Abstract
Among women with a mutation in BRCA1 or BRCA2, the risk of breast cancer is high, but it may be modified by exogenous and endogenous factors. There is concern that exposure to carcinogens in cigarette smoke may increase the risk of cancer in mutation carriers. We conducted a matched case–control study of 2,538 cases of breast cancer among women with a BRCA1 (n = 1,920) or a BRCA2 (n = 618) mutation. One non-affected mutation carrier control was selected for each case, matched on mutation, country of birth, and year of birth. Odds ratios were calculated using conditional logistic regression, adjusted for oral contraceptive use and parity. Ever-smoking was not associated with an increased breast cancer risk among BRCA1 carriers (OR = 1.09; 95% CI 0.95–1.24) or among BRCA2 carriers (OR = 0.81; 95% CI 0.63–1.05). The result did not differ when cases were restricted to women who completed the questionnaire within two years of diagnosis. A modest, but significant increase in risk was seen among BRCA1 carriers with a past history of smoking (OR = 1.27; 95% CI 1.06–1.50), but not among current smokers (OR = 0.95; 0.81–1.12). There appears to be no increase in the risk of breast cancer associated with current smoking in BRCA1 or BRCA2 carriers. There is a possibility of an increased risk of breast cancer among BRCA1 carriers associated with past smoking. There may be different effects of carcinogens in BRCA mutation carriers, depending upon the timing of exposure.
Keywords: BRCA1, BRCA2, Smoking
Introduction
Currently, it is estimated that the lifetime risk of breast cancer in female carriers of a BRCA1 or BRCA2 mutation is between 50% and 80% [1–3]. Not all mutation carriers develop breast cancer and there is interest in identifying non-genetic and genetic factors, which modify risk. Knowledge of these factors-may help us to predict, and ultimately to prevent cancer, in this high-risk population. It has been proposed that cigarette smoking may modify breast cancer risk in mutation carriers, but reports to date have been conflicting.BRCA1 and BRCA2 play important roles in DNA repair, and it is plausible that carcinogens of the type that are contained in cigarette smoke may increase the risk of breast cancer in BRCA mutation carriers through DNA breakage. It is also possible that the anti-estrogenic effects of smoking may be protective.
In 2004, we reported that there was no association between cigarette smoking and breast cancer [4]. In this case–control study of 1,097 breast cancer cases and matched controls, we did not distinguish between incident and prevalent cases. It has recently been suggested that the use of prevalent cases may mask a hazardous effect if women who smoke are more likely to die after a diagnosis of breast cancer than are women with breast cancer, but who do not smoke. Since 2004, we have expanded our database of mutation carriers and we are now able to re-examine the association between cigarette smoking and breast cancer risk.
Methods
Study population
Eligible subjects are women who carry a pathogenic mutation in either the BRCA1 or BRCA2 gene. Information on study subjects was submitted from 52 participating centers in 11 countries. These women participated in clinical and research protocols at the host institutions. All study subjects (with the exception of those at the University of Utah) received genetic counseling and provided written consent for genetic testing. In most cases, testing was offered initially to women who were affected with breast or ovarian cancer. When a BRCA1 or BRCA2 mutation was detected in an affected individual (proband), genetic testing was then offered to other unaffected and affected women in the family. However, in a few families (<10%) only unaffected carriers were identified. Genetic testing for BRCA mutations was performed using a range of techniques, and all abnormal nucleotide sequences were confirmed by direct sequencing of DNA. A subject was eligible for the current study if the molecular analysis confirmed the presence of a pathogenic BRCA mutation. The great majority of the mutations were either nonsense or missense mutations, deletions, insertions, or small frame-shifts and resulted in a truncated protein.
Information was submitted to the data center on a total of 8,208 mutation carriers. 931 women were excluded because information was missing regarding their smoking history and 84 women were excluded because other key information was missing (mutation status, cancer history or date of birth) and 68 women diagnosed with ovarian cancer prior to breast cancer were excluded. After these exclusions, a total of 7,095 subjects was eligible for the analysis, including 3271 women with breast cancer (potential cases) and 3,824 women without breast cancer (potential controls).
An attempt was made to identify a single matched control for each of the 3,271 eligible breast cancer cases. Controls were matched to a case according to the mutated gene (BRCA1 or BRCA2), year of birth (within one year), place of residence (USA, Quebec, other Canada, Poland, other Europe, or Israel) and date of questionnaire completion. In order to be matched to a given case, the control had to be at least as old as the case at the time of diagnosis of breast cancer and a control was not eligible if she had a bilateral prophylactic mastectomy prior to the age of diagnosis of breast cancer of the matched case.
A total of 2,538 matched case–control pairs was generated for the analysis (representing 78% of the eligible cases), including 1,920 BRCA1 pairs and 618 BRCA2 pairs. For five cases (0.2%) the matched control was related to the case, and for 2,533 pairs, the case and control were unrelated. Many of the study subjects who were in our previous report are also included in the present report, but the previous matching was not retained.
All study subjects were asked to complete a self-administered questionnaire which asked about medical and reproductive histories and selected lifestyle factors, including smoking. In most cases, the questionnaire was completed when blood was drawn for genetic testing, or within a year of receiving the test result. In some centers, the questionnaire was compiled by a face-to-face interview. Smoking-related questions included: ever smoking, if current smoker, age at commencement of smoking, age at smoking cessation and the average smoking consumption during the smoking period. The number of pack-years was determined by the average number of packs per day over the smoking period, multiplied by the number of years smoked. Only smoking exposures that occurred prior to the diagnosis of breast cancer were considered, for both the cases and matched controls. “Ever-smokers” were divided into past and current smokers. A current smoker was defined as one who reported smoking in the same calendar year as the year of diagnosis of breast cancer (others were past smokers). The questionnaires were completed after the diagnosis of breast cancer. On average, 7.7 years had elapsed between the diagnosis of breast cancer and the completion of the questionnaire (range 0–45 years). 764 of the breast cancer cases (33.1%) completed the questionnaire within two years of the diagnosis of breast cancer (incident cases).
Statistical analysis
Smoking histories were compared between case subjects and control subjects. The odds ratios for breast cancer associated with smoking were estimated by use of conditional logistic regression for matched sets. Odds ratios were estimated by univariate analysis and by multivariate analysis, adjusting for parity and oral contraceptive use (ever/never). Analyses were conducted using SAS (SAS Institute Inc. NY, USA).
Results
A total of 2,538 matched pairs was included in the analysis (Table 1). The mean age of the cases at the time of completion of the questionnaire was 48.5 years (range 18–82 years), and the mean age at diagnosis of breast cancer was 40.8 (range 19–75 years). Cases were matched for age, place of residence and year of birth. The majority of the cases and controls resided in North America. Cases and controls were similar in terms of parity, age of first birth, and oral contraceptive use. The mean age of menarche was slightly younger for cases (12.9 years) than for controls (13.0 years) (P = 0.001).
Table 1.
Characteristic of Study Subjects
| Value | Cases N (%) |
Controls N (%) |
P-value |
|---|---|---|---|
| Mean age | 48.5 | 48.7 | 0.46 |
| Year of birth (mean) | 1953 | 1953 | |
| Place of residence | |||
| Poland | 565 (22.3) | 565 (22.3) | |
| Other Europe | 281 (11.1) | 281 (11.1) | |
| Israel | 101 (4.0) | 101 (4.0) | |
| Quebec | 163 (6.4) | 163 (6.4) | |
| Canada (other than Quebec) | 511 (20.1) | 511 (20.1) | |
| USA | 917 (36.1) | 917 (36.1) | |
| BRCA mutation | |||
| BRCA1 | 1,920 (75.7) | 1,920 (75.7) | |
| BRCA2 | 618 (24.4) | 618 (24.4) | |
| Mean age at menarche | 12.9 | 13.0 | 0.001 |
| Mean number of children | 1.9 | 2.0 | 0.14 |
| Mean age at 1st birth | 24.5 | 24.3 | 0.14 |
| Oral contraceptive (ever) | 1,562 (61.5) | 1566 (61.7) | 0.91 |
For BRCA1 and BRCA2 carriers combined, there was no significant difference between the proportions of cases and controls who reported ever having smoked regularly (39.2 vs. 38.6%, respectively) (Table 2). Among smokers, the mean number of pack-years was 10.8 for cases, and 11.1 for controls (P = 0.5). No difference was seen between cases and controls in either the age at which smoking commenced, the age at cessation of smoking, or total years of smoking.
Table 2.
Association of smoking and breast cancer in carriers of BRCA gene mutations with history of smoking
| Smoking (cigarette) |
Cases | Controls | OR (95% CI) | P-value |
|---|---|---|---|---|
| Never | 1,543 | 1,558 | 1.0 | |
| Ever | 995 | 980 | 1.02 (0.61–1.15) | 0.73 |
| Mean age started | 18.8 | 19.0 | 0.33 | |
| Mean age last smoked | 35.1 | 35.1 | 0.96 | |
| Mean year smoked | 16.3 | 16.1 | 0.55 | |
| Mean pack years (smokers) | 10.8 | 11.1 | 0.51 |
Pcyear = pack years (age stopped − age started) × package per week/7
Table 3a and b shows the odds ratios for breast cancer by current and past smoking histories for BRCA1 (Table 3a) and BRCA2 (Table 3b) mutation carriers. There were no significant associations between breast cancer and current or ever-smoking. However, among BRCA1 carriers who smoked in the past, but no longer smoked, a positive association with breast cancer was observed, in both the unadjusted and adjusted analyses. Furthermore, among past smokers, there was a significant trend between risk and total lifetime cigarette consumption. Among BRCA2 carriers, no significant effect was observed with past smoking and current smoking was marginally protective (OR = 0.71; 95% CI 0.5–1.0). To address the question of whether or not early exposure to cigarette smoke is hazardous, we subdivided the cases by age at initiation of smoking (Table 4). There did not appear to be an increase in breast cancer risk among women who started smoking before age 18, compared to those who never smoked.
Table 3.
Odds ratios for breast cancer associated with smoking in (a) BRCA1 carriers and (b) BRCA2 carriers
| Smoking related factors 1920 pairs |
Univariate analysis OR (95% CI) P |
Multivariate analysis* OR (95% CI) P |
|---|---|---|
| (a) BRCA1 carriers | ||
| Never | 1 | 1 |
| Ever | 1.10 (0.96–1.25) 0.17 | 1.09 (0.95–1.24) 0.22 |
| Never | 1 | 1 |
| 0 < pcyear ≤ 5 | 1.04 (0.85–1.29) 0.69 | 1.03 (0.83–1.28) 0.77 |
| 3 > pcyear ≤ 10 | 1.12 (0.86–1.47) 0.40 | 1.12 (0.85–1.46) 0.42 |
| 10 pcyear | 1.11 (0.95–1.30) 0.19 | 1.10 (0.94–1.29) 0.22 |
| Ptrend = 0.15 | Ptrend = 0.18 | |
| Never | 1 | 1 |
| Ever past | 1.28 (1.08–1.52) 0.005 | 1.27 (1.06–1.50) 0.008 |
| Ever current | 0.96 (0.81–1.12) 0.58 | 0.95 (0.81–1.12) 0.54 |
| Never | 1 | 1 |
| Ever past, 0 < pcyear ≤ 5 | 1.20 (0.94–1.54) 0.14 | 1.19 (0.93–1.52) 0.17 |
| Ever past, 5 < pcyear ≤ 10 | 1.28 (0.88–1.87) 0.20 | 1.27 (0.87–1.86) 0.22 |
| Ever past, 10 < pcyear | 1.36 (1.06–1.74) 0.01 | 1.34 (1.05–1.72) 0.02 |
| Ptrend = 0.005 | Ptrend = 0.007 | |
| Smoking related factors 618 pairs |
Univariate analysis OR (95% CI) P |
Multivariate analysis* OR (95% CI) P |
| (b) BRCA2 carriers | ||
| Never | 1 | 1 |
| Ever | 0.81 (0.63–1.04) 0.10 | 0.81 (0.63–1.05) 0.11 |
| Never | 1 | 1 |
| 0 < pcyear ≤ 5 | 0.81 (0.55–1.18) 0.26 | 0.80 (0.55–1.18) 0.26 |
| 3 > pcyear ≤ 10 | 0.98 (0.60–1.58) 0.92 | 0.98 (0.60–1.58) 0.92 |
| 10 pcyear | 0.77 (0.57–1.04) 0.09 | 0.77 (0.57–1.05) 0.10 |
| Ptrend = 0.11 | Ptrend = 0.12 | |
| Never | 1 | 1 |
| Ever past | 0.89 (0.66–1.20) 0.44 | 0.89 (0.66–1.20) 0.45 |
| Ever current | 0.71 (0.50–1.00) 0.05 | 0.71 (0.50–1.00) 0.05 |
| Never | 1 | 1 |
| Ever past, 0 < pcyear ≤ 5 | 0.80 (0.54–1.19) 0.26 | 0.80 (0.54–1.19) 0.27 |
| Ever past, 5 < pcyear ≤ 10 | 1.03 (0.58–1.85) 0.91 | 1.03 (0.58–1.85) 0.91 |
| Ever past, 10 < pcyear | 0.95 (0.62–1.46) 0.82 | 0.96 (0.63–1.47) 0.85 |
| Ptrend = 0.74 | Ptrend = 0.77 | |
The multivariate odds ratios are adjusted for oral contraceptive use (yes/no) and parity (0, 1, 2, 3, 4, … 9)
Table 4.
Association between smoking and breast cancer by age of initiation of smoking
| Age of initiation | Univariate analysis OR (95% CI) P |
Multivariate analysis* OR (95% CI) P |
|---|---|---|
| BRCA1 | ||
| Never | 1.0 | 1.0 |
| ≤18 | 1.12 (0.95–1.32) 0.17 | 1.11 (0.89–1.18) 0.75 |
| >18 | 1.07 (0.89–1.27) 0.48 | 1.05 (0.87–1.11) 0.83 |
| BRCA2 | ||
| Never | 1.0 | 1.0 |
| ≤18 | 0.78 (0.58–1.04) 0.09 | 0.78 (0.59–1.04) 0.10 |
| >18 | 0.87 (0.61–1.23) 0.43 | 0.87 (0.61–1.24) 0.44 |
The multivariate odds ratios are adjusted for oral contraceptive use (yes/no) and parity (0, 1, 2, 3, 4, … 9)
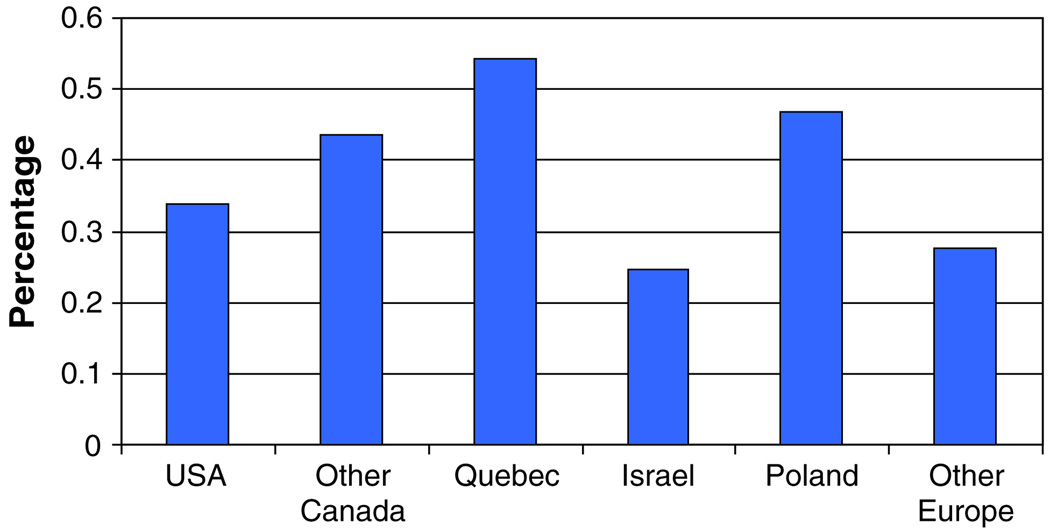
We also examined the association between smoking and breast cancer by age at diagnosis (Table 5) and by country/province of residence (Table 6). No significant differences were seen in smoking exposures among breast cancer cases in any of the three age categories. Smoking habits varied from country to country (Fig. 1). No significant effects were seen in any of the countries studied, with the exception of Quebec. Among Quebec women with BRCA2 mutations, smoking was found to be protective (OR = 0.33); however this sample size was small (n = 70 pairs). Smoking was not found to be protective for breast cancer elsewhere in Canada or in the other countries.
Table 5.
Association between ever-smoking and breast cancer by age of diagnosis (BRCA1 and BRCA2 combined)
| Age of diagnosis | Univariate analysis OR (95% CI) P |
Multivariate analysis* OR (95% CI) P |
|---|---|---|
| ≤40 (1,324 pairs) | 1.00 (0.85–1.18) 1.00 | 1.00 (0.85–1.18) 1.00 |
| 41–50 (914 pairs) | 1.01 (0.84–1.21) 0.96 | 1.00 (0.83–1.21) 0.98 |
| Case dxed >50 (300 pairs) | 1.22 (0.88–1.71) 0.24 | 1.19 (0.85–1.66) 0.32 |
The multivariate odds ratios are adjusted for oral contraceptive use (yes/no) and parity (0, 1, 2, 3, 4, … 9)
Table 6.
Association between smoking and breast cancer by residence
| By country (region) | Univariate analysis OR (95% CI) P |
Multivariate analysis* OR (95% CI) P |
|---|---|---|
| USA (all) (917 pairs) | 1.12 (0.92–1.37) 0.25 | 1.11 (0.91–1.36) 0.30 |
| BRCA1 (633 pairs) | 1.28 (1.02–1.61) 0.04 | 1.25 (0.98–1.58) 0.07 |
| BRCA2 (284 pairs) | 0.78 (0.54–1.15) 0.21 | 0.79 (0.54–1.16) 0.23 |
| Other Canada (all) (511 pairs) | 1.02 (0.80–1.31) 0.85 | 1.03 (0.80–1.32) 0.83 |
| BRCA1 (313 pairs) | 1.06 (0.78–1.450 0.70 | 1.06 (0.78–1.45) 0.70 |
| BRCA2 (198 pairs) | 0.96 (0.63–1.45) 0.83 | 0.99 (0.65–1.50) 0.96 |
| Quebec (all) (101 pairs) | 0.55 (0.34–0.89) 0.02 | 0.51 (0.31–0.83) 0.008 |
| BRCA1 (93 pairs) | 0.69 (0.38–1.26) 0.23 | 0.67 (0.35–1.27) 0.21 |
| BRCA2 (70 pairs) | 0.38 (0.17–0.86) 0.02 | 0.33 (0.14–0.78) 0.01 |
| Israel (all) (101 pairs) | 0.58 (0.28–1.22) 0.15 | 0.65 (0.30–1.40) 0.28 |
| BRCA1 (77 pairs) | 0.50 (0.21–1.17) 0.11 | 0.57 (0.24–1.37) 0.21 |
| BRCA2 (24 pairs) | 1.00 (0.20–4.96) 1.00 | 1.47 (0.25–8.76) 0.68 |
| Poland (all) (565 pairs) | 1.01 (0.80–1.27) 0.95 | 1.02 (0.81–1.28) 0.89 |
| BRCA1 (564 pairs) | 1.01 (0.80–1.27) 0.95 | 1.02 (0.81–11.28) 0.89 |
| BRCA2 (1 pair) | NA | NA |
| Other Europe (all) (281 pairs) | 1.40 (0.94–2.06) 0.10 | 1.38 (0.93–2.04) 0.11 |
| BRCA1 (240 pairs) | 1.42 (0.93–2.17) 0.11 | 1.41 (0.92–2.16) 0.12 |
| BRCA2 (41 pairs) | 1.29 (0.48–3.45) 0.62 | 1.28 (0.45–3.66) 0.64 |
The multivariate odds ratios are adjusted for oral contraceptive use (yes/no) and parity (0, 1, 2, 3, 4, … 9)
Fig. 1.
Prevalence of smoking by country
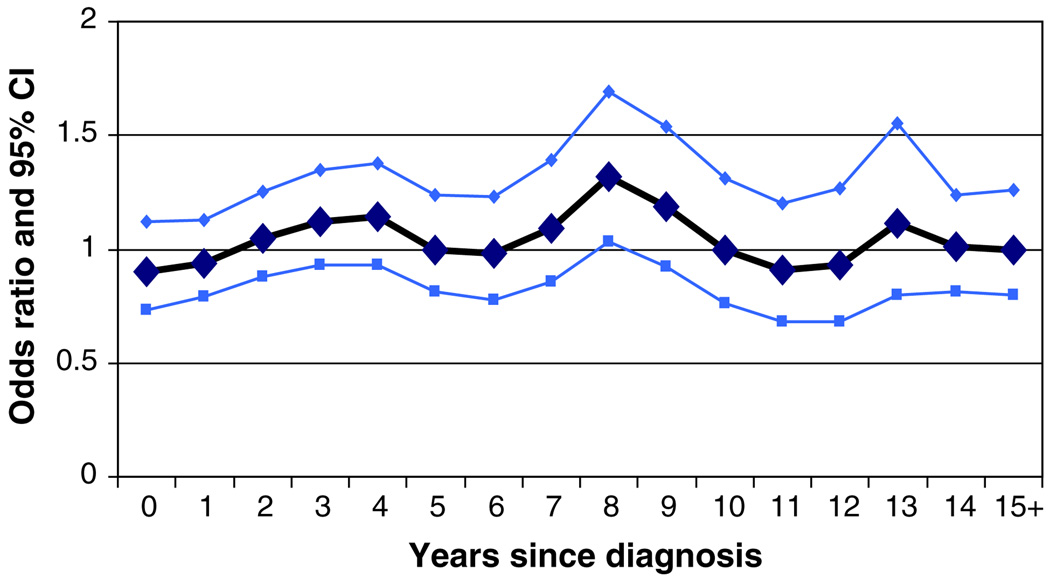
It is possible that breast cancer cases who smoke (or who had smoked in the past) may experience decreased survival following the diagnosis of breast cancer. If this were the case, we would expect to see fewer smokers than expected among long-term survivors of breast cancer. This would result in a spurious protective effect associated with smoking (survivor bias), and the size of the effect would increase with time since diagnosis. This bias could potentially mask a hazardous effect of smoking. We examined the association among incident cases of breast cancer, defined as those who were within 2 years of their diagnosis at the time of questionnaire completion (Table 7). Among the 764 matched pairs who were interviewed within two years of diagnosis of breast cancer, there was no association between smoking and breast cancer risk (OR = 0.90; 95% CI 0.72–1.11, P = 0.33). The odds ratio for breast cancer among ever-smokers was relatively constant according to time since diagnosis (Fig. 2).
Table 7.
Association between smoking and breast cancer by time elapsed from diagnosis to interview (BRCA1 and BRCA2 combined)
| Time elapsed from diagnosis to interview |
Controls/Cases | Univariate analysis OR (95% CI) P |
Multivariate analysis* OR (95% CI) P |
|---|---|---|---|
| 0–2 years (764 pairs) | |||
| Never | 475/494 | 1 | 1 |
| Ever | 289/270 | 0.89 (0.72–1.11) 0.30 | 0.90 (0.72–1.11) 0.33 |
| 3–5 years (556 pairs) | |||
| Never | 215/198 | 1 | 1 |
| Ever | 137/154 | 1.23 (0.96–1.57) 0.10 | 1.23 (0.96–1.57) 0.10 |
| 6–10 years (542 pairs) | |||
| Never | 325/310 | 1 | 1 |
| Ever | 217/232 | 1.13 (0.88–1.44) 0.35 | 1.12 (0.88–1.44) 0.36 |
| >10 years (676) pairs | |||
| Never | 419/427 | 1 | 1 |
| Ever | 257/249 | 0.95 (0.75–1.19) 0.64 | 0.94 (0.75–1.18) 0.58 |
The multivariate odds ratios are adjusted for oral contraceptive use (yes/no) and parity (0, 1, 2, 3, 4, … 9)
Fig. 2.
Association of smoking and breast cancer by years of interview after the dx of cases
Discussion
In this report, we present an updated analysis of smoking exposures and breast cancer risk among our set of BRCA1 and BRCA2 carriers. In our first publication [5] we reported an apparent protective effect of smoking on breast cancer risk among carriers (OR = 0.46 for four or more pack-years versus nonsmoker). However, this small study included only 186 matched pairs. In our 2004 update (1,097 matched pairs) we reported a null association between smoking and breast cancer risk [4]. In that study we did not separate incident and prevalent cases and we did not consider past and current smoking separately.
Our current report includes 2,538 matched pairs. The results of this much larger study do not differ substantially from those of our 2004 report. However, in the present study, we found a significant positive association for BRCA1 carriers who had at least six pack-years of smoking, but who no longer smoked. There was also a marginally significant protective effect of smoking among French-Canadian women with BRCA2 mutations, but not among BRCA2 carriers from other countries. This may be a spurious finding, as the number of mutation carriers from this province was small. However, French-Canadian women had the highest prevalence of smoking of any of the regions studied. Also, multiple comparisons were made in the study.
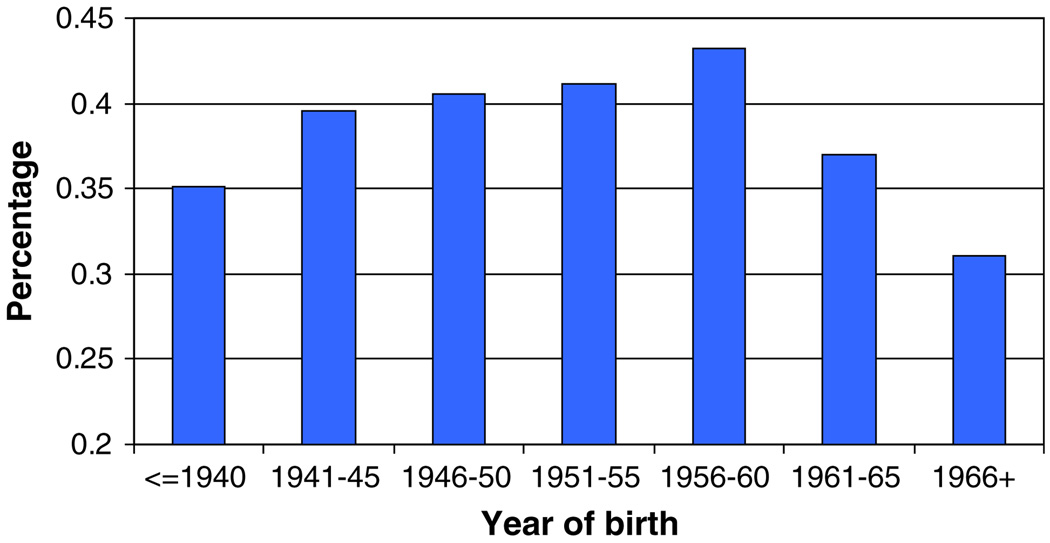
Boyd and his colleagues [6] reported that smoking was associated with breast cancer risk in a historical cohort study of 804 mutation carriers (323 affected and 481 unaffected). They reported an odds ratio of 2.3 (95% CI 1.6–3.5) for BRCA1 carriers with more than five pack-years of exposure and an odds ratio of 2.6 for BRCA2 carriers (95% CI 1.8–3.9). They estimated that the risk of breast cancer increased by 7% for each pack-year. When they restricted cases to subjects who completed the questionnaire within three years of the diagnosis of breast cancer, the results were similar. There are several possible reasons for the different findings in our report and that of Boyd et al. Our updated analysis included 764 matched pairs who completed their questionnaires less than two years after the diagnosis of breast cancer. The lack of a difference in the effect size in the incident and prevalent cases discounts the possibility that our results are influenced by prevalence or survivor bias (Neyman bias). We observed strong correlations between ever/never smoking country of residence (Fig. 1) and birth cohort (Fig. 3). The proportion of smokers ranged from 31% among carriers born after 1965 to 43% for women born between 1956 and 1960. Small differences in the year of birth may influence the prevalence of ever-smoking in the two groups. Because of the associations between smoking and year of birth and country of origin, we matched cases and controls on these variables. In the Boyd study, 26% of the controls and 4% of the cases were born after 1970. Given the decreased prevalence in smoking in young women, it is likely that their cases, which are derived from earlier birth cohorts, contain a higher proportion of smokers than their controls. Similarly, in that study a higher proportion of cases than controls came from Canada, and smoking appears to be more prevalent in Canada than elsewhere in North America and Europe (Fig. 1). In contrast, there were fewer cases than controls from Utah, where the prevalence of smoking is low. It is possible that the reported positive association in their study is the result of residual confounding.
Fig. 3.
Percentage of smoking by year of birth
In addition to our two previous reports, there have been other case–control studies of smoking and breast cancer risk in BRCA mutation carriers. Two of these studies reported a null association [7, 8] and one study reported a negative (protective) association [9]; however, many of the cases and controls in these earlier reports are also included in the present series.
In addition to the studies of BRCA mutation carriers, several studies have examined the effect of smoking on breast cancer risk in women with a family history of breast cancer, but who were not tested for BRCA mutations. Couch et al. [10] examined smoking and breast cancer risk in a historical cohort study of 426 high-risk breast cancer families, ascertained through a consecutive series of probands, between 1944 and 1952. Among 132 families at high risk (at least three breast or ovarian cancers) women who had ever smoked were at 2.4-fold increased risk, compared with never-smokers (RR = 2.4; 95% CI 1.2–5.1). When the analysis was restricted to the 35 families at highest risk, an even stronger association was seen (RR = 5.8 for daughters and mothers of probands).
In a cohort study of 116,544 members of the California Teachers Study [11], the incidence of breast cancer was higher among current smokers than among never smokers (HR 1.3, 95% CI 1.1–1.6). Significant trends were with a duration and intensity of smoking. The risk was accentuated for women who started smoking at a young age; however, the association was not seen among women with a family history of breast cancer.
There are many published reports on the effects of smoking on breast cancer risk in the general population [12–22]. In 2002, Terry and Rohan [23] reviewed the published studies and concluded that smoking probably did not increase the risk of breast cancer, although there may be an increased risk for women with a long duration of exposure and in those who smoked prior to a first full-term pregnancy. A re-analysis of 53 epidemiological studies by The Collaborative Group on Hormonal Factors in Breast Cancer [20] reported that smoking had no independent effect on the risk of developing breast cancer. In contrast, a 2005 meta-analysis concluded that both active and passive smoking were associated with an increased risk of premenopausal breast cancer [22]. Three recent cohort studies, including The Nurses Health study [24], The California Teachers Study [11], and a study from Norway [25] showed positive associations between smoking and breast cancer risk. At least two reports suggest smoking may preferentially increase the risk of estrogen-receptor positive breast cancer [24, 26, 27].
Several biological mechanisms have been postulated which may explain either an increased or a decreased risk of breast cancer for smokers in the general population. Animal and human studies support an increased risk from the carcinogenic effects of metabolites in cigarette smoke, such as N-nitrosamines, aromatic amines, and polycyclic hydrocarbons [28–32]. Some of these fat-soluble compounds have been found to induce mammary tumours in rodents [33], and DNA adducts of some chemicals in cigarette smoke have been found in the breast tissue of smokers as well as in the breast tumours of smokers with breast cancer [34]. In contrast, smoking is associated with lower levels of luteal phase urinary estrogens, lower body fat, an earlier menopause, a lower risk of endometrial cancer and a higher risk of osteoporosis, all of which suggest biologically significant effects on estrogen metabolism and a decreased risk of breast cancer [35–39].
The idea of a null association or protective effect of smoking on breast cancer risk in mutation carriers is unexpected, given the known mutagenic/carcinogenic effects of smoking and the importance of BRCA1 and BRCA2 in DNA repair. However, both these genes have additional functions, and BRCA1 is also important in the regulation of estrogen receptor [40]. Our data suggest that the relationship between smoking and breast cancer risk may be complex, i.e. that the effect of smoking may depend on the timing of the exposure. It is possible that carcinogens in cigarette smoke induce DNA mutations that later manifest as cancer, but that current smoking has an anti-estrogenic effect that inhibits to growth of existing tumours or pre-neoplastic lesions. The net effect of smoking on breast cancer risk in BRCA1 and BRCA2 carriers may therefore result from a balance between the dose, duration and time of exposure of mutagenic carcinogens and the effect on circulating levels of estrogen. Similarly, there is emerging evidence that early estrogen exposure may be hazardous, but that estrogen in the postmenopausal period may reduce the risk of breast cancer in BRCA1 carriers (unpublished data). It is hoped that future studies may elucidate the effects and the underlying mechanisms.
Acknowledgement
SNL was supported by grant R01 CA744175.
Abbreviations
- BRCA1
Breast cancer susceptibility gene 1
- BRCA2
Breast cancer susceptibility gene 2
- OR
Odds ratio
- CI
Confidence interval
- DNA
Deoxyribonucleic acid
- RR
Relative risk
- HR
Hazard ratio
Footnotes
Other members of the Hereditary Breast Cancer Clinical Study Group: Olufunmilayo Olopade, Shelly Cummings, Fergus Couch, Barry Rosen, Dominique Stoppa-Lyonnet, Ruth Gershoni-Baruch, David Horsman, Teresa Wagner, Howard Saal, Ellen Warner, Wendy Meschino, Kenneth Offit, Amber Trivedi, Michael Osborne, Dawna Gilchrist, Charis Eng, Jeffrey Weitzel, Wendy McKinnon, Marie Wood, Christine Maugard, Barbari Pasini, Peter Ainsworth, Kevin Sweet, Boris Pasche, Beth Karlan, Raluca N. Kurz, Anna Tulman, Ed Lemire, Jane Mclennan, Gareth Evans, Tomas Byrski, Tomas Huzarski, Jacek Gronwald, Bohdan Gorski, Eitan Friedman, Andrea Eisen, Mary Daly, Judy Garber, Sofia Merajver.
Contributor Information
Ophira Ginsburg, The Campbell Family Institute for Breast Cancer Research at Princess Margaret Hospital, Toronto, ON, Canada.
Parviz Ghadirian, Epidemiology Research Unit, Centre Hospitalier de l’Universite de Montreal (CHUM) Hotel-Dieu, Faculty of Medicine, Universite de Montreal, Montreal, QC, Canada.
Jan Lubinski, Pomeranian Medical University, Szczecin, Poland.
Cezary Cybulski, Pomeranian Medical University, Szczecin, Poland.
Henry Lynch, Department of Preventive Medicine and Public Health, Creighton University School of Medicine, Omaha, NE, USA.
Susan Neuhausen, Department of Epidemiology, University of California, Irvine, CA, USA.
Charmaine Kim-Sing, British Columbia Cancer Agency, Vancouver, BC, Canada.
Mark Robson, Clinical Genetics, Department of Medicine, Memorial-Sloan Kettering, New York, NY, USA.
Susan Domchek, Departments of Medicine and Genetics, University of Pennsylvania, Philadelphia, PA, USA.
Claudine Isaacs, Lombardi Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Jan Klijn, Department of Medical Oncology, (Dr. Daniel den Hoed Kliniek) Rotterdam Cancer Institute, University Hospital Rotterdam, Rotterdam, The Netherlands.
Susan Armel, Department of Obstetrics and Gynecology, University Health Network, Toronto, ON, Canada.
William D. Foulkes, Program in Cancer Genetics, Departments of Oncology and Human Genetics, McGill University, Montreal, QC, Canada
Nadine Tung, Beth Israel Deaconess Hospital, Boston, MA, USA.
Pal Moller, Department for Cancer Genetics, The Norwegian Radium Hospital, Oslo, Norway.
Ping Sun, Womens College Research Institute, Women’s College Hospital, University of Toronto, 790 Bay Street, 7th Floor, Toronto, ON, Canada M5G 1N8.
Steven A. Narod, Email: steven.narod@wchospital.ca, Womens College Research Institute, Women’s College Hospital, University of Toronto, 790 Bay Street, 7th Floor, Toronto, ON, Canada M5G 1N8.
Hereditary Breast Cancer Clinical Study Group, Womens College Research Institute, Toronto, ON, Canada.
References
- 1.Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1- mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:256–271. [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24:863–871. doi: 10.1200/JCO.2005.03.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghadirian P, Lubinski J, Lynch H, et al. Smoking and the risk of breast cancer among carriers of BRCA mutations. Int J Cance. 2004;110:413–416. doi: 10.1002/ijc.20106. [DOI] [PubMed] [Google Scholar]
- 5.Brunet JS, Ghadirian P, Rebbeck TR, et al. Effect of smoking on breast cancer in carriers of mutant BRCAI or BRCA2 genes. JNCI. 1998;90:761–766. doi: 10.1093/jnci/90.10.761. [DOI] [PubMed] [Google Scholar]
- 6.Breast Cancer Family Registry; Kathleen Cuningham Consortium for Research into Familial Breast Cancer (Australasia); Ontario Cancer Genetics Network (Canada) (2007) Smoking and risk of breast cancer in carriers of mutations in BRCA1 or BRCA2 aged less than 50 years. Breast Cancer Res Treat. doi: 10.1007/s10549-007-9621-9. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronwald J, Byrski T, Huzarski T, et al. Influence of selected lifestyle factors on breast and ovarian cancer in BRCA1 mutation carriers from Poland. Breast Cancer Res Treat. 2006;95:105–109. doi: 10.1007/s10549-005-9051-5. [DOI] [PubMed] [Google Scholar]
- 8.Nkondjock A, Robidoux A, Paredes Y, et al. Diet, lifestyle and BRCA-related breast cancer risk among French-Canadians. Breast Cancer Res Treat. 2006;98:285–294. doi: 10.1007/s10549-006-9161-8. [DOI] [PubMed] [Google Scholar]
- 9.Collia S, Kantoff PW, Neuhausen SL, et al. The joint effect of smoking and AIB1 on breast cancer risk in BRCA1 mutation carriers. Carcinogenesis. 2006;27:599–605. doi: 10.1093/carcin/bgi246. [DOI] [PubMed] [Google Scholar]
- 10.Couch FJ, Cerhan JR, Vierkant RA, et al. Cigarette smoking increases risk for breast cancer in high-risk breast cancer families. Cancer Epidemiol Biomarkers Prev. 2001;10:327–332. [PubMed] [Google Scholar]
- 11.Reynolds P, Hurley S, Goldberg D, et al. Active smoking, passive smoking, and breast cancer: evidence from the California Teachers Study. J Natl Cancer Inst. 2004;96:29–37. doi: 10.1093/jnci/djh002. [DOI] [PubMed] [Google Scholar]
- 12.Bennicke K, Conrad C, Sabrae S, et al. Cigarette smoking and breast cancer. Br Med J. 1995;310:1431–1432. doi: 10.1136/bmj.310.6992.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braga C, Negri E, La Vecchia C, et al. Cigarette smoking and risk of breast cancer. Eur J Cancer Prev. 1996;5:159–164. doi: 10.1097/00008469-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Lash TL, Aschengrau A. Active and passive cigarette smoking and the occurrence of breast cancer. Am J Epidemiol. 1999;149:5–12. doi: 10.1093/oxfordjournals.aje.a009727. [DOI] [PubMed] [Google Scholar]
- 15.Marcus PM, Newman B, Millikan RC, et al. The associations of adolescent cigarette smoking, alcohol beverage consumption, environmental tobacco smoke, and ionizing radiation with subsequent breast cancer risk (United States) Cancer Causes Control. 2000;11:271–278. doi: 10.1023/a:1008911902994. [DOI] [PubMed] [Google Scholar]
- 16.Johnson KC, Hu J, Mao Y. Passive and active smoking and breast cancer risk in Canada, 1994–97. The Canadian Cancer Registries Epidemiology Research Group. Cancer Causes Control. 2000;11:211–221. doi: 10.1023/a:1008906105790. [DOI] [PubMed] [Google Scholar]
- 17.Band PR, Le ND, Fang R, et al. Carcinogenic and endocrine disrupting effects of cigarette smoke and risk of breast cancer. Lancet. 2002;360:1044–1049. doi: 10.1016/S0140-6736(02)11140-8. [DOI] [PubMed] [Google Scholar]
- 18.Kropp S, Chang-Claude J. Active and passive smoking and risk of breast cancer by age 50 years among German women. Am J Epidemiol. 2002;156:616–626. doi: 10.1093/aje/kwf093. [DOI] [PubMed] [Google Scholar]
- 19.Egan KM, Stampfer MJ, Hunter D, et al. active and passive smoking in breast cancer: prospective results from the Nurses’ Health Study. Epidemiology. 2002;13:138–145. doi: 10.1097/00001648-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Hamajima N, Hirose K, Tajima K, et al. Alcohol, tobacco, and breast cancer-collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87(11):1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor DA, Ebrahim S, Smith GD. Smoking before the birth of a first child is not associated with increased risk of breast cancer: findings from the British Women Heart and Health Cohort Study and a meta-analysis. Br J Cancer. 2004;91:512–518. doi: 10.1038/sj.bjc.6601916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JC. Accumulating evidence on passive and active smoking and breast cancer risk. Int J Cancer. 2005;117:619–628. doi: 10.1002/ijc.21150. [DOI] [PubMed] [Google Scholar]
- 23.Terry PD, Rohan TE. Cigarette smoking and the risk of breast cancer in women: a review of the literature. Cancer Epidemiol Biomarkers Prev. 2002;11:953–971. [PubMed] [Google Scholar]
- 24.Al-Delaimy WK, Cho E, Chen W, et al. A prospective study of smoking and risk of breast cancer in young adult women. Cancer Epidemiol Biomarkers Prev. 2004;13:398–404. [PubMed] [Google Scholar]
- 25.Gram IT, Braaten T, Terry PD, et al. Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol Biomarkers Prev. 2005;14:61–66. [PubMed] [Google Scholar]
- 26.London SJ, Colditz GA, Stampfer MJ, et al. Prospective study of smoking and the risk of breast cancer. J Natl Cancer Inst. 1989;81:1625–1631. doi: 10.1093/jnci/81.21.1625. [DOI] [PubMed] [Google Scholar]
- 27.Morabia A, Bernstein M, Ruiz J, et al. Relation of smoking to breast cancer by estrogen receptor status. Int J Cancer. 1998;75:339–342. doi: 10.1002/(sici)1097-0215(19980130)75:3<339::aid-ijc2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Obana H, Hori S, Kashimoto T, Kunita N. Polycyclic aromatic hydrocarbons in human fat and liver. Bull Environ Contam Toxicol. 1981;27:23–27. doi: 10.1007/BF01610981. [DOI] [PubMed] [Google Scholar]
- 29.IARC. IARC Sci Publ. Vol. 38. France: IARC Lyon; 1986. Tobacco smoking. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans; pp. 1–397. [PubMed] [Google Scholar]
- 30.el-Bayoumy K. Environmental carcinogens that may be involved in human breast cancer etiology. Chem Res Toxicol. 1992;5:585–590. doi: 10.1021/tx00029a001. [DOI] [PubMed] [Google Scholar]
- 31.Morris JJ, Seifter E. The role of aromatic hydrocarbons in the genesis of breast cancer. Med Hypotheses. 1992;38:177–184. doi: 10.1016/0306-9877(92)90090-y. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman D, Hoffman I, el-Bayoumy K. The less harmful cigarette: a controversial issue. A tribute to Ernst L Wynder. Chem Res Toxicol. 2001;14:767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- 33.Phillips DH, Martin FL, Grover PL, Williams JA. Toxicological basis for a possible association of breast cancer with smoking and other sources of environmental carcinogens. J Women’s Cancer. 2001;2:9–16. [Google Scholar]
- 34.Perera FP, Estabrook A, Hewer A. Carcinogen-DNA adducts in human breast tissue. Cancer Epidemiol Biomarkers Prev. 1995;4:233–238. [PubMed] [Google Scholar]
- 35.Kaufman DW, Slone D, Rosenberg L, Miettinen OS, Shapiro S. Cigarette smoking, age at natural menopause. Am J Public Health. 1980;70:420–422. doi: 10.2105/ajph.70.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams AR, Weiss NS, Ure CI, et al. Effect of weight, smoking, and estrogen use on the risk of hip and forearm fractures in postmenopausal women. Obstet Gynecol. 1982;60:695–699. [PubMed] [Google Scholar]
- 37.MacMahon B, Trichopoulos D, Cole P, et al. Cigarette smoking and urinary estrogens. N Engl J Med. 1982;307:1062–1065. doi: 10.1056/NEJM198210213071707. [DOI] [PubMed] [Google Scholar]
- 38.Baron JA, La Vecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gyneco1. 1990;162:502–514. doi: 10.1016/0002-9378(90)90420-c. [DOI] [PubMed] [Google Scholar]
- 39.Lesko SM, Rosenberg L, Kaufman DW, et al. Cigarette smoking and the risk of endometrial cancer. N Engl J Med. 1985;313:593–596. doi: 10.1056/NEJM198509053131001. [DOI] [PubMed] [Google Scholar]
- 40.Hosey AM, Gorski JJ, Murray MM, et al. Molecular basis for estrogen receptor α deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst. 2007;99:1683–1694. doi: 10.1093/jnci/djm207. [DOI] [PMC free article] [PubMed] [Google Scholar]