mean visual gain is approximately halved.6 There is therefore a need for a more durable treatment modality to reduce the cost and burden of treatment, while maintaining or improving vision.
Radiation and neovascular AMD
Certain biological principles suggest that radiation may be an effective treatment modality for wet AMD. This is based on the concept that the development of neovascular AMD is similar to a proliferative wound healing process, and that proliferating cells are known to be sensitive to the effects of radiation. Radiotherapy causes irreparable damage to DNA and protein synthesis, preventing further replication, while still maintaining cellular integrity. Although all cells in the area are affected, radiation has a selective effect, because nondividing cells are able to repair the damage to DNA, while rapidly proliferating cells discontinue the cell division cycle and undergo apoptosis.7
Radiation used for medical therapies can be divided into 2 main categories depending on its method of delivery to the tissue. Brachytherapy uses a radiation source delivered directly to the lesion by surgery. The source is usually an isotope which produces ionizing radiation as it decays and emits energy. Teletherapy (external beam therapy) uses radiation formed into a beam which can be projected at an internal body tissue from an external source. The source can also be an isotope, but more recently electronically produced ionizing radiation has been used.8
Initial ophthalmology studies investigated external beam radiation to treat wet AMD. These used high energy radiation to penetrate the ocular and periocular tissue, and target the macula. While some studies showed results better than the natural history, the results do not compare favorably to those in the anti-VEGF era.9–11 This may be because of collateral damage to ocular tissue, and difficulty targeting macular lesions using technology that was designed for lesions that are usually several orders of magnitude larger. Another factor could have been the time-delay before radiation has an effect. In the era before anti-VEGF therapy this meant the disease progressed before the benefit of radiation occurred. A disadvantage of early external beam therapy was that linear accelerators were used to generate the energy. These accelerators produce extremely high levels of energy which are tightly regulated and need special precautions to prevent escape of the radiation: usually lead-lined concrete walls, large power supplies, and cooling measures.12
Currently 2 different approaches to radiation therapy in the treatment of neovascular AMD are being investigated: epimacular brachytherapy (VIDION; NeoVista Inc. Fremont, CA) and stereotactic radiosurgery (IRay system; Oraya Therapeutics Inc. Newark, CA).
Synergistic effect
Anti-VEGF agents have a rapid onset of action but limited durability in many patients. In general, disease activity tends to recur as they are eliminated from the eye. By contrast, radiotherapy produces a delayed response but has a much longer duration of action. Therefore, there is good scientific rationale for a synergistic response when a combination approach is used, because both therapies target the disease in different ways.13 The anti-VEGF therapy inhibits growth factors in the local area while the radiotherapy disables the local inflammatory cell population and induces an apoptotic effect on the vascular endothelium. The overall result from these 2 approaches has the potential to bring a faster and more complete recovery of functional vision. This rationale is supported by experience from oncology, in which anti-VEGF agents and radiotherapy were combined to treat colon cancer.14,15
Epimacular brachytherapy
Introduction
Unlike previous attempts using external beam radiation, epimacular brachytherapy was developed to deliver intraocular radiation. Brachytherapy, from the Greek brachy, meaning ‘short-distance’, places the source of radiation close to the CNV complex at the macula. In addition, this device uses beta radiation from a strontium-90/yttrium-90 source. Beta radiation has a rapid decline in dose with increasing distance from the source, which limits radiation exposure to a defined region, with little damage to adjacent normal tissue. To date, no serious ocular or systemic complications have been reported in small uncontrolled clinical trials using a prototype device.16,17
Details of the procedure
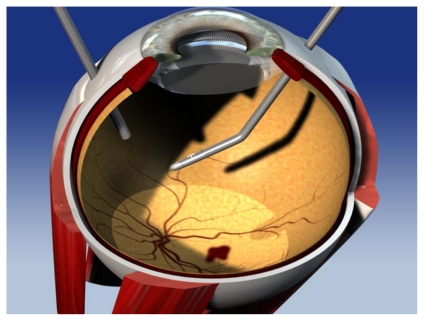
The beta radiation used in epimacular brachytherapy is delivered via a pars plana vitrectomy, a well-established surgical procedure (Figures 1, 2, and 3). Once the vitreous has been removed the surgeon positions the probe over the CNV lesion. A preoperative fundus fluorescein angiogram is used to ensure that the area of maximum dosage is directed over the area of greatest disease activity. The probe is held in position for approximately four minutes and then removed. Surgery is usually undertaken using local anesthetic in a day case setting. Because beta radiation decreases with increasing distance from the source, the delivery of radiation to neighboring structures is low. Hence the macular lesion receives 24 gray (Gy), the optic nerve receives 2.4 G, and the lens 0.00056 Gy.17
Figure 1.
Epimacular brachytherapy probe.
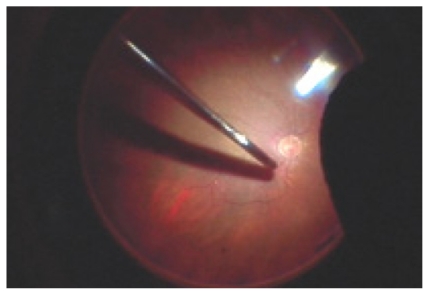
Figure 2.
Intraocular probe in mid vitreous cavity prior to placement on the retinal surface.
Figure 3.
Probe positioned on the retinal surface.
In the published trials of epimacular brachytherapy, patients received an anti-VEGF injection at the time of surgery and again 1 month later, to treat any pre-existing disease activity at the time of surgery. Thereafter they had anti-VEGF therapy as needed, based on disease activity.16,17
The potential risks of intraocular radiation include retinopathy, optic neuropathy, and cataract. The dose delivered to the macula is 24 Gy. The dose delivered to nearby structures during epimacular brachytherapy is below the reported safety threshold for each of these ocular structures (Table 1).17–22 The use of beta radiation also ensures that the total dose received by the patients is less than a routine chest X-ray.23
Table 1.
Clinically observable radiation damage thresholds for ocular structures and the calculated doses for epimacular brachytherapy and stereotactic radiosurgery17–22
| Tissue | Effect | Reported thresholds for clinically observable radiation damage | Dose delivered during epimacular brachytherapy | Dose delivered during stereotactic radiosurgery |
|---|---|---|---|---|
| Lens | Cataract | 2 Gy | 0.00056 Gy | 0.12–0.13 Gy |
| Retina | Radiation retinopathy | 35–55 Gy | 24 Gy | 16–24 Gy |
| Optic nerve | Optic neuropathy | >55 Gy | 2.4 Gy | 0.2–0.37 Gy |
Abbreviation: Gy, gray.
Epimacular brachytherapy is performed as part of a vitrectomy procedure and this combination of anti-VEGF therapy, vitrectomy, and radiation may be uniquely suited to the treatment of AMD. It has been proposed that the removal of the vitreous increases the level of oxygen available to the inner layers of the retina via diffusion from the aqueous humor.24–26 A reduced oxygen tension may play a role in the initial CNV formation. In addition, by increasing the oxygenation in the local area at the time of brachytherapy, it may increase the formation of free radicals and therefore the double-stranded DNA breaks required to prevent further CNV formation.27,28
Epimacular brachytherapy studies
Published trials
Two key preliminary studies provided the early data on the safety and efficacy of epimacular brachytherapy. The first (NVI-68) trial was a nonrandomized multicenter feasibility study with 34 treatment-naive subjects enrolled. Subjects received either 15 Gy (8 patients) or 24 Gy (26 patients) of beta radiation. Twelve months after treatment, no radiation-related adverse events had been recorded. However, there was a significant difference in the visual acuity result between the 2 groups, when tested using the ETDRS visual chart. In the 24 Gy group, the mean change in visual acuity was a gain of 10.3 letters (approximately 2 Snellen lines) while the 15 Gy group had a loss of −1.0 letters.16
The second (NV-111) trial was a prospective, nonrandomized, multicenter study that enrolled 34 treatment-naive subjects. Subjects were treated with a single dose of 24 Gray epiretinal brachytherapy and two injections of anti-VEGF therapy, 1 at the time of surgery, and another 1 month later. Thereafter anti-VEGF therapy was administered as needed. Twelve months after treatment, there were no reported cases of radiation retinopathy. ETDRS visual acuity showed a mean improvement of 8.9 letters (approximately 2 Snellen lines), with 91% maintaining vision (<3 lines of vision loss/15 ETDRS letters) and 68% having stable or improved vision.17 Approximately three-quarters of patients required no further anti-VEGF therapy over the first year. By comparison, NICE anticipates patients would require 8 anti-VEGF injections over this interval, if they were receiving ranibizumab monotherapy. These early feasibility trials showed promising short-term results compared to anti-VEGF therapy, and demonstrated the preliminary safety and efficacy of epimacular brachytherapy that prompted the larger ongoing studies.
Ongoing clinical trials
MERITAGE study
MERITAGE is a multicenter investigator-initiated study to evaluate the safety and efficacy of focal delivery of radiation in patients that require persistent injections of anti-VEGF therapy. This study was initiated in the UK and was subsequently expanded to include 5 international sites in the United States and Israel. A total of 53 participants have now completed 12 months of follow-up, and results are expected to be published shortly.
CABERNET study
CABERNET is a commercial, multinational, pivotal randomized controlled trial evaluating the safety and efficacy of epimacular brachytherapy in treatment-naive patients. This study has now completed recruitment, having enrolled 495 subjects across 17 sites. Results will be released when patients reach 24 months follow-up, and are expected in late 2011.
MERLOT study
MERLOT is a noncommercial, investigator-initiated, UK multicenter randomized controlled trial in patients who have already commenced anti-VEGF therapy. It is has been adopted on to the National Institute of Health Research (NIHR) Comprehensive Clinical Research Network (CCRN) portfolio, an organization that provides assistance to studies addressing areas of importance to the National Health Service (NHS). MERLOT is actively recruiting at 16 UK NHS hospitals with further sites to join soon. In total 363 patients will be randomized in a 2:1 ratio, comparing epimacular brachytherapy and as required ranibizumab, to ranibizumab monotherapy.
Whereas most studies of epimacular brachytherapy target patients who have not yet commenced any treatment, MERITAGE and MERLOT both target those who are requiring regular eye injections. The rationale is that there are limited surgical resources and these resources are best directed to those who have not fully responded to ranibizumab therapy, or whose response is short-lived. These patients have the most to gain from a therapy that may reduce their frequency of anti-VEGF retreatment.
Stereotactic radiosurgery
Introduction
More recently investigators have revisited external beam therapy, using a technique called stereotactic radiotherapy or radiosurgery. This treatment directs beams from different angles relative to the target area, thereby minimizing the exposure to surrounding healthy tissue, and at the same time precisely targeting the radiation energy onto the lesion. The IRay system uses a low voltage X-ray source that does not require the same degree of radiation shielding as the early linear accelerators. The system is designed to overcome the traditional disadvantages of external beam therapy by dividing the dose into several separate beams that pass into the eye via different locations on the sclera. An added benefit is the use of the lower energy X-ray source allowing treatment within the clinical environment, without expensive safety precautions.21,29
Details of the procedure
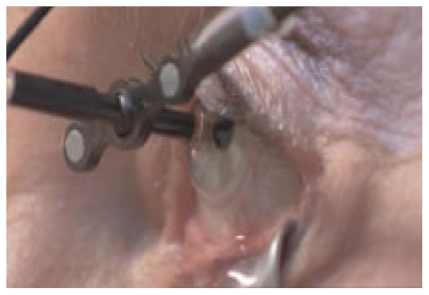
The radiation source uses a robotically controlled delivery system connected to the patient using a contact lens and 25 mmHg suction (Figure 4). The system is designed to fit in a standard clinical environment and runs off a 220- to 240-V wall socket, without the need for room shielding. It delivers two to three separate beams through the inferior pars plana region of the sclera (5-, 6- and 7-o’clock) to overlap on the predicted foveal center, therefore dispersing the scleral entry dose and minimizing exposure of the lens and optic nerve30 (Figure 5). The patient is secured in position with a head restraint that also contains a lead backing to prevent radiation traveling beyond the patient. Exposure to the lower lid is avoided by a lid retractor. The operator is separated from the patient during treatment via a lead-lined glass shield, which allows the operator to monitor the patient (Figure 6). 21,29 The patient’s eye is then secured in position with a vacuum-coupled contact lens interface with suction; the system can detect any eye motion and stabilizes the eye during treatment. The eye is then continually tracked during treatment and an inbuilt safety feature interrupts the radiation treatment if the eye moves out of position.
Figure 4.
The robotically controlled system connected to the patient via a contact lens.
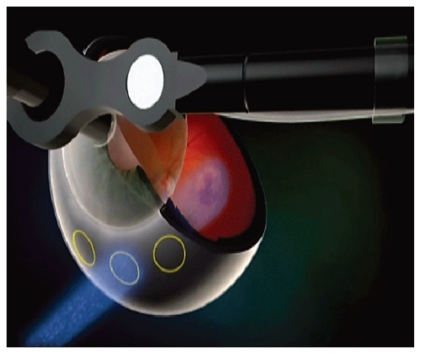
Figure 5.
Illustration of the trajectory of the external beam radiation through the pars plana into the macula, avoiding the lens and optic nerve.
Figure 6.
The IRay system set up within the clinical environment with the operator controls separated by the lead-lined glass screen.
Stereotactic radiosurgery studies
CLH001
CLH001 was a single-center uncontrolled pilot study that included 62 participants with neovascular AMD. The study included patients who had already received anti-VEGF therapy, and others who were treatment naïve. It investigated 2 radiation doses (16 Gy and 24 Gy) and different induction treatment regimens. Induction involved anti-VEGF therapy at baseline and month 1 with the radiotherapy in between, or radiotherapy only at baseline. All the regimens then administered anti-VEGF as required. At 12 months visual acuity had stabilized or improved for the majority of patients, and the mean gain has been 8 to 10 ETDRS letters (approximately 2 Snellen lines) (the 12-month data were presented by Peter Kaiser at Angiogenesis Feb 20, 2010 and again by Darius Moshfeghi at Macula Society Feb 24–27, 2010).
Ongoing clinical trials
CLH002 study
CLH002 is a commercial, multinational, randomized controlled feasibility study evaluating the safety and effectiveness of low voltage stereotactic radiosurgery in patients who have previously been treated with anti-VEGF therapy. CLH002 is actively recruiting at more than 20 hospitals in five countries. In total, 210 patients will be randomized to 16 Gy, 24 Gy, or sham stereotactic radiosurgery, with as required ranibizumab in all groups.
CLH003 study
CLH003 is a multinational pivotal RCT, planned to start in 2011.
Conclusion
The ongoing management of patients with wet AMD represents a considerable challenge to eye departments across the country. The ideal treatment for this sight-threatening condition would maintain or improve a patient’s vision, whilst limiting the number of treatment follow-ups. The introduction of anti-VEGF therapy represents an important advance in treatment. However, patients require frequent hospital review, and most require regular intravitreal injections to maintain the benefit. This course of treatment imposes a considerable burden for affected older adults, and their carers. In addition the injections may elicit patient anxiety and discomfort, and there is a cumulative risk of complications such as endophthalmitis and retinal detachment.
Both epimacular brachytherapy and stereotactic radiosurgery have the potential to significantly improve the quality of life for patients suffering with wet AMD, by reducing their reliance on frequent injections and therefore the need for such regular long-term follow-up.
If the results of the early studies are replicated in large RCTs, then these radiotherapy treatments could offer a useful alternative for those patients whose response to anti-VEGF therapy is incomplete or short-lived. It is however important that these new treatments are thoroughly assessed with long-term follow-up and robust analysis of large clinical trials. In particular, any reduction in demand for anti-VEGF treatment needs to occur in the context of an acceptable visual outcome, and a favorable safety profile.
Footnotes
Disclosure
Mr Timothy Jackson has received research funding from NeoVista Inc, Oraya Therapeutics Inc, and Novartis.
References
- 1.Fletcher A, Donoghue M, Owen C. Low vision services for people with age-related macular degeneration in the UK: a review of services needed and provision 2001. Macular Disease Society Report [Google Scholar]
- 2.NICE: HTA Ranibizumab and pegaptanib for the treatment of AMD. 2006. [Accessed Nov 18, 2010]. Final scope: www.nice.org.uk.
- 3.Ferris FL, III, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verte-porfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 6.Spaide R. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143(4):679–680. doi: 10.1016/j.ajo.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Kirwan JF, Constable PH, Murdoch IE, Khaw PT. Beta irradiation: new uses for an old treatment: a review. Eye. 2003;17(2):207–215. doi: 10.1038/sj.eye.6700306. [DOI] [PubMed] [Google Scholar]
- 8.Barnhard HJ. Supervoltage therapy comes of age. N Engl J Med. 1958;258(6):275–277. doi: 10.1056/NEJM195802062580605. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarthy U, MacKenzie G. External beam radiotherapy in exudative age-related macular degeneration: a pooled analysis of phase I data. Br J Radiol. 2000;73(867):305–313. doi: 10.1259/bjr.73.867.10817048. [DOI] [PubMed] [Google Scholar]
- 10.Jaakkola A, Heikkonen J, Tommila P, Laatikainen L, Immonen I. Strontium plaque brachytherapy for exudative age-related macular degeneration: three-year results of a randomized study. Ophthalmology. 2005;112(4):567–573. doi: 10.1016/j.ophtha.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 11.Evans JR, Sivagnanavel V, Chong V. Radiotherapy for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2010;(5):CD004004. doi: 10.1002/14651858.CD004004.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rokni SH, Fassò A, Liu JC. Operational radiation protection in high-energy physics accelerators. Radiat Prot Dosimetry. 2009;137(1–2):3–17. doi: 10.1093/rpd/ncp194. [DOI] [PubMed] [Google Scholar]
- 13.Woodward BW. Comparison of efficacy of proton beam and 90Sr/90Y beta radiation in treatment of exudative age-related macular degeneration. NeoVista Inc.; 47865 Fremont Blvd., Fremont, CA, 94538: 2008. [Google Scholar]
- 14.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senan S, Smit EF. Design of clinical trials of radiation combined with antiangiogenic therapy. Oncologist. 2007;12(4):465–477. doi: 10.1634/theoncologist.12-4-465. [DOI] [PubMed] [Google Scholar]
- 16.Avila MP, Farah ME, Santos A, et al. Twelve-month safety and visual acuity results from a feasibility study of intraocular, epiretinal radiation therapy for the treatment of subfoveal CNV secondary to AMD. Retina. 2009;29(2):157–169. doi: 10.1097/IAE.0b013e3181985915. [DOI] [PubMed] [Google Scholar]
- 17.Ávila MP, Farah ME, Santos A, Duprat JP, Woodward BW, Nau J. Twelve-month short-term safety and visual-acuity results from a multi-centre prospective study of epiretinal strontium-90 brachytherapy with bevacizumab for the treatment of subfoveal choroidal neovascularisation secondary to age-related macular degeneration. Br J Ophthalmol. 2009;93(3):305–309. doi: 10.1136/bjo.2008.145912. [DOI] [PubMed] [Google Scholar]
- 18.Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation retinopathy after external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30(4):765–773. doi: 10.1016/0360-3016(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 19.Boozalis GT, Schachat AP, Green WR. Subretinal neovascularization from the retina in radiation retinopathy. Retina. 1987;7(3):156–161. doi: 10.1097/00006982-198700730-00004. [DOI] [PubMed] [Google Scholar]
- 20.Gordon KB, Char DH, Sagerman RH. Late effects of radiation on the eye and ocular adnexa. Int J Radiat Oncol Biol Phys. 1995;31(5):1123–1139. doi: 10.1016/0360-3016(95)00062-4. [DOI] [PubMed] [Google Scholar]
- 21.Hanlon J, Lee C, Chell E, et al. Kilovoltage stereotactic radiosurgery for age-related macular degeneration: assessment of optic nerve dose and patient effective dose. Med Phys. 2009;36(8):3671–3681. doi: 10.1118/1.3168554. [DOI] [PubMed] [Google Scholar]
- 22.Finger PT, Berson A, Ng T, Szechter A. Ophthalmic plaque radiotherapy for age-related macular degeneration associated with subretinal neovascularization. Am J Ophthalmol. 1999;127(2):170–177. doi: 10.1016/s0002-9394(98)00389-4. [DOI] [PubMed] [Google Scholar]
- 23.Timke C, Zieher H, Roth A, et al. Combination of vascular endothelial growth factor receptor/platelet-derived growth factor receptor inhibition markedly improves radiation tumor therapy. Clin Cancer Res. 2008;14(7):2210–2219. doi: 10.1158/1078-0432.CCR-07-1893. [DOI] [PubMed] [Google Scholar]
- 24.Stefansson E, Landers MB, 3rd, Wolbarsht ML. Increased retinal oxygen supply following pan-retinal photocoagulation and vitrectomy and lensectomy. Trans Am Ophthalmol Soc. 1981;79:307–334. [PMC free article] [PubMed] [Google Scholar]
- 25.Jampol LM. Oxygen therapy and intraocular oxygenation. Trans Am Ophthalmol Soc. 1987;85:407–437. [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto E, Hirakata A, Hotta K, Shinoda K, Miki D, Hida T. Unusual macular retinal detachment associated with vitreomacular traction syndrome. Br J Ophthalmol. 1998;82(3):326. doi: 10.1136/bjo.82.3.326a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41(1):31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 28.Brizel DM, Scully SP, Harrelson JM, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56(5):941–943. [PubMed] [Google Scholar]
- 29.Moshfeghi DM, Kaiser PK, Gertner M. Stereotactic low-voltage x-ray irradiation for age-related macular degeneration. Br J Ophthalmol. 2010 Sep 18; doi: 10.1136/bjo.2009.163907. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Lee C, Chell E, Gertner M, et al. Dosimetry characterization of a multibeam radiotherapy treatment for age-related macular degeneration. Med Phys. 2008;35(11):5151–5160. doi: 10.1118/1.2990780. [DOI] [PubMed] [Google Scholar]