Abstract
The identification and production of recombinant morphogens and growth factors that play key roles in tissue regeneration have generated much enthusiasm and numerous clinical trials, but the results of many of these trials have been largely disappointing. Interestingly, the trials that have shown benefit all contain a common denominator, the presence of a material carrier, suggesting strongly that spatio-temporal control over the location and bioactivity of factors after introduction into the body is crucial to achieve tangible therapeutic effect. Sophisticated materials systems that regulate the biological presentation of growth factors represent an attractive new generation of therapeutic agents for the treatment of a wide variety of diseases. This review provides an overview of growth factor delivery in tissue engineering. Certain fundamental issues and design strategies relevant to the material carriers that are being actively pursued to address specific technical objectives are discussed. Recent progress highlights the importance of materials science and engineering in growth factor delivery approaches to regenerative medicine.
Keywords: regenerative medicine, biomaterials, synthetic extracellular matrix, stimuli-responsive materials, nanoparticles
1. Introduction
Regenerative medicine, which is a term often used interchangeably with tissue engineering, merges the fields of life sciences and engineering and aims to orchestrate body regeneration by specifically controlling the biological environment [1]. Regeneration in the adult often represents a recapitulation of developmental processes, and strives to maintain and/or restore tissue integrity and functionality. Regenerative medicine provides alternatives to organ transplantation, which is limited in applicability owing to immune responses against allografts and the large disparity between the need for organs and tissues and the number available for transplantation. Inspiration for regenerative medicine strategies commonly derive from our increasing understanding of how cells and biological systems decipher cues, and aims to replicate biological concepts and instructions expressed during embryonic development, including signal transduction pathways, transcription factor instructions and protein regulation. Growth factors are critical signalling molecules that instruct cells during development, and one may achieve tissue regeneration in the adult by enabling control over growth factor delivery.
Tissue-regeneration strategies are often broken down into three categories: (i) direct injection of bolus cells into the tissue of interest or the systematic circulation, (ii) implantation of cells after they have been combined to form a three-dimensional tissue structure, often within a bioreactor, and (iii) scaffold-based delivery of signalling molecules such as low-molecular-weight drugs, proteins and oligonucleotides that stimulate cell migration, growth and differentiation. These signalling molecules, which are the focus of this review, are broadly grouped into the overlapping categories of mitogens (stimulate cell division), growth factors (originally identified by their proliferation-inducing effects, but have multiple functions) and morphogens (control generation of tissue form). Precise control over the signalling of these factors in a local area may potentially allow control over a regenerative process. As is typical in this field, the term growth factor will be broadly used in this review for proteins, which affects cell migration, proliferation and cellular differentiation. In this review, we will discuss general approaches to the strategic use of these factors and specific applications in regenerative medicine. This review is focused on the materials science and chemistry aspects of this topic, not the polymer physics. In particular, we first will discuss the types of growth factors, their mechanisms of action and variables affecting growth factor delivery. Section 2 summarizes strategies for biomaterial presentation of growth factors including: (i) chemical immobilization and (ii) physical encapsulation. The former will discuss not only covalent conjugation of growth factors, but also growth factor incorporation through secondary interactions between growth factors and the biomaterials. The latter section will cover a variety of physical encapsulation strategies aimed at pre-programmed release and diffusion of growth factors into surrounding tissues. Section 3 of this review covers release on demand of growth factors.
2. Growth factor interactions with natural and synthetic extracellular matrices
2.1. Types of growth factors and their mechanism of action
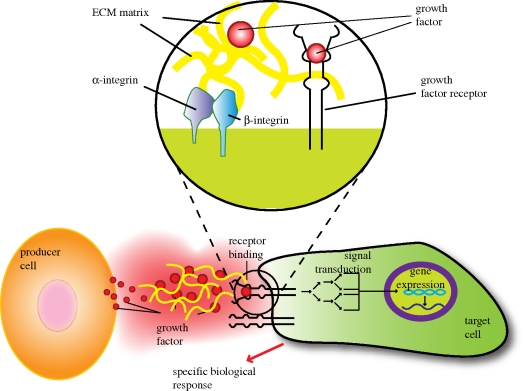
Growth factors are soluble-secreted signalling polypeptides capable of instructing specific cellular responses in a biological environment ([2]; table 1). The specific cellular response trigged by growth factor signalling can result in a very wide range of cell actions, including cell survival, and control over migration, differentiation or proliferation of a specific subset of cells. Prior to addressing strategic delivery of growth factors, understanding the biological functions and roles of these proteins in the extracellular matrix is first of all required because the extracellular matrix contain numerous components such as adhesive molecules, notch signalling molecules, traction-enabling adhesion molecules and proteoglycan molecules to bind and modulate the activity of a number of growth factors [3–5]. The signal transmission mechanism initiates with growth factor secretion by the producer cell. The growth factor instructs cell behaviour through binding to specific transmembrane receptors on the target cells (figure 1). The machinery that transduces the growth factor-binding signal to the cell nucleus involves a complex array of events involving cytoskeleton protein phosphorylation, ion fluxes, changes in metabolism, gene expression, protein synthesis and ultimately an integrated biological response [6].
Table 1.
Popular growth factors in tissue regeneration. Ang, angiopoietin; bFGF, basic fibroblast growth factor; BMP, bone morphogenetic protein; EGF, epidermal growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IGF, insulin-like growth factor; NGF, nerve growth factor, PDGF, platelet-derived growth factor; TFG, transforming growth factor; VEGF, vascular endothelial growth factor.
| abbreviation | tissues treated | representative function |
|---|---|---|
| Ang-1 | blood vessel, heart, muscle | blood vessel maturation and stability |
| Ang-2 | blood vessel | destabilize, regress and disassociate endothelial cells from surrounding tissues |
| FGF-2 | blood vessel, bone, skin, nerve, spine, muscle | migration, proliferation and survival of endothelial cells, inhibition of differentiation of embryonic stem cells |
| BMP-2 | bone, cartilage | differentiation and migration of osteoblasts |
| BMP-7 | bone, cartilage, kidney | differentiation and migration of osteoblasts, renal development |
| EGF | skin, nerve | regulation of epithelial cell growth, proliferation and differentiation |
| EPO | nerve, spine, wound healing | promoting the survival of red blood cells and development of precursors to red blood cells. |
| HGF | bone, liver, muscle | proliferation, migration and differentiation of mesenchymal stem cells |
| IGF-1 | muscle, bone, cartilage, bone liver, lung, kidney, nerve, skin | cell proliferation and inhibition of cell apoptosis |
| NGF | nerve, spine, brain | survival and proliferation of neural cells |
| PDGF-AB (or -BB) | blood vessel, muscle, bone, cartilage, skin | embryonic development, proliferation, migration, growth of endothelial cells |
| TGF-α | brain, skin | proliferation of basal cells or neural cells |
| TGF-β | bone, cartilage | proliferation and differentiation of bone-forming cells, anti-proliferative factor for epithelial cells |
| VEGF | blood vessel | migration, proliferation and survival of endothelial cells. |
Figure 1.
Cross talk between cells mediated by growth factors and ECM. The producer cell secretes soluble growth factors that bind to target cell receptors. The instructions are translated into the cell through complex signal transduction networks resulting in a specific biological cellular response. Insert illustrates how ECM can control growth factor presentation in a temporal and spatial fashion. Cell migration towards gradients of growth factors, bonded to ECM, can also be ECM mediated, whereas cells will use integrin machinery to follow growth factor gradients. Upon degradation, ECM growth factors become available for cell binding via cell membrane growth factor receptors and will ultimately induce a specific biological cellular response.
Growth factors differ from other oligo-/polypeptide molecules, such as insulin and hormones, in the mode of delivery and response elicited. Typically, growth factors do not act in an endocrine fashion; they exhibit short-range diffusion through the extracellular matrix and act locally owing to their short half-lives and slow diffusion. The ability of a growth factor to deliver a particular message to a distinct subpopulation of cells is not exclusively determined by the identity of the growth factor and its ability to diffuse through the extracellular matrices (ECMs); it is also determined by the target cell number, type of receptors and the intracellular signal transduction subsequent to factor binding. The same growth factor can convey different instructions depending on the receptor type to which it binds, and on the cell type to which it binds. Moreover, the same receptor can translate different messages depending on the intracellular transduction pathways, which can differ from one cell type to another. The ultimate response of a target cell to a particular soluble growth factor can also be governed by external factors, including the ability of the factors to bind to ECM, ECM degradation and growth factor concentration and cell target location [7].
2.2. Variables affecting growth factor interaction with ECM
2.2.1. ECM-mimicking polymer carriers
The insoluble matrix cues presented by the ECM are also critical to govern tissue formation and regeneration in spite of the emphasis on growth factor signalling in mediating cellular fate. The ECM can regulate the spatial presentation of growth factors by controlling the extent of binding of these factors to the matrix. For example, the presence of heparin-binding domains in certain growth factor molecules is crucial to mediate specific interactions with the ECM. Growth factors that exhibit ECM-binding domains frequently are present in spatio-temporal gradients that provide essential cues to elicit specific cellular responses [8]. In contrast, growth factors lacking ECM binding capabilities are much more highly diffusible in tissues. The ECM, although not the focus of this review, also plays critical roles in mediating cellular migration and maintaining cells in a quiescent state, by the local presentation of physical and structural cues allowing the anchorage of the cellular motility machinery (e.g. actin fibres, integrins; [7]). For example, integrins, a large family of cell surface ECM receptors, are active in regulating angiogenic signalling and endothelial cell behaviour in physiological and pathological events [9,10].
Control over the overall growth factor regulatory system is critical to instruct specific cellular decisions. The response of cells to growth factors can be regulated by cell–cell signalling, and together these processes affect cell proliferation, differentiation and stem cell fate decisions. One recent report suggests that the local concentration of growth factors influences cadherin-mediated cell–cell contact, which suppresses cell proliferation only when a specific growth factor recedes below a threshold level [11]. Similarly, angiogenesis promoted via vascular endothelial growth factor (VEGF) was effectively controlled by notch signalling, which is known to suppress the uptake of VEGF. Moreover, local notch inhibition by a small molecule (DAPT, N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine-t-butyl ester, γ-secretase inhibitor IX) assists the effective uptake of VEGF by endothelial cells, resulting in the promotion of regional angiogenesis [3].
Owing to the critical role of growth factors in controlling basic cellular functions, and their ability to directly elicit and orchestrate tissue regeneration, a wide range of growth factors has been tested for distinct therapeutic applications, including bone regeneration and neovascularization of ischaemic tissues. The ability of angiogenic factors to treat ischaemic diseases was first examined by intracoronary injections of fibroblast growth factor (FGF), and animal studies demonstrated improvement of cardiac systolic function and reduction in the infarcted area [12]. A number of growth factors have been tested in clinical trials using a similar delivery approach, including VEGF [13–16]. Phase I trials typically have reported promising results [17,18]. However, the results obtained in the larger phase II trials have not shown the expected benefit to patients ([19]; table 2). These disappointing clinical results with angiogenic factors demonstrate the need for the development of alternative strategies capable of inducing a sustainable and physiologically meaningful neovascularization.
Table 2.
Results of some clinical studies using different growth factor strategies for therapeutic applications. n.a., not applicable.
| study | no. of patients | growth factor | administration | clinical target | result | commercially available |
|---|---|---|---|---|---|---|
| VIVA [23] | 178 | VEGF165 | infusions (intravenous and intracoronary) | cardiovascular diseases | neutral | n.a. |
| FIRST [19,24] | 337 | FGF-2 | infusions (intracoronary) | cardiovascular diseases | neutral | n.a. |
| ‘Polymer’ [181,182] | 24 | FGF-2 | alginate microcapsules | cardiovascular diseases | positive | n.a. |
| BESTT [183,184] | 450 | BMP-2 | collagen sponge | bone fractures | positive | yes (https://www.infusebonegraft.com/) |
| OP-1 Putty [185,186] | 336 | BMP-7 | collagen matrix | bone defects | positive | yes (http://www.stryker.com/en-us/products/Orthobiologicals/index.htm) |
The relatively unsuccessful experience with the phase II angiogenesis clinical trials could be attributable to several causes, including the formulation of the growth factor used, the dose used, the route of administration and/or inappropriate clinical trial design (e.g. selection of patients). Although largely neglected by the clinical community, one particular limitation is probably the mode of delivery. Large doses of potent growth factors, formulated in solution form, were directly injected into the body. This administration of supraphysiological concentrations of growth factors may lead to severe side effects owing to the extremely high initial concentration, and conversely may not allow sufficient levels of the factors to be sensed by target tissue for the necessary time frame owing to their rapid degradation and cleaving. Degradation of growth factors in vivo can occur via several distinct pathways, including denaturation, oxidation or proteolysis [20,21]. For example, VEGF presents a biological half-life of less than 30 min when infused intravenously [22], resulting in the need for massive doses and multiple injections [19,23,24]. However, the use of large quantities of VEGF should be avoided because it could lead to catastrophic pathological vessel formation at non-target sites (e.g. dormant tumours). The classical delivery strategies that use infusions of factor cocktails intrinsically lack targeting of specific cell populations, and probably result in a transient and inadequate biological response.
To improve unsatisfactory outcomes in classical delivery of growth factors, polymer matrices with relevant modifications for the presentation of growth factors could be good platforms as delivery substrates. Bioactive factors can be chemically immobilized or physically encapsulated into polymer matrices, preventing their denaturation, and their release can be controlled by the degradation rate of the polymer matrices, their diffusion through the polymer construct or external triggers [25,26]. During polymer/growth factor complex preparation, features such as chemical modification of polymer matrices (e.g. adhesion cues) and physical encapsulation of growth factors with secondary polymer carriers may be critical to increase the therapeutic efficiency. Elaborate engineering of delivery matrices composed of polymers showing distinct physical properties can also provide a dramatic enhancement of therapeutic efficiency. The degradation kinetics of polymer-based delivery systems enable one to control the release profile of growth factors, resulting in optimized concentrations of growth factors, which is a main goal of these systems.
The design and control of extracellular matrix-mimicking scaffolds for effective growth factor-relating treatments have attracted wide attention in tissue engineering. From a biophysical perspective, polymer matrices used for growth factor delivery substrate must also be considered to be a biological compartment in tissue development because the matrices will affect not only the factor's efficiency but also cell fate. In addition to designing polymer structures to control chemotactic responses of cells with growth factor delivery, the physical properties of the scaffolds may influence subsequent cellular growth factor secretion and related cell signalling [27]. There are many reports that physical parameters, such as shape, elasticity, hardness, stiffness, pore size, elastic reversibility and degradation rate of matrices, can alter cellular processes [28,29]. For example, short filaments showed higher persistence in vivo than spherical counterparts, and cellular uptake was one order of magnitude higher than longer filaments [30]. A further example is the finding that increasing the degree of cross-linking of three-dimensional polymer networks incorporating bone morphogenetic protein-2 (BMP-2) used in the healing of critical-sized defects in rat calvaria caused a decrease in the cell migration rate [31–33].
2.2.2. Spatio-temporal control over the multiple growth factors
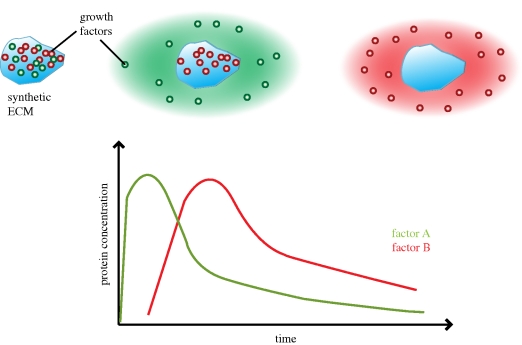
In addition to localized delivery of a single component or factor, simultaneous or sequential delivery of multiple growth factors has also been exploited to enhance the therapeutic efficiency. The complex processes of cell migration, differentiation and proliferation are typically dependent on both the presence of specific growth factors, and their time-dependent and spatial distributions. Composite polymer systems can be designed to induce the spatio-temporal delivery of multiple growth factors. Complex polymer systems showing distinct release kinetics for growth factors may be critical to control biological processes. For example, the first step of angiogenesis requires VEGF, FGF and angiopoietin-2 (Ang-2) to disrupt the structure of pre-existing blood vessels, and to promote the proliferation and migration of new cells to form new immature vessels. Next, angiopoietin-1 and PDGF-BB stabilize these newly formed blood vessels [34–36]. Sequential and simultaneous delivery of these growth factors has been shown to enhance vascularization ([27,37,38]; figure 2). Degradable alginate hydrogel-based delivery systems have also provided the simultaneous delivery of an osteogenic growth factor and other morphogens and showed enhanced effects [39,40]. In a similar manner, sequential delivery of BMP-2 and BMP-7 encapsulated in (poly(lactide-co-glycolide) (PLG) acid-based nanoparticles through chitosan-based three-dimensional fibre scaffolds was also recently reported [41–43]. Different combinations of sequential delivery with BMP-2 and insulin-like growth factor (IGF-1) from a layered structure, BMP-2 and transforming growth factor-β3 (TGF-β3) from alginate hydrogels and VEGF and BMP-2 from gelatin microparticles have recently shown enhanced healing effects compared with single growth factor delivery [40,44,45]. Also, sequential delivery of three factors from alginate scaffolds, VEGF, PDGF-BB and TGF-β1, showed superior vascularization to only basic fibroblast growth factor (bFGF). Each protein has a different association with alginate, and the difference in affinity governs the release rate [46,47].
Figure 2.
Illustration displays spatio-temporal delivery of distinct factors. A material system loaded with different bioactive factors can be tailored to display a sequential delivery of these factors over time, resulting in controlled sequential waves of factor delivery over extended periods of time.
3. Strategies for biomaterial presentation of growth factors
Two distinct strategies for biomaterial presentation of growth factors in tissue engineering have been pursued: (i) chemical immobilization of the growth factor into or onto the matrix and (ii) physical encapsulation of growth factors in the delivery system (figure 3). The former approach typically involves chemical binding or affinity interaction between the growth factor-containing polymer substrate and a cell or a tissue. The latter approach is achieved by the encapsulation, diffusion and pre-programmed release of growth factor from substrate into the surrounding tissue. The efficacy of factor delivery can be significantly enhanced by three-dimensional patterning of the growth factors on scaffolds [48–50]. Both general concepts and recent advances in these two strategies will be discussed in this section. In addition, growth factor release on demand by external/internal triggering provides another level of control and will be discussed in the diffusion-based growth factor release section.
Figure 3.
Schematic of two tissue engineering approaches using synthetic ECMs to present growth factors to tissues. (a) Physically encapsulated bioactive factors can be released from synthetic ECMs to target specific cell populations to migrate and direct tissue regeneration. (b) Alternatively, growth factors can be chemically bound to the material system, making them available to cells that infiltrate the material.
3.1. Chemical conjugation of growth factors or morphogens to scaffold materials
A variety of techniques to conjugate growth factors to natural or synthetic biomaterials and chemicals have been developed. These immobilized factors will be available to cells that come in contact with the matrix, providing a highly localized signal to control cell fate (e.g. stem cell differentiation; [51,52]). Growth factors may be active in the bound state or be activated by cleavage from the matrix. Site-specific tethering of growth factors and other biological molecules enables one to control multiple functions of growth factors and their delivery. There are two main strategies for direct presentation of growth factors on extracelluar matrices: (i) physical adsorption owing to protein–protein hydrogen bonding or hydrophobic interactions with excipient molecules often acting as a molecular chaperone (non-covalent approaches) and (ii) direct covalent immobilization of the growth factor or growth factor-mimicking molecules to the matrix (covalent approach). Regardless of which technique is used to immobilize growth factors, making the scaffolds also adhesive to cells will aid in bringing the cells into close contact. The potential benefit of this concept has been further demonstrated by the finding that the effects of growth factors such as VEGF-A and bFGF (or FGF-2) are influenced by extracellular matrix receptors as integrin binding can increase the activation of growth factor receptors [53–56]. Based on these findings, a ligand for αvβ3-integrin has been used to increase growth factor receptor expression and cellular response [57].
3.1.1. Non-covalent incorporation
Absorption of growth factors typically exploits direct charge–charge or other secondary interactions between growth factors and matrices, or indirect interaction via intermediate proteins or other biological molecules [58–60]. Proteins such as heparin, fibronectin, gelatin and small oligopeptides mimicking large proteins can be chemically or physically coated to provide specific biological sites to immobilize the growth factors or morphogens. Small molecules mimicking key fragments and functions of these large proteins can also be used in their place to immobilize growth factors.
3.1.1.1. Biopolymeric gels for immobilization of growth factors
Biopolymeric gels containing fibronectin, laminin, collagen, elastin or the glycosaminoglycans heparin sulphate, chondroitin sulphate, hyaluronic acid or a variety of synthetic hydrogels have been used as extracellular matrix-mimicking materials to immobilize growth factor-inducing moieties or growth factors directly [61,62]. For example, fibrin, which plays an important role in wound healing, has been used to link and deliver peptides or growth factors into the area of interest. Bioactive molecules have been covalently coupled to fibrin using enzymes [63]. The β-nerve growth factor (NGF) fusion protein immobilized to fibrin in this manner is released by means of the proteolytic activity of plasmin, and this mode of delivery showed enhanced nerve regeneration [64]. Release of similarly coupled BMP-2 from fibrin gels via cell-activated plasmin induced bone defect healing [65]. Similarly, an engineered variant of VEGF bound to a fibrin network was developed for induction of local angiogenesis [48,50,66]. However, growth factor coupling and release can show deleterious effects, suggesting that delivery must be optimized for each situation [67].
For example, the surface of extracellular matrix-mimicking substrates can be chemically or physically modified to immobilize heparin, in order to bind growth factors via their affinity to the grafted heparin. Heparin-based growth factor-delivery systems have demonstrated the ability to provide sustained release of growth factors [68]. The kinetics of release is dependent not only on the constant association between the electrostatically bound molecules, but also on the environmental conditions (e.g. temperature, acidity and hydrophobicity). There are several recent reports showing affinity and release results of platelet-derived growth factor (PDGF), basic FGF, VEGF, TGF-β and bone morphogenetic proteins (BMPs) in heparin or other materials-coated ECMs [58,69–75].
3.1.1.2. Small oligopeptides mimicking proteins
The coupling of small oligopeptides mimicking the adhesion properties of large matrix molecules to a delivery vehicle can aid in obtaining effective responses to delivered growth factors [76–80]. For example, arginine–glycine–aspartic acid (RGD)-presenting macroporous scaffolds have been used to transplant myoblasts to muscle laceration sites as well as to provide a sustained delivery of hepatocyte growth factor (HGF) and fibroblast growth factor-2 (FGF-2). Compared with blank scaffold or delivery of only cells or growth factors, the combined system enhanced the survival and migration of the transplanted myoblasts, and overall muscle regeneration [81]. Similarly, macroporous alginate scaffolds releasing VEGF and containing covalently linked cell-adhesion peptides dramatically increased the survival rate of endothelial progenitor cells delivered into the body, and the return of blood perfusion to ischaemic tissue [82]. This approach also provides a platform for immune-modulating function. One recent example involves the immobilization of a peptide (YCWSQYLCY) to a hydrogel, in order to mimic the tumour necrosis factor-α (TNF-α) recognition loop to enhance the survival and function of encapsulated cells [83]. The use of peptide amphiphile molecules to form self-assembled factor delivery vehicles has also been reported [84]. This strategy exploits self-assembling oligopeptides composed of alternating hydrophilic and hydrophobic amino acids (RARADADARARADADA) as building blocks. The conjugation of biotin molecules at the end of the peptides allows incorporation of tetravalent streptavidin molecules, and subsequent tethering of a biotinylated IGF-1 has been used for prolonged factor delivery and activity.
3.1.2. Covalent incorporation
Covalent bonding of growth factors to material carriers can provide more prolonged release than that achieved by physical immobilization. There are other potential advantages in presenting growth factors as matrix-tethered molecules for applications in tissue engineering, as well. The factors remain competent to bind and activate the growth factor receptors, but are more slowly degraded and internalized. Factors can be conjugated to the polymers via functional groups, which are incorporated by copolymerization or chemical or physical treatment [85]. For example, epidermal growth factor (EGF) which was covalently coupled to amino-silane glass via star poly(ethyleneoxide) (PEO), which allows the ligand to retain better mobility, is more effective in promoting cell-growth responses in primary rat hepatocytes than physically adsorbed EGF [86]. Similarly, TGF-β1 conjugated covalently to poly(ethylene glycol) PEG hydrogels leads to increased matrix production, and can counteract the effect of adhesive RGD ligands bound to the same PEG, resulting in enhanced matrix production [87]. However, there are some limitations to this approach, as the specificity of the coupling site on the conjugated protein can be difficult to assign selectively. Also, proteins may lose their bioactivity during immobilization owing to screening of the active pocket of the protein or damage to bioactive functional groups.
In summary, chemical immobilization strategies for factor delivery allow for localized and strong interactions between growth factors delivered into tissues, and the resident cells. Challenges in this approach include finding the correct balance between the factor dose and physical and chemical properties of the scaffold, which can regulate cell behaviours.
3.2. Physical encapsulation of growth factors with pre-programmed release, and diffusion into surrounding tissues
3.2.1. Polymeric scaffold for physical encapsulation of growth factors
Physical encapsulation of growth factors for the purpose of their controlled release is a prominent strategy for local growth factor delivery in tissue engineering, and its simplicity can provide an appealing alternative to chemical conjugation. Similar to chemical conjugation methods, though, factor delivery can be combined with other variables of scaffolds to provide favourable tissue interactions and elicit a desirable cellular response. Delivery materials that are injectable or transplantable, with relevant mechanical strength, porosity and degradation rates, can be readily fabricated. Fabrication methods actively used for physical encapsulation of growth factors include solvent casting and particulate leaching, freeze drying, phase separation, melt moulding, phase emulsion, in situ polymerization and gas foaming [1]. A key issue is minimizing exposure of factors to harsh conditions during processing in order to protect the activity of the biomolecules. For example, gas-foaming processes have been developed to avoid the exposure of growth factors to hazardous solvents that is common in many other techniques (both probably lead to protein denaturation; [88–90]). CO2, when present at high pressure, will dissolve in an appropriate polymer, but, when the pressure is decreased, nucleation and CO2 pore formation will result in expansion of the polymer matrix leading to factor encapsulation. Combining gas foaming with a particle-leaching technique can lead to open-pore networks, and has been used for the delivery of growth factors in vivo in a bioactive form, resulting in sustainable release of intact growth factors that can lead to new tissue formation [91]. The various scaffold fabrication techniques used for physical incorporation of growth factors into scaffold materials have advantages and disadvantages (table 3).
Table 3.
Popular scaffold preparation techniques used for physical association of growth factors.
| technique | advantages | potential disadvantages |
|---|---|---|
| solvent casting/particulate leaching [118,187] | control over porosity, pore sizes and crystallinity; high porosity | residual solvents and porogen materials; limited mechanical properties |
| freeze drying [188,189] | high porosity and interconnectivity | limited pore sizes range (15–35 µm) |
| phase separation [190,191] | high porosity | limited pore sizes, residual solvents (1–10 µm) |
| melt moulding [192] | control over macrogeometry, porosity and pore size; free of harsh organic solvents | high temperatures |
| high internal-phase emulsion [193] | control over porosity, pore size and interconnectivity | limited polymer types and mechanical properties |
| in situ polymerization [194,195] | injectable; control over mechanical properties | residual monomers and cross-linking agents, limited porosity |
| gas foaming [88,89] | free of organic solvents; control over porosity | pore interconnectivity |
Combining methods is also often pursued to bypass limitations. For example, a high initial burst release of growth factor results from several techniques, but growth factors can first be encapsulated in the bulk of a polymer using a technique such as solvent casting, and subsequently incorporated into a scaffold using gas foaming, leading to a sustained release of the growth factor. The surface area of the material system will typically influence growth factor release with physical encapsulation as release is controlled by diffusion. For example, nanospheres (1–100 nm) or microparticles (1–100 µm) as a carrier of individual or multiple growth factors will lead to varying delivery times as the size of the carrier particles controls the surface-to-volume ratio. Further, the rate of intracellular uptake is influenced by particle size [92,93]. Further control over release with encapsulating strategies can be achieved by altering various physical and chemical parameters, including the polymer molecular weight distribution, hydrophobicity and porocity.
3.2.2. Types of biomaterials for physical encapsulation
3.2.2.1. Synthetic polymers
The selection of the specific biomaterial used for encapsulation is a key variable in the design and development of encapsulation systems. A multiplicity of synthetic polymers, including poly(α-hydroxy acids), poly(orthoesters), poly(anhydrides), poly(amino acids), dextrin, poly(glycoside) (PGA), poly(l-lactide) (PLA) and their copolymers (PLG acid) have been used for growth-factor encapsulation [43,90,94–99]. These types of implantable materials or nano-/microparticles may be particularly advantageous for applications that require a very well-defined physical space over time. For example, PLG-based nano- or microcapsules containing growth factors prepared by a double emulsion–solvent evaporation technique are widely used in this field [99,100]. Sustained delivery of VEGF encapsulated in PLG microspheres can upregulate angiogenesis [101]. The degree of encapsulation and mechanism of growth factor entrapment are dependent on hydrophobic–hydrophobic or hydrophilic–hydrophilic interactions among the molecules and polymers. Blending of materials can allow more complex structures and patterns of factor encapsulation. For example, a blend of PLG and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) microspheres prepared by an emulsion technique showed a core-shell structure. PHBV molecules, which are more hydrophobic and less degradable, were distributed within the shell. This prevents the loss of growth factors during fabrication and washing processes, and produced a better encapsulating material than PLG- or PHBV-alone microspheres [102].
Liposome-based particles represent another approach to encapsulate growth factors [103–106]. Liposome-based delivery systems avoid the use of harsh organic solvents during preparation, potentially enhancing biocompatibility. One recent example demonstrates that VEGF delivery from liposomes in a myocardial infarction model can improve cardiac function [105]. One limitation of liposome-based delivery, in contrast to cross-linked polymer systems, is the relatively short duration of release.
Synthetic polymeric vehicles can also be used to investigate the role of combinations of growth factors in tissue formation, as synergistic effects between different growth factors have been demonstrated in several processes [38,107,108]. For example, a three-dimensional PLG scaffold has been developed which allows the sequential release of VEGF followed by PDGF, and this led both to a significant increase in the local blood vessel density and to maturation of the newly formed vessels [27]. The two growth factors were incorporated together into the same scaffolds by mixing polymer microspheres containing pre-encapsulated PDGF with lyophilized VEGF before processing into scaffolds. Simultaneous delivery of the two factors led to little or no revascularization. The systematic integration of quantitative biological measurements and mathematical modelling provided a design principle for the delivery vehicles through understanding of the growth factor-driven process of angiogenesis. In addition, quantitative information about spatio-temporal control of VEGF presentation on microvascular endothelial cells can be obtained by FRET or radio-labelling assays [38,107]. Similarly, PEG-based hyrogel has also been used to incorporate multiple neurotrophin family members, and to release them at different rates [109].
Growth factors encapsulated in synthetic nanoporous/macroporous materials can be exploited not only to deliver factors to cells in the surrounding tissue, but also to actively rearrange and recruit the cells and regulate their proliferation and differentiation [110]. For example, a recent report demonstrates how infection-mimicking materials can be used to programme dendritic cells via delivery of cytokines from scaffolds; the system recruits, activates and homes the cells to the lymph nodes [111]. Granulocyte macrophage colony-stimulating factor (GM–CSF) released from the scaffolds first recruits the dendritic cells. The recruited cells are activated by danger signals immobilized on the scaffold and subsequently home to lymph nodes to activate T-cells. These biomaterials can potentially be exploited as a cancer vaccine or immune-modulating therapy.
3.2.2.2. Natural polymers
Naturally occurring materials such as silk, keratin, collagen, gelatin, fibrinogen, elastin, chitosan, hyaluronic acid, starch, carrageenan, cellulose and alginate have also gained wide attention as drug carriers [112–115]. The natural origin of these materials allows one to design and engineer biomaterial systems that function at the molecular level, and often minimizes chronic inflammation. They are often soluble in water, allowing mild fabrication conditions that are relatively harmless to the bioactivity of the growth factors. Promoting cross-linking when the growth factor is dispersed in the polymer solution represents one of the most popular methods for encapsulation of factors in these materials [93,116,117]. Gelatin microspheres and combinations of glycidyl methacrylated dextran with gelatin are examples that have successfully been used to deliver distinct growth factors, including BMP-2 and IGF-1 [94]. These polymers can be formulated into a variety of physical structures relevant to drug-delivery using several processes [89,90,118], but a common crucial issue is maintenance of the bioactivity of the encapsulated growth factors [91].
Factor release from degradable gels can be tuned by controlling either factor diffusion or gel degradation. Fast degradation will lead to rapid release of growth factors, while slowing degradation will retard factor release [119]. A potential problem in the use of natural polymers as delivery vehicles is that some physical properties, such as the degradation rate, can be challenging to control. Chemical modifications of the polymer are often performed to control the degradation rate. For example, typical alginate hydrogels present a slow and unpredictable degradation in vivo. Recent studies have addressed this limitation by partially oxidizing the polymer chains with sodium periodate to enable hydrolytic degradation [120] and modifying the polymer molecular weight distribution [121]. Similarly, injectable hydrogels prepared by a blend of chitosan, phopholipid and lauric aldehyde, and lauric chloride show tunable degradation rates and biocompatibility for factor delivery [122]. Blends of hyaluronan and methylcellulose containing NGF, FGF-2 and EGF or erythropoietin (EPO) have also been reported to provide local gradients of neurotrophic factors to injured nervous tissue [123–127]. Biodegradable hydrogels containing hydrophobic subunits such as cholesterol provide new types of stable and monodisperse platforms of gelation for incorporation of growth factors, and have been used in clinical trials [128–132].
3.3. Growth factor release on demand
Matrix degradation and subsequent diffusion-based delivery systems with pre-programmed kinetics are appealing for growth factor/morphogen delivery, as they can provide sustained release for tunable times [8,91,112,133]. However, in many situations there is a need for delivery systems that respond to local environmental signals or externally applied cues in order to control release, so-called ‘release on demand’. Release on demand by external triggering can be offered by the introduction of stimuli-responsive components into delivery systems [134]. The most commonly used triggering mechanisms involve changes in the local environment, including (i) pH or temperature, (ii) proteins such as enzymes that cleave a cross-linker used to immobilize a growth factor, or (iii) drugs or ions that trigger cleavage of an engineered substrate, resulting in the release of encapsulated growth factors. Externally applied light or electric or magnetic fields, and ultrasound can also modify the vehicle structure or factor immobilization and regulate release.
3.3.1. pH and/or temperature-triggered release
One of the most common triggers to activate release on demand is local pH. This concept is based on the varying pH found in different tissues or targets of drug delivery (e.g. low pH in tumours). Hydrogels are composed of a highly cross-linked polymer network immersed in a solvent, and moieties that exhibit large reversible volume transitions upon pH changes are often used to provide control over release. Stimuli-sensitive hydrogels or polymeric micelles triggered by pH have been investigated for use in the gastrointestinal tract and in cancer targeting [135–139]. These systems show good stability at physiological pH, but protonation of their functional groups triggered by acidification reduces their stability, leading to release of the drug. Also, the swelling ratio and swelling/de-swelling kinetics are strongly dependent on environment signals such as pH or temperature, which produce another means to regulate drug release. Popular pH-sensitive materials include those containing pH-sensitive moieties, such as sulphamethazine oligomers (SMOs), sulphonamide and methacrylic acid, and are often based on PEG [140,141], poly(ε-caprolactone)-based copolymers [139,140,142], poly(l-lactic acid) (PLLA; [143]), polyHis [144–146], poly(glutamic acid) [147] and their derivatives. Recently, pH-sensitive poly(β amino ester)-containing hydrogels were also developed for controlled DNA [148–150] or insulin delivery [148]. An interesting recent report is based on hydrogels that show pH sensitivity and degradation in the presence of a specific bacterial (Pseudomonas cepacia) lipase [151]. In addition, other types of polymers that respond to pH change can be used to initiate release on demand. pH-sensitive liposome-mediated drug-delivery systems in vivo have been developed to regulate the endocytotic transportation of drugs or protein [152]. Also, vinyl-type macromolecule-based hydrogels containing acid-degradable units can be incorporated to deliver and release protein-based vaccines upon pH change [153]. Recently cross-linked methacrylatated dextran-based hydrogels showing pH stimulus behaviour and colon-specific degradation were reported for the potential treatment of pathological conditions such as inflammatory disease, infection and carcinoma [154].
A change in temperature is also often used to trigger the release of drugs from polymeric carriers [155]. The most commonly exploited materials include poly(N-isopropylacrylamide) (PNIPAAM), as it exhibits LCST behaviours, or di-/triblock copolymer-based hydrogels made of PLA/PEG showing sol-to-gel transition under physiological conditions (37°C, pH 7.4; [139,156,157]). Exploration of PNIPAAM moieties for targeted delivery offers the advantages of good sensitivity, reversible transitions and low cytotoxicity when compared with many cationic polymers. Drug release can be readily tuned to vary with pH and temperature [158,159]. The degradation of these polymers can be altered by changing the hydrophilicity through alteration of the chemical structure of the PNIPAAM. In addition, most pH-sensitive materials mentioned above, including poly(propylene glycol) [160], poly(propylene fumarate) [161], poly(organophosphazenes) [162] and their derivatives, also show temperature-dependent gelation behaviours.
3.3.2. Protease-triggered release
Protease action can also be used as a triggering mechanism to initiate release on demand. Most popular systems are based on catalytic matrix metalloproteinase (MMP) interactions [163]. A number of MMP inhibitor-containing systems have been developed for cytostatic and anti-angiogenic agents. Also, small oligopeptide (GPLGVRG) molecules cleaved by MMP (specifically MMP-2), a protease upregulated in angiogenesis, invasion and metastasis, were developed and furthermore conjugated to polymers for in vivo cancer imaging [164]. Synthetic materials have also been used for the coupling and local delivery of BMP-2. PEG-based hydrogels containing pendant integrin-binding oligopeptide molecules (arginine–glycine–aspartic acid–serine–proline; RGD) can be formed using amine-functionalized MMP ligands as a cross-linker. Close proximity of cells to the gels was mediated by the pendant RGD peptides, and a recombinant human bone morphogenetic protein-2 (rhBMP-2) bound to the matrix was delivered to the site of bone defects [163]. The biodegradability of the synthetic matrix and its biofunctional characteristics can be tuned by chemical modification of the hydrogel network, and modulation of proteases to increase the efficiency of growth-factor delivery [33,49]. With a similar concept, VEGF-conjugated, biofunctionalized PEG-peptide hydrogels that release VEGF only upon local cellular demand were formed for local, controlled induction of angiogenesis [115]. Recently, iron oxide nanoparticles containing both cell-internalizing oligopeptides and PEG polymers tethered by an MMP-2-cleavable peptide substrate have been reported [165]. Once activated upon cleavage by MMP-2, the unveiled peptide domain associates with cell membranes, resulting in the penetration of nanoparticles into cells. Similarly, proteolytically degradable PEG hydrogel networks can also be used to incorporate and release growth factor proteins [49]. It has been recently demonstrated that a series of enzyme-cleavable linker-containing hydrogel beads can specifically respond to target enzymes for the controlled release of growth factors [166]. Hydrogel formation can also be triggered by enzyme-catalysed reactions. DNA hydrogels, formed by ligase-mediated cross-linking, can be easily moulded into desired shapes and sizes [167]. It was also reported that similar hydrogels can be constructed using DNA stem–loop structures [168].
3.3.3. Control over release with drugs, ions, light, external magnetic or electric field
Exciting progress has recently been made in release-on-demand drug-delivery systems in which cleavage can be initiated by small drugs, antigens [169] and antibiotics [165,170]. For example, antibiotic-detecting hydrogels have been developed for the inducible release and spatio-temporal control of entrapped human growth factors [170]. Hydrogels formed by the interaction between bacterial gyrase subunit B coupled to a polymer chain, and a coumermycin cross-linking unit changed to a sol state upon adding novobiocin, which interrupts their interaction, resulting in the release of VEGF121. Similarly, addition of calcium to hydrogels containing ion-binding proteins also can initiate a sol-to-gel transition, and change binding affinities for proteins [171].
There are elegant approaches for smart materials in which triggering can be induced by changes in ion concentration [171], light [172–174], electric fields [135,175–177], magnetic fields [178] and polysaccharides [169] that can be potentially applicable to growth factor-delivery systems. PEG-based hydrogels can be photodegradable, and channels formed by polymer degradation triggered by light can be used for the release of growth factors or migration of cells [179]. However, the majority of these systems to date are only prototypes, because commonly applied wavelength ranges or electric fields used for triggering are not suitable for in vivo use. Finally, the dynamic mechanical environment of the delivery site can also affect the release rate of growth factors [180]. This concept can be potentially used to design a triggering system to deliver bioactive molecules to locations under mechanical stress conditions (e.g. the heart).
In summary, these stimuli-sensitive delivery systems hold great promise as smart materials for factor release. The appropriate choice of triggering systems may enable on–off switches with fast and precise responses in tightly defined local areas. Applications of these systems will be enhanced by developing triggering in conditions applicable to in vivo use.
4. Conclusions and future directions
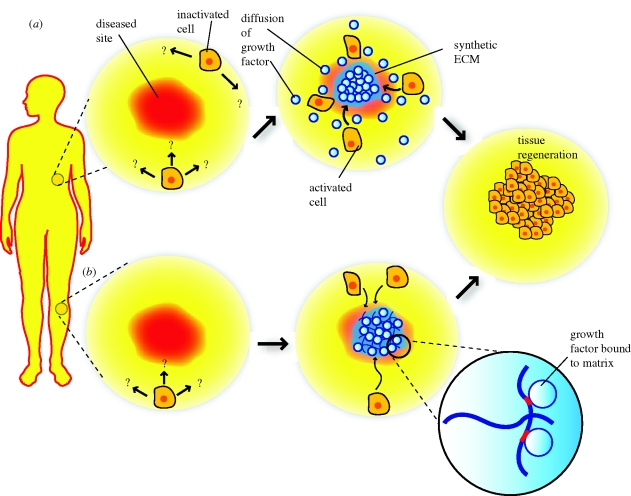
This review illustrates the utility of using material systems to obtain control over growth factor delivery, and the importance that spatio-temporal control has over these factors in tissue formation and regeneration. Synthetic ECM supporting cells transplanted or recruited to a target must provide an environment for cell development to be regulated appropriately and this function can be delicately tuned or engineered through suitable delivery of growth factors/morphogens. The polymer systems reviewed here tend to be quite versatile, and could easily be used in the future for more complex applications. For example, systems that can recruit cells into a scaffold, programme the cells appropriately and stimulate cells to leave the scaffolds may allow one to bypass the ex vivo culture and manipulation common to current cell therapies (figure 4). The polymer systems reviewed here also provide novel models to study and manipulate a multiplicity of physiological events that rely on the signalling of multiple growth factors.
Figure 4.
Sophisticated material systems capable of using growth factors to programme native cells. Polymer systems can be functionalized to recruit specific subsets of cells, where the cells are programmed to execute a specific task after leaving the material that can be to maintain, regenerate or even to destroy a particular tissue or subset of cells at a local or distant site.
Major advances have been made over recent years in the construction of polymer-based growth factor-delivery systems that allow the controlled release of growth factors, but there remain a number of challenges that will need to be addressed in the future. These include enhancing the stability of encapsulated proteins to allow release for extended times (e.g. weeks to months), the difficulty in scaling up certain approaches, determining the appropriate structure/compartmentalization of delivery materials to allow multiple factors to be released with distinct kinetics and the release of certain factors owing to their hydrophobic nature or strong charge–charge interactions between the polymers and the growth factors. Also, a major challenge is the adaptation of these approaches to clinical use. The majority of work carried out to date has been done with animal models and it is unclear how well much of this work will translate to the use of human recombinant growth factors in human patients. In a broader perspective, further advances in the field will rely on multi-disciplinary approaches that combine medicine, chemistry, engineering and pathology to develop effective strategies to treat complex wounds and pathologies.
Acknowledgements
The National Institutes of Health provides financial support for this research in the authors' laboratory.
References
- 1.Lanza R. P., Langer R. S., Vacanti J. 2007. Principles of tissue engineering. Amsterdam, The Netherlands: Elsevier Academic Press [Google Scholar]
- 2.Cross M., Dexter T. M. 1991. Growth factors in development, transformation, and tumorigenesis. Cell 64, 271–280 10.1016/0092-8674(91)90638-F (doi:10.1016/0092-8674(91)90638-F) [DOI] [PubMed] [Google Scholar]
- 3.Cao L., Arany P. R., Wang Y. S., Mooney D. J. 2009. Promoting angiogenesis via manipulation of VEGF responsiveness with notch signaling. Biomaterials 30, 4085–4093 10.1016/j.biomaterials.2009.04.051 (doi:10.1016/j.biomaterials.2009.04.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Discher D. E., Janmey P., Wang Y. L. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 10.1126/science.1116995 (doi:10.1126/science.1116995) [DOI] [PubMed] [Google Scholar]
- 5.Ramirez F., Rifkin D. B. 2003. Cell signaling events: a view from the matrix. Matrix Biol. 22, 101–107 10.1016/S0945-053X(03)00002-7 (doi:10.1016/S0945-053X(03)00002-7) [DOI] [PubMed] [Google Scholar]
- 6.Cohen G. B., Ren R., Baltimore D. 1995. Modular binding domains in signal transduction proteins. Cell 80, 237–248 10.1016/0092-8674(95)90406-9 (doi:10.1016/0092-8674(95)90406-9) [DOI] [PubMed] [Google Scholar]
- 7.Lamalice L., Le Boeuf F., Huot J. 2007. Endothelial cell migration during angiogenesis. Circ. Res. 100, 782–794 10.1161/01.RES.0000259593.07661.1e (doi:10.1161/01.RES.0000259593.07661.1e) [DOI] [PubMed] [Google Scholar]
- 8.Cao L., Mooney D. J. 2007. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv. Drug Deliv. Rev. 59, 1340–1350 10.1016/j.addr.2007.08.012 (doi:10.1016/j.addr.2007.08.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W., Giancotti F. G. 2004. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 5, 816–826 10.1038/nrm1490 (doi:10.1038/nrm1490) [DOI] [PubMed] [Google Scholar]
- 10.Tucker G. C. 2006. Integrins: molecular targets in cancer therapy. Curr. Oncol. Rep. 8, 96–103 10.1007/s11912-006-0043-3 (doi:10.1007/s11912-006-0043-3) [DOI] [PubMed] [Google Scholar]
- 11.Kim J. H., Kushiro K., Graham N. A., Asthagiri A. R. 2009. Tunable interplay between epidermal growth factor and cell–cell contact governs the spatial dynamics of epithelial growth. Proc. Natl. Acad. Sci. USA 106, 11 149–11 153 10.1073/pnas.0812651106 (doi:10.1073/pnas.0812651106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagisawa-Miwa A., et al. 1992. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science 257, 1401–1403 10.1126/science.1382313 (doi:10.1126/science.1382313) [DOI] [PubMed] [Google Scholar]
- 13.Bauters C., Asahara T., Zheng L. P., Takeshita S., Bunting S., Ferrara N., Symes J. F., Isner J. M. 1994. Physiological assessment of augmented vascularity induced by VEGF in ischemic rabbit hindlimb. Am. J. Physiol. 267, H1263–H1271 [DOI] [PubMed] [Google Scholar]
- 14.Freedman S. B., Isner J. M. 2002. Therapeutic angiogenesis for coronary artery disease. Ann. Intern. Med. 136, 54–71 [DOI] [PubMed] [Google Scholar]
- 15.Takeshita S., Pu L. Q., Stein L. A., Sniderman A. D., Bunting S., Ferrara N., Isner J. M., Symes J. F. 1994. Intramuscular administration of vascular endothelial growth factor induces dose-dependent collateral artery augmentation in a rabbit model of chronic limb ischemia. Circulation 90, II228–II234 [PubMed] [Google Scholar]
- 16.Takeshita S., Zheng L. P., Brogi E., Kearney M., Pu L. Q., Bunting S., Ferrara N., Symes J. F., Isner J. M. 1994. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J. Clin. Invest. 93, 662–670 10.1172/JCI117018 (doi:10.1172/JCI117018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosengart T. K., Lee L. Y., Patel S. R., Kligfield P. D., Okin P. M., Hackett N. R., Isom O. W., Crystal R. G. 1999. Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA. Ann. Surg. 230, 466–470 (Discussion 470–472.) (doi:10.1097/00000658-199910000-00002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacher B., Pecher P., Von Specht B. U., Stegmann T. 1998. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation 97, 645–650 [DOI] [PubMed] [Google Scholar]
- 19.Simons M., et al. 2002. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation 105, 788–793 10.1161/hc0802.104407 (doi:10.1161/hc0802.104407) [DOI] [PubMed] [Google Scholar]
- 20.Krishnamurthy R., Manning M. C. 2002. The stability factor: importance in formulation development. Curr. Pharm. Biotechnol. 3, 361–371 10.2174/1389201023378229 (doi:10.2174/1389201023378229) [DOI] [PubMed] [Google Scholar]
- 21.Manning M. C., Patel K., Borchardt R. T. 1989. Stability of protein pharmaceuticals. Pharm. Res. 6, 903–918 10.1023/A:1015929109894 (doi:10.1023/A:1015929109894) [DOI] [PubMed] [Google Scholar]
- 22.Eppler S. M., Combs D. L., Henry T. D., Lopez J. J., Ellis S. G., Yi J. H., Annex B. H., Mccluskey E. R., Zioncheck T. F. 2002. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin. Pharmacol. Ther. 72, 20–32 10.1067/mcp.2002.126179 (doi:10.1067/mcp.2002.126179) [DOI] [PubMed] [Google Scholar]
- 23.Henry T. D., et al. 2003. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 107, 1359–1365 10.1161/01.CIR.0000061911.47710.8A (doi:10.1161/01.CIR.0000061911.47710.8A) [DOI] [PubMed] [Google Scholar]
- 24.Simons M., Ware J. A. 2003. Therapeutic angiogenesis in cardiovascular disease. Nat. Rev. Drug Discov. 2, 863–871 10.1038/nrd1226 (doi:10.1038/nrd1226) [DOI] [PubMed] [Google Scholar]
- 25.Fischbach C., Mooney D. J. 2007. Polymers for pro- and anti-angiogenic therapy. Biomaterials 28, 2069–2076 10.1016/j.biomaterials.2006.12.029 (doi:10.1016/j.biomaterials.2006.12.029) [DOI] [PubMed] [Google Scholar]
- 26.Mitragotri S., Lahann J. 2009. Physical approaches to biomaterial design. Nat. Mater. 8, 15–23 10.1038/nmat2344 (doi:10.1038/nmat2344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson T. P., Peters M. C., Ennett A. B., Mooney D. J. 2001. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19, 1029–1034 10.1038/nbt1101-1029 (doi:10.1038/nbt1101-1029) [DOI] [PubMed] [Google Scholar]
- 28.Boontheekul T., Hill E. E., Kong H. J., Mooney D. J. 2007. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 13, 1431–1442 10.1089/ten.2006.0356 (doi:10.1089/ten.2006.0356) [DOI] [PubMed] [Google Scholar]
- 29.Datta N., Pham Q. P., Sharma U., Sikavitsas V. I., Jansen J. A., Mikos A. G. 2006. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc. Natl Acad. Sci. USA 103, 2488–2493 10.1073/pnas.0505661103 (doi:10.1073/pnas.0505661103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng Y., Dalhaimer P., Cai S., Tsai R., Tewari M., Minko T., Discher D. E. 2007. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2, 249–255 10.1038/nnano.2007.70 (doi:10.1038/nnano.2007.70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lutolf M. P., Lauer-Fields J. L., Schmoekel H. G., Metters A. T., Weber F. E., Fields G. B., Hubbell J. A. 2003. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl Acad. Sci. USA 100, 5413–5418 10.1073/pnas.0737381100 (doi:10.1073/pnas.0737381100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raeber G. P., Lutolf M. P., Hubbell J. A. 2005. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys. J. 89, 1374–1388 10.1529/biophysj.104.050682 (doi:10.1529/biophysj.104.050682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizzi S. C., Ehrbar M., Halstenberg S., Raeber G. P., Schmoekel H. G., Hagenmuller H., Muller R., Weber F. E., Hubbell J. A. 2006. Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part II. Biofunctional characteristics. Biomacromolecules 7, 3019–3029 10.1021/bm060504a (doi:10.1021/bm060504a) [DOI] [PubMed] [Google Scholar]
- 34.Carmeliet P. 2003. Angiogenesis in health and disease. Nat. Med. 9, 653–660 10.1038/nm0603-653 (doi:10.1038/nm0603-653) [DOI] [PubMed] [Google Scholar]
- 35.Carmeliet P. 2005. Angiogenesis in life, disease and medicine. Nature 438, 932–936 10.1038/nature04478 (doi:10.1038/nature04478) [DOI] [PubMed] [Google Scholar]
- 36.Risau W. 1997. Mechanisms of angiogenesis. Nature 386, 671–674 10.1038/386671a0 (doi:10.1038/386671a0) [DOI] [PubMed] [Google Scholar]
- 37.Chapanian R., Amsden B. G. 2010. Combined and sequential delivery of bioactive VEGF(165) and HGF from poly(trimethylene carbonate) based photo-cross-linked elastomers. J. Control. Release 143, 53–63 10.1016/j.jconrel.2009.11.025 (doi:10.1016/j.jconrel.2009.11.025) [DOI] [PubMed] [Google Scholar]
- 38.Chen R. R., Silva E. A., Yuen W. W., Brock A. A., Fischbach C., Lin A. S., Guldberg R. E., Mooney D. J. 2007. Integrated approach to designing growth factor delivery systems. FASEB J. 21, 3896–3903 10.1096/fj.06-7873com (doi:10.1096/fj.06-7873com) [DOI] [PubMed] [Google Scholar]
- 39.Marui A., et al. 2005. Simultaneous application of basic fibroblast growth factor and hepatocyte growth factor to enhance the blood vessels formation. J. Vasc. Surg. 41, 82–90 10.1016/j.jvs.2004.10.029 (doi:10.1016/j.jvs.2004.10.029) [DOI] [PubMed] [Google Scholar]
- 40.Simmons C. A., Alsberg E., Hsiong S., Kim W. J., Mooney D. J. 2004. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 35, 562–569 10.1016/j.bone.2004.02.027 (doi:10.1016/j.bone.2004.02.027) [DOI] [PubMed] [Google Scholar]
- 41.Basmanav F. B., Kose G. T., Hasirci V. 2008. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials 29, 4195–4204 [DOI] [PubMed] [Google Scholar]
- 42.Yilgor P., Hasirci N., Hasirci V. 2010. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J. Biomed. Mater. Res. A 93, 528–536 [DOI] [PubMed] [Google Scholar]
- 43.Yilgor P., Tuzlakoglu K., Reis R. L., Hasirci N., Hasirci V. 2009. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials 30, 3551–3559 10.1016/j.biomaterials.2009.03.024 (doi:10.1016/j.biomaterials.2009.03.024) [DOI] [PubMed] [Google Scholar]
- 44.Patel Z. S., Young S., Tabata Y., Jansen J. A., Wong M. E., Mikos A. G. 2008. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43, 931–940 10.1016/j.bone.2008.06.019 (doi:10.1016/j.bone.2008.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raiche A. T., Puleo D. A. 2004. In vitro effects of combined and sequential delivery of two bone growth factors. Biomaterials 25, 677–685 10.1016/S0142-9612(03)00564-7 (doi:10.1016/S0142-9612(03)00564-7) [DOI] [PubMed] [Google Scholar]
- 46.Freeman I., Cohen S. 2009. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials 30, 2122–2131 10.1016/j.biomaterials.2008.12.057 (doi:10.1016/j.biomaterials.2008.12.057) [DOI] [PubMed] [Google Scholar]
- 47.Freeman I., Kedem A., Cohen S. 2008. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials 29, 3260–3268 10.1016/j.biomaterials.2008.04.025 (doi:10.1016/j.biomaterials.2008.04.025) [DOI] [PubMed] [Google Scholar]
- 48.Ehrbar M., et al. 2004. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ. Res. 94, 1124–1132 10.1161/01.RES.0000126411.29641.08 (doi:10.1161/01.RES.0000126411.29641.08) [DOI] [PubMed] [Google Scholar]
- 49.Ehrbar M., Rizzi S. C., Hlushchuk R., Djonov V., Zisch A. H., Hubbell J. A., Weber F. E., Lutolf M. P. 2007. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials 28, 3856–3866 10.1016/j.biomaterials.2007.03.027 (doi:10.1016/j.biomaterials.2007.03.027) [DOI] [PubMed] [Google Scholar]
- 50.Zisch A. H., Schenk U., Schense J. C., Sakiyama-Elbert S. E., Hubbell J. A. 2001. Covalently conjugated VEGF–fibrin matrices for endothelialization. J. Control. Release 72, 101–113 10.1016/S0168-3659(01)00266-8 (doi:10.1016/S0168-3659(01)00266-8) [DOI] [PubMed] [Google Scholar]
- 51.Dawson E., Mapili G., Erickson K., Taqvi S., Roy K. 2008. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 60, 215–228 10.1016/j.addr.2007.08.037 (doi:10.1016/j.addr.2007.08.037) [DOI] [PubMed] [Google Scholar]
- 52.Lutolf M. P., Hubbell J. A. 2005. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 23, 47–55 10.1038/nbt1055 (doi:10.1038/nbt1055) [DOI] [PubMed] [Google Scholar]
- 53.Cross M. J., Dixelius J., Matsumoto T., Claesson-Welsh L. 2003. VEGF-receptor signal transduction. Trends Biochem. Sci. 28, 488–494 10.1016/S0968-0004(03)00193-2 (doi:10.1016/S0968-0004(03)00193-2) [DOI] [PubMed] [Google Scholar]
- 54.Hood J. D., Frausto R., Kiosses W. B., Schwartz M. A., Cheresh D. A. 2003. Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J. Cell Biol. 162, 933–943 10.1083/jcb.200304105 (doi:10.1083/jcb.200304105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hutchings H., Ortega N., Plouet J. 2003. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J. 17, 1520–1522 [DOI] [PubMed] [Google Scholar]
- 56.Tsou R., Isik F. F. 2001. Integrin activation is required for VEGF and FGF receptor protein presence on human microvascular endothelial cells. Mol. Cell. Biochem. 224, 81–89 10.1023/A:1011947301849 (doi:10.1023/A:1011947301849) [DOI] [PubMed] [Google Scholar]
- 57.Hall H., Hubbell J. A. 2004. Matrix-bound sixth Ig-like domain of cell adhesion molecule L1 acts as an angiogenic factor by ligating alphavbeta3-integrin and activating VEGF-R2. Microvasc. Res. 68, 169–178 10.1016/j.mvr.2004.07.001 (doi:10.1016/j.mvr.2004.07.001) [DOI] [PubMed] [Google Scholar]
- 58.Li B., Davidson J. M., Guelcher S. A. 2009. The effect of the local delivery of platelet-derived growth factor from reactive two-component polyurethane scaffolds on the healing in rat skin excisional wounds. Biomaterials 30, 3486–3494 10.1016/j.biomaterials.2009.03.008 (doi:10.1016/j.biomaterials.2009.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X., Won Y., Ma P. X. 2005. Surface modification of interconnected porous scaffolds. J. Biomed. Mater. Res. A 74, 84–91 10.1002/jbm.a.30367 (doi:10.1002/jbm.a.30367) [DOI] [PubMed] [Google Scholar]
- 60.Park H., Temenoff J. S., Holland T. A., Tabata Y., Mikos A. G. 2005. Delivery of TGF-beta1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials 26, 7095–7103 10.1016/j.biomaterials.2005.05.083 (doi:10.1016/j.biomaterials.2005.05.083) [DOI] [PubMed] [Google Scholar]
- 61.Ikeuchi M., Dohi Y., Horiuchi K., Ohgushi H., Noshi T., Yoshikawa T., Yamamoto K., Sugimura M. 2002. Recombinant human bone morphogenetic protein-2 promotes osteogenesis within atelopeptide type I collagen solution by combination with rat cultured marrow cells. J. Biomed. Mater. Res. 60, 61–69 10.1002/jbm.1281 (doi:10.1002/jbm.1281) [DOI] [PubMed] [Google Scholar]
- 62.Silva A. K., Richard C., Bessodes M., Scherman D., Merten O. W. 2009. Growth factor delivery approaches in hydrogels. Biomacromolecules 10, 9–18 10.1021/bm801103c (doi:10.1021/bm801103c) [DOI] [PubMed] [Google Scholar]
- 63.Schense J. C., Hubbell J. A. 1999. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug. Chem. 10, 75–81 10.1021/bc9800769 (doi:10.1021/bc9800769) [DOI] [PubMed] [Google Scholar]
- 64.Sakiyama-Elbert S. E., Panitch A., Hubbell J. A. 2001. Development of growth factor fusion proteins for cell-triggered drug delivery. FASEB J. 15, 1300–1302 [DOI] [PubMed] [Google Scholar]
- 65.Schmoekel H. G., Weber F. E., Schense J. C., Gratz K. W., Schawalder P., Hubbell J. A. 2005. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol. Bioeng. 89, 253–262 10.1002/bit.20168 (doi:10.1002/bit.20168) [DOI] [PubMed] [Google Scholar]
- 66.Ehrbar M., Metters A., Zammaretti P., Hubbell J. A., Zisch A. H. 2005. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J. Control. Release 101, 93–109 10.1016/j.jconrel.2004.07.018 (doi:10.1016/j.jconrel.2004.07.018) [DOI] [PubMed] [Google Scholar]
- 67.Walpoth B. H., et al. 2007. Enhanced intimal thickening of expanded polytetrafluoroethylene grafts coated with fibrin or fibrin-releasing vascular endothelial growth factor in the pig carotid artery interposition model. J. Thorac. Cardiovasc. Surg. 133, 1163–1170 10.1016/j.jtcvs.2007.01.029 (doi:10.1016/j.jtcvs.2007.01.029) [DOI] [PubMed] [Google Scholar]
- 68.Sakiyama-Elbert S. E., Hubbell J. A. 2000. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J. Control. Release 65, 389–402 10.1016/S0168-3659(99)00221-7 (doi:10.1016/S0168-3659(99)00221-7) [DOI] [PubMed] [Google Scholar]
- 69.Nillesen S. T., Geutjes P. J., Wismans R., Schalkwijk J., Daamen W. F., Van Kuppevelt T. H. 2007. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials 28, 1123–1131 10.1016/j.biomaterials.2006.10.029 (doi:10.1016/j.biomaterials.2006.10.029) [DOI] [PubMed] [Google Scholar]
- 70.Ruppert R., Hoffmann E., Sebald W. 1996. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur. J. Biochem. 237, 295–302 10.1111/j.1432-1033.1996.0295n.x (doi:10.1111/j.1432-1033.1996.0295n.x) [DOI] [PubMed] [Google Scholar]
- 71.Sakiyama-Elbert S. E., Hubbell J. A. 2000. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J. Control. Release 69, 149–158 10.1016/S0168-3659(00)00296-0 (doi:10.1016/S0168-3659(00)00296-0) [DOI] [PubMed] [Google Scholar]
- 72.Scheufler C., Sebald W., Hulsmeyer M. 1999. Crystal structure of human bone morphogenetic protein-2 at 2.7 A resolution. J. Mol. Biol. 287, 103–115 10.1006/jmbi.1999.2590 (doi:10.1006/jmbi.1999.2590) [DOI] [PubMed] [Google Scholar]
- 73.Sieron A. L., Louneva N., Fertala A. 2002. Site-specific interaction of bone morphogenetic protein 2 with procollagen II. Cytokine 18, 214–221 10.1006/cyto.2002.1035 (doi:10.1006/cyto.2002.1035) [DOI] [PubMed] [Google Scholar]
- 74.Wissink M. J., Beernink R., Pieper J. S., Poot A. A., Engbers G. H., Beugeling T., Van Aken W. G., Feijen J. 2001. Binding and release of basic fibroblast growth factor from heparinized collagen matrices. Biomaterials 22, 2291–2299 10.1016/S0142-9612(00)00418-X (doi:10.1016/S0142-9612(00)00418-X) [DOI] [PubMed] [Google Scholar]
- 75.Yoon J. J., Chung H. J., Park T. G. 2007. Photo-crosslinkable and biodegradable Pluronic/heparin hydrogels for local and sustained delivery of angiogenic growth factor. J. Biomed. Mater. Res. A 83, 597–605 10.1002/jbm.a.31271 (doi:10.1002/jbm.a.31271) [DOI] [PubMed] [Google Scholar]
- 76.Lin C. C., Anseth K. S. 2009. Controlling affinity binding with peptide-functionalized poly(ethylene glycol) hydrogels. Adv. Funct. Mater. 19, 2325. 10.1002/adfm.200900107 (doi:10.1002/adfm.200900107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin C. C., Boyer P. D., Aimetti A. A., Anseth K. S. 2010. Regulating MCP-1 diffusion in affinity hydrogels for enhancing immuno-isolation. J. Control. Release 142, 384–391 10.1016/j.jconrel.2009.11.022 (doi:10.1016/j.jconrel.2009.11.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maynard H. D., Hubbell J. A. 2005. Discovery of a sulfated tetrapeptide that binds to vascular endothelial growth factor. Acta Biomater. 1, 451–459 10.1016/j.actbio.2005.04.004 (doi:10.1016/j.actbio.2005.04.004) [DOI] [PubMed] [Google Scholar]
- 79.Soontornworajit B., Zhou J., Shaw M. T., Fan T. H., Wang Y. 2010. Hydrogel functionalization with DNA aptamers for sustained PDGF-BB release. Chem. Commun. 46, 1857–1859 10.1039/b924909e (doi:10.1039/b924909e) [DOI] [PubMed] [Google Scholar]
- 80.Willerth S. M., Johnson P. J., Maxwell D. J., Parsons S. R., Doukas M. E., Sakiyama-Elbert S. E. 2007. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J. Biomed. Mater. Res. A 80, 13–23 10.1002/jbm.a.30844 (doi:10.1002/jbm.a.30844) [DOI] [PubMed] [Google Scholar]
- 81.Hill E., Boontheekul T., Mooney D. J. 2006. Regulating activation of transplanted cells controls tissue regeneration. Proc. Natl Acad. Sci. USA 103, 2494–2499 10.1073/pnas.0506004103 (doi:10.1073/pnas.0506004103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silva E. A., Kim E. S., Kong H. J., Mooney D. J. 2008. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc. Natl Acad. Sci. USA 105, 14 347–14 352 10.1073/pnas.0803873105 (doi:10.1073/pnas.0803873105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin C. C., Metters A. T., Anseth K. S. 2009. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials 30, 4907–4914 10.1016/j.biomaterials.2009.05.083 (doi:10.1016/j.biomaterials.2009.05.083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davis M. E., Hsieh P. C., Takahashi T., Song Q., Zhang S., Kamm R. D., Grodzinsky A. J., Anversa P., Lee R. T. 2006. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc. Natl Acad. Sci. USA 103, 8155–8160 10.1073/pnas.0602877103 (doi:10.1073/pnas.0602877103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ito Y. 2008. Covalently immobilized biosignal molecule materials for tissue engineering. Soft Matter 4, 46–56 10.1039/b708359a (doi:10.1039/b708359a) [DOI] [PubMed] [Google Scholar]
- 86.Kuhl P. R., Griffithcima L. G. 1996. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat. Med. 2, 1022–1027 10.1038/nm0996-1022 (doi:10.1038/nm0996-1022) [DOI] [PubMed] [Google Scholar]
- 87.Mann B. K., Schmedlen R. H., West J. L. 2001. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials 22, 439–444 10.1016/S0142-9612(00)00196-4 (doi:10.1016/S0142-9612(00)00196-4) [DOI] [PubMed] [Google Scholar]
- 88.Harris L. D., Kim B. S., Mooney D. J. 1998. Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 42, 396–402 (doi:10.1002/(SICI)1097-4636(19981205)42:3<396::AID-JBM7>3.0.CO;2-E) [DOI] [PubMed] [Google Scholar]
- 89.Mooney D. J., Baldwin D. F., Suh N. P., Vacanti J. P., Langer R. 1996. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials 17, 1417–1422 10.1016/0142-9612(96)87284-X (doi:10.1016/0142-9612(96)87284-X) [DOI] [PubMed] [Google Scholar]
- 90.Sheridan M. H., Shea L. D., Peters M. C., Mooney D. J. 2000. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J. Control. Release 64, 91–102 10.1016/S0168-3659(99)00138-8 (doi:10.1016/S0168-3659(99)00138-8) [DOI] [PubMed] [Google Scholar]
- 91.Chen R. R., Mooney D. J. 2003. Polymeric growth factor delivery strategies for tissue engineering. Pharm. Res. 20, 1103–1112 10.1023/A:1025034925152 (doi:10.1023/A:1025034925152) [DOI] [PubMed] [Google Scholar]
- 92.Desai M. P., Labhasetwar V., Walter E., Levy R. J., Amidon G. L. 1997. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 14, 1568–1573 10.1023/A:1012126301290 (doi:10.1023/A:1012126301290) [DOI] [PubMed] [Google Scholar]
- 93.Panyam J., Labhasetwar V. 2003. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 55, 329–347 10.1016/S0169-409X(02)00228-4 (doi:10.1016/S0169-409X(02)00228-4) [DOI] [PubMed] [Google Scholar]
- 94.Chen F. M., Chen R., Wang X. J., Sun H. H., Wu Z. F. 2009. In vitro cellular responses to scaffolds containing two microencapulated growth factors. Biomaterials 30, 5215–5224 10.1016/j.biomaterials.2009.06.009 (doi:10.1016/j.biomaterials.2009.06.009) [DOI] [PubMed] [Google Scholar]
- 95.Hubbell J. A. 1999. Bioactive biomaterials. Curr. Opin. Biotechnol. 10, 123–129 10.1016/S0958-1669(99)80021-4 (doi:10.1016/S0958-1669(99)80021-4) [DOI] [PubMed] [Google Scholar]
- 96.Meinel L., et al. 2003. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone 33, 660–672 10.1016/S8756-3282(03)00207-2 (doi:10.1016/S8756-3282(03)00207-2) [DOI] [PubMed] [Google Scholar]
- 97.Shin H., Jo S., Mikos A. G. 2003. Biomimetic materials for tissue engineering. Biomaterials 24, 4353–4364 10.1016/S0142-9612(03)00339-9 (doi:10.1016/S0142-9612(03)00339-9) [DOI] [PubMed] [Google Scholar]
- 98.Siccardi F. J. 1975. Considerations concerning the host–parasite relationship of Escherichia coli in poultry. Am. J. Vet. Res. 36, 572. [PubMed] [Google Scholar]
- 99.Wenk E., Meinel A. J., Wildy S., Merkle H. P., Meinel L. 2009. Microporous silk fibroin scaffolds embedding PLGA microparticles for controlled growth factor delivery in tissue engineering. Biomaterials 30, 2571–2581 10.1016/j.biomaterials.2008.12.073 (doi:10.1016/j.biomaterials.2008.12.073) [DOI] [PubMed] [Google Scholar]
- 100.Meinel L., Illi O. E., Zapf J., Malfanti M., Peter Merkle H., Gander B. 2001. Stabilizing insulin-like growth factor-I in poly(D,L-lactide-co-glycolide) microspheres. J. Control. Release 70, 193–202 10.1016/S0168-3659(00)00352-7 (doi:10.1016/S0168-3659(00)00352-7) [DOI] [PubMed] [Google Scholar]
- 101.Rocha F. G., Sundback C. A., Krebs N. J., Leach J. K., Mooney D. J., Ashley S. W., Vacanti J. P., Whang E. E. 2008. The effect of sustained delivery of vascular endothelial growth factor on angiogenesis in tissue-engineered intestine. Biomaterials 29, 2884–2890 10.1016/j.biomaterials.2008.03.026 (doi:10.1016/j.biomaterials.2008.03.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu X. H., Wang C. H., Tong Y. W. 2009. In vitro characterization of hepatocyte growth factor release from PHBV/PLGA microsphere scaffold. J. Biomed. Mater. Res. A 89, 411–423 10.1002/jbm.a.31978 (doi:10.1002/jbm.a.31978) [DOI] [PubMed] [Google Scholar]
- 103.Jain R. A. 2000. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 21, 2475–2490 10.1016/S0142-9612(00)00115-0 (doi:10.1016/S0142-9612(00)00115-0) [DOI] [PubMed] [Google Scholar]
- 104.Johnson M. R., Lee H. J., Bellamkonda R. V., Guldberg R. E. 2009. Sustained release of BMP-2 in a lipid-based microtube vehicle. Acta Biomater. 5, 23–28 10.1016/j.actbio.2008.09.001 (doi:10.1016/j.actbio.2008.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scott R. C., Rosano J. M., Ivanov Z., Wang B., Chong P. L., Issekutz A. C., Crabbe D. L., Kiani M. F. 2009. Targeting VEGF-encapsulated immunoliposomes to MI heart improves vascularity and cardiac function. FASEB J. 23, 3361–3367 10.1096/fj.08-127373 (doi:10.1096/fj.08-127373) [DOI] [PubMed] [Google Scholar]
- 106.Shive M. S., Anderson J. M. 1997. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 28, 5–24 [DOI] [PubMed] [Google Scholar]
- 107.Chen R. R., Silva E. A., Yuen W. W., Mooney D. J. 2007. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm. Res. 24, 258–264 10.1007/s11095-006-9173-4 (doi:10.1007/s11095-006-9173-4) [DOI] [PubMed] [Google Scholar]
- 108.Pepper M. S., Ferrara N., Orci L., Montesano R. 1992. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem. Biophys. Res. Commun. 189, 824–831 10.1016/0006-291X(92)92277-5 (doi:10.1016/0006-291X(92)92277-5) [DOI] [PubMed] [Google Scholar]
- 109.Burdick J. A., Ward M., Liang E., Young M. J., Langer R. 2006. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials 27, 452–459 10.1016/j.biomaterials.2005.06.034 (doi:10.1016/j.biomaterials.2005.06.034) [DOI] [PubMed] [Google Scholar]
- 110.Discher D. E., Mooney D. J., Zandstra P. W. 2009. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 10.1126/science.1171643 (doi:10.1126/science.1171643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ali O. A., Huebsch N., Cao L., Dranoff G., Mooney D. J. 2009. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 8, 151–158 10.1038/nmat2357 (doi:10.1038/nmat2357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boontheekul T., Mooney D. J. 2003. Protein-based signaling systems in tissue engineering. Curr. Opin. Biotechnol. 14, 559–565 10.1016/j.copbio.2003.08.004 (doi:10.1016/j.copbio.2003.08.004) [DOI] [PubMed] [Google Scholar]
- 113.Santo V. E., Frias A. M., Carida M., Cancedda R., Gomes M. E., Mano J. F., Reis R. L. 2009. Carrageenan-based hydrogels for the controlled delivery of PDGF-BB in bone tissue engineering applications. Biomacromolecules 10, 1392–1401 10.1021/bm8014973 (doi:10.1021/bm8014973) [DOI] [PubMed] [Google Scholar]
- 114.Lee K. Y., Mooney D. J. 2001. Hydrogels for tissue engineering. Chem. Rev. 101, 1869–1879 10.1021/cr000108x (doi:10.1021/cr000108x) [DOI] [PubMed] [Google Scholar]
- 115.Zisch A. H., et al. 2003. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 17, 2260–2262 [DOI] [PubMed] [Google Scholar]
- 116.Holland T. A., Tabata Y., Mikos A. G. 2005. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Control. Release 101, 111–125 10.1016/j.jconrel.2004.07.004 (doi:10.1016/j.jconrel.2004.07.004) [DOI] [PubMed] [Google Scholar]
- 117.Tabata Y., Ikada Y., Morimoto K., Katsumata H., Yabuta T., Iwanaga K., Kakemi M. 1999. Surfactant-free preparation of biodegradable hydrogel microspheres for protein release. J. Bioactive Compatible Polym. 14, 371–384 [Google Scholar]
- 118.Mikos A. G., Lyman M. D., Freed L. E., Langer R. 1994. Wetting of poly(L-lactic acid) and poly(DL-lactic-co-glycolic acid) foams for tissue culture. Biomaterials 15, 55–58 10.1016/0142-9612(94)90197-X (doi:10.1016/0142-9612(94)90197-X) [DOI] [PubMed] [Google Scholar]
- 119.Fournier E., Passirani C., Montero-Menei C. N., Benoit J. P. 2003. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials 24, 3311–3331 10.1016/S0142-9612(03)00161-3 (doi:10.1016/S0142-9612(03)00161-3) [DOI] [PubMed] [Google Scholar]
- 120.Bouhadir K. H., Lee K. Y., Alsberg E., Damm K. L., Anderson K. W., Mooney D. J. 2001. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 17, 945–950 10.1021/bp010070p (doi:10.1021/bp010070p) [DOI] [PubMed] [Google Scholar]
- 121.Boontheekul T., Kong H. J., Mooney D. J. 2005. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 26, 2455–2465 10.1016/j.biomaterials.2004.06.044 (doi:10.1016/j.biomaterials.2004.06.044) [DOI] [PubMed] [Google Scholar]
- 122.De Souza R., Zahedi P., Allen C. J., Piquette-Miller M. 2009. Biocompatibility of injectable chitosan–phospholipid implant systems. Biomaterials 30, 3818–3824 10.1016/j.biomaterials.2009.04.003 (doi:10.1016/j.biomaterials.2009.04.003) [DOI] [PubMed] [Google Scholar]
- 123.Gupta D., Tator C. H., Shoichet M. S. 2006. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials 27, 2370–2379 10.1016/j.biomaterials.2005.11.015 (doi:10.1016/j.biomaterials.2005.11.015) [DOI] [PubMed] [Google Scholar]
- 124.Jimenez Hamann M. C., Tator C. H., Shoichet M. S. 2005. Injectable intrathecal delivery system for localized administration of EGF and FGF-2 to the injured rat spinal cord. Exp. Neurol. 194, 106–119 [DOI] [PubMed] [Google Scholar]
- 125.Jimenez Hamann M. C., Tsai E. C., Tator C. H., Shoichet M. S. 2003. Novel intrathecal delivery system for treatment of spinal cord injury. Exp. Neurol. 182, 300–309 10.1016/S0014-4886(03)00040-2 (doi:10.1016/S0014-4886(03)00040-2) [DOI] [PubMed] [Google Scholar]
- 126.Kang C. E., Poon P. C., Tator C. H., Shoichet M. S. 2009. A new paradigm for local and sustained release of therapeutic molecules to the injured spinal cord for neuroprotection and tissue repair. Tissue Eng. Part A 15, 595–604 10.1089/ten.tea.2007.0349 (doi:10.1089/ten.tea.2007.0349) [DOI] [PubMed] [Google Scholar]
- 127.Kapur T. A., Shoichet M. S. 2004. Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. J. Biomed. Mater. Res. A 68, 235–243 [DOI] [PubMed] [Google Scholar]
- 128.Akiyoshi K., Deguchi S., Moriguchi N., Yamaguchi S., Sunamoto J. 1993. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules 26, 3062–3068 10.1021/ma00064a011 (doi:10.1021/ma00064a011) [DOI] [Google Scholar]
- 129.Kageyama S., et al. 2008. Humoral immune responses in patients vaccinated with 1-146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci. 99, 601–607 10.1111/j.1349-7006.2007.00705.x (doi:10.1111/j.1349-7006.2007.00705.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kato N., Hasegawa U., Morimoto N., Saita Y., Nakashima K., Ezura Y., Kurosawa H., Akiyoshi K., Noda M. 2007. Nanogel-based delivery system enhances PGE2 effects on bone formation. J. Cell. Biochem. 101, 1063–1070 10.1002/jcb.21160 (doi:10.1002/jcb.21160) [DOI] [PubMed] [Google Scholar]
- 131.Morimoto N., Endo T., Iwasaki Y., Akiyoshi K. 2005. Design of hybrid hydrogels with self-assembled nanogels as cross-linkers: interaction with proteins and chaperone-like activity. Biomacromolecules 6, 1829–1834 10.1021/bm050156x (doi:10.1021/bm050156x) [DOI] [PubMed] [Google Scholar]
- 132.Nomura Y., Sasaki Y., Takagi M., Narita T., Aoyama Y., Akiyoshi K. 2005. Thermoresponsive controlled association of protein with a dynamic nanogel of hydrophobized polysaccharide and cyclodextrin: heat shock protein-like activity of artificial molecular chaperone. Biomacromolecules 6, 447–452 10.1021/bm049501t (doi:10.1021/bm049501t) [DOI] [PubMed] [Google Scholar]
- 133.Silva E. A., Mooney D. J. 2004. Synthetic extracellular matrices for tissue engineering and regeneration. Curr. Top. Dev. Biol. 64, 181–205 10.1016/S0070-2153(04)64008-7 (doi:10.1016/S0070-2153(04)64008-7) [DOI] [PubMed] [Google Scholar]
- 134.Ulijn R. V., Bibi N., Jayawarna V., Thornton P. D., Todd S. J., Mart R. J., Smith A. M., Gough J. E. 2007. Bioresponsive hydrogels. Mater. Today 10, 40–48 10.1016/S1369-7021(07)70049-4 (doi:10.1016/S1369-7021(07)70049-4) [DOI] [Google Scholar]
- 135.Casanovas J., Zanuy D., Aleman C. 2006. Conducting polymer actuator mechanism based on the conformational flexibility of calix[4]arene. Angew. Chem. Int. Ed. Engl. 45, 1103–1105 10.1002/anie.200503304 (doi:10.1002/anie.200503304) [DOI] [PubMed] [Google Scholar]
- 136.Domb A. J., Turovsky L., Nudelman R. 1994. Chemical interactions between drugs containing reactive amines with hydrolyzable insoluble biopolymers in aqueous solutions. Pharm. Res. 11, 865–868 10.1023/A:1018985909777 (doi:10.1023/A:1018985909777) [DOI] [PubMed] [Google Scholar]
- 137.Fu K., Pack D. W., Klibanov A. M., Langer R. 2000. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm. Res. 17, 100–106 10.1023/A:1007582911958 (doi:10.1023/A:1007582911958) [DOI] [PubMed] [Google Scholar]
- 138.Middaugh C. R., Evans R. K., Montgomery D. L., Casimiro D. R. 1998. Analysis of plasmid DNA from a pharmaceutical perspective. J. Pharm. Sci. 87, 130–146 10.1021/js970367a (doi:10.1021/js970367a) [DOI] [PubMed] [Google Scholar]
- 139.Shim W. S., Kim S. W., Lee D. S. 2006. Sulfonamide-based pH- and temperature-sensitive biodegradable block copolymer hydrogels. Biomacromolecules 7, 1935–1941 10.1021/bm0600567 (doi:10.1021/bm0600567) [DOI] [PubMed] [Google Scholar]
- 140.Kim H. K., Shim W. S., Kim S. E., Lee K. H., Kang E., Kim J. H., Kim K., Kwon I. C., Lee D. S. 2009. Injectable in situ-forming pH/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng. Part A 15, 923–933 10.1089/ten.tea.2007.0407 (doi:10.1089/ten.tea.2007.0407) [DOI] [PubMed] [Google Scholar]
- 141.Shim W. S., Yoo J. S., Bae Y. H., Lee D. S. 2005. Novel injectable pH and temperature sensitive block copolymer hydrogel. Biomacromolecules 6, 2930–2934 10.1021/bm050521k (doi:10.1021/bm050521k) [DOI] [PubMed] [Google Scholar]
- 142.Gong C. Y., et al. 2009. Biodegradable thermosensitive injectable PEG-PCL-PEG hydrogel for bFGF antigen delivery to improve humoral immunity. Growth Factors 27, 377–383 10.3109/08977190903159938 (doi:10.3109/08977190903159938) [DOI] [PubMed] [Google Scholar]
- 143.Jeong B., Lee K. M., Gutowska A., An Y. H. 2002. Thermogelling biodegradable copolymer aqueous solutions for injectable protein delivery and tissue engineering. Biomacromolecules 3, 865–868 10.1021/bm025536m (doi:10.1021/bm025536m) [DOI] [PubMed] [Google Scholar]
- 144.Lee E. S., Na K., Bae Y. H. 2003. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release 91, 103–113 10.1016/S0168-3659(03)00239-6 (doi:10.1016/S0168-3659(03)00239-6) [DOI] [PubMed] [Google Scholar]
- 145.Lee E. S., Na K., Bae Y. H. 2005. Super pH-sensitive multifunctional polymeric micelle. Nano. Lett. 5, 325–329 10.1021/nl0479987 (doi:10.1021/nl0479987) [DOI] [PubMed] [Google Scholar]
- 146.Putnam D. 2006. Polymers for gene delivery across length scales. Nat. Mater. 5, 439–451 10.1038/nmat1645 (doi:10.1038/nmat1645) [DOI] [PubMed] [Google Scholar]
- 147.Matsusaki M., Akashi M. 2005. Novel functional biodegradable polymer IV: pH-sensitive controlled release of fibroblast growth factor-2 from a poly(gamma-glutamic acid)-sulfonate matrix for tissue engineering. Biomacromolecules 6, 3351–3356 10.1021/bm050369m (doi:10.1021/bm050369m) [DOI] [PubMed] [Google Scholar]
- 148.Huynh D. P., Nguyen M. K., Pi B. S., Kim M. S., Chae S. Y., Lee K. C., Kim B. S., Kim S. W., Lee D. S. 2008. Functionalized injectable hydrogels for controlled insulin delivery. Biomaterials 29, 2527–2534 10.1016/j.biomaterials.2008.02.016 (doi:10.1016/j.biomaterials.2008.02.016) [DOI] [PubMed] [Google Scholar]
- 149.Little S. R., Lynn D. M., Ge Q., Anderson D. G., Puram S. V., Chen J., Eisen H. N., Langer R. 2004. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc. Natl Acad. Sci. USA 101, 9534–9539 10.1073/pnas.0403549101 (doi:10.1073/pnas.0403549101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lynn D. M., Langer R. 2000. Degradable poly(beta-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J. Am. Chem. Soc. 122, 10 761–10 768 10.1021/ja0015388 (doi:10.1021/ja0015388) [DOI] [Google Scholar]
- 151.Wu D. Q., Sun Y. X., Xu X. D., Cheng S. X., Zhang X. Z., Zhuo R. X. 2008. Biodegradable and pH-sensitive hydrogels for cell encapsulation and controlled drug release. Biomacromolecules 9, 1155–1162 10.1021/bm7010328 (doi:10.1021/bm7010328) [DOI] [PubMed] [Google Scholar]
- 152.Simoes S., Moreira J. N., Fonseca C., Duzgunes N., De Lima M. C. 2004. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 56, 947–965 10.1016/j.addr.2003.10.038 (doi:10.1016/j.addr.2003.10.038) [DOI] [PubMed] [Google Scholar]
- 153.Murthy N., Xu M., Schuck S., Kunisawa J., Shastri N., Frechet J. M. 2003. A macromolecular delivery vehicle for protein-based vaccines: acid-degradable protein-loaded microgels. Proc. Natl Acad. Sci. USA 100, 4995–5000 10.1073/pnas.0930644100 (doi:10.1073/pnas.0930644100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Casadei M. A., Pitarresi G., Calabrese R., Paolicelli P., Giammona G. 2008. Biodegradable and pH-sensitive hydrogels for potential colon-specific drug delivery: characterization and in vitro release studies. Biomacromolecules 9, 43–49 10.1021/bm700716c (doi:10.1021/bm700716c) [DOI] [PubMed] [Google Scholar]
- 155.Klouda L., Mikos A. G. 2008. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 68, 34–45 10.1016/j.ejpb.2007.02.025 (doi:10.1016/j.ejpb.2007.02.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hacker M. C., Klouda L., Ma B. B., Kretlow J. D., Mikos A. G. 2008. Synthesis and characterization of injectable, thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers. Biomacromolecules 9, 1558–1570 10.1021/bm8000414 (doi:10.1021/bm8000414) [DOI] [PubMed] [Google Scholar]
- 157.Reddy T. T., Kano A., Maruyama A., Hadano M., Takahara A. 2008. Thermosensitive transparent semi-interpenetrating polymer networks for wound dressing and cell adhesion control. Biomacromolecules 9, 1313–1321 10.1021/bm701390f (doi:10.1021/bm701390f) [DOI] [PubMed] [Google Scholar]
- 158.Fundueanu G., Constantin M., Ascenzi P. 2008. Preparation and characterization of pH- and temperature-sensitive pullulan microspheres for controlled release of drugs. Biomaterials 29, 2767–2775 10.1016/j.biomaterials.2008.03.025 (doi:10.1016/j.biomaterials.2008.03.025) [DOI] [PubMed] [Google Scholar]
- 159.Stover T. C., Kim Y. S., Lowe T. L., Kester M. 2008. Thermoresponsive and biodegradable linear-dendritic nanoparticles for targeted and sustained release of a pro-apoptotic drug. Biomaterials 29, 359–369 10.1016/j.biomaterials.2007.09.037 (doi:10.1016/j.biomaterials.2007.09.037) [DOI] [PubMed] [Google Scholar]
- 160.Saim A. B., Cao Y., Weng Y., Chang C. N., Vacanti M. A., Vacanti C. A., Eavey R. D. 2000. Engineering autogenous cartilage in the shape of a helix using an injectable hydrogel scaffold. Laryngoscope 110, 1694–1697 10.1097/00005537-200010000-00023 (doi:10.1097/00005537-200010000-00023) [DOI] [PubMed] [Google Scholar]
- 161.Fisher J. P., Jo S., Mikos A. G., Reddi A. H. 2004. Thermoreversible hydrogel scaffolds for articular cartilage engineering. J. Biomed. Mater. Res. A 71, 268–274 [DOI] [PubMed] [Google Scholar]
- 162.Seong J. Y., Jun Y. J., Jeong B., Sohn Y. S. 2005. New thermogelling poly (organophosphazenes) with methoxypoly(ethylene glycol) and oligopeptide as side groups. Polymer 46, 5075–5081 10.1016/j.polymer.2005.04.024 (doi:10.1016/j.polymer.2005.04.024) [DOI] [Google Scholar]
- 163.Lutolf M. P., Weber F. E., Schmoekel H. G., Schense J. C., Kohler T., Muller R., Hubbell J. A. 2003. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 21, 513–518 10.1038/nbt818 (doi:10.1038/nbt818) [DOI] [PubMed] [Google Scholar]
- 164.Bremer C., Tung C. H., Weissleder R. 2001. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat. Med. 7, 743–748 10.1038/89126 (doi:10.1038/89126) [DOI] [PubMed] [Google Scholar]
- 165.Harris T. J., Von Maltzahn G., Lord M. E., Park J. H., Agrawal A., Min D. H., Sailor M. J., Bhatia S. N. 2008. Protease-triggered unveiling of bioactive nanoparticles. Small 4, 1307–1312 10.1002/smll.200701319 (doi:10.1002/smll.200701319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Thornton P. D., Mcconnell G., Ulijn R. V. 2005. Enzyme responsive polymer hydrogel beads. Chem. Commun. 5913–5915 10.1039/b511005j (doi:10.1039/b511005j) [DOI] [PubMed] [Google Scholar]
- 167.Um S. H., Lee J. B., Park N., Kwon S. Y., Umbach C. C., Luo D. 2006. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 5, 797–801 10.1038/nmat1741 (doi:10.1038/nmat1741) [DOI] [PubMed] [Google Scholar]
- 168.Murakami Y., Maeda M. 2005. DNA-responsive hydrogels that can shrink or swell. Biomacromolecules 6, 2927–2929 10.1021/bm0504330 (doi:10.1021/bm0504330) [DOI] [PubMed] [Google Scholar]
- 169.Miyata T., Asami N., Uragami T. 1999. A reversibly antigen-responsive hydrogel. Nature 399, 766–769 10.1038/21619 (doi:10.1038/21619) [DOI] [PubMed] [Google Scholar]
- 170.Ehrbar M., Schoenmakers R., Christen E. H., Fussenegger M., Weber W. 2008. Drug-sensing hydrogels for the inducible release of biopharmaceuticals. Nat. Mater. 7, 800–804 10.1038/nmat2250 (doi:10.1038/nmat2250) [DOI] [PubMed] [Google Scholar]
- 171.Ehrick J. D., Deo S. K., Browning T. W., Bachas L. G., Madou M. J., Daunert S. 2005. Genetically engineered protein in hydrogels tailors stimuli-responsive characteristics. Nat. Mater. 4, 298–302 10.1038/nmat1352 (doi:10.1038/nmat1352) [DOI] [PubMed] [Google Scholar]
- 172.Kobatake S., Takami S., Muto H., Ishikawa T., Irie M. 2007. Rapid and reversible shape changes of molecular crystals on photoirradiation. Nature 446, 778–781 10.1038/nature05669 (doi:10.1038/nature05669) [DOI] [PubMed] [Google Scholar]
- 173.Lendlein A., Jiang H., Junger O., Langer R. 2005. Light-induced shape-memory polymers. Nature 434, 879–882 10.1038/nature03496 (doi:10.1038/nature03496) [DOI] [PubMed] [Google Scholar]
- 174.Sumaru K., Kameda M., Kanamori T., Shinbo T. 2004. Reversible and efficient proton dissociation of spirobenzopyran-functionalized poly(N-isopropylacrylamide) in aqueous solution triggered by light irradiation and temporary temperature rise. Macromolecules 37, 7854–7856 10.1021/ma0487249 (doi:10.1021/ma0487249) [DOI] [Google Scholar]
- 175.Jager E. W., Smela E., Inganas O. 2000. Microfabricating conjugated polymer actuators. Science 290, 1540–1545 10.1126/science.290.5496.1540 (doi:10.1126/science.290.5496.1540) [DOI] [PubMed] [Google Scholar]
- 176.Welch P. M. 2005. A tunable dendritic molecular actuator. Nano Lett. 5, 1279–1283 10.1021/nl050422c (doi:10.1021/nl050422c) [DOI] [PubMed] [Google Scholar]
- 177.Yun Y., Shanov V., Tu Y., Schulz M. J., Yarmolenko S., Neralla S., Sankar J., Subramaniam S. 2006. A multi-wall carbon nanotube tower electrochemical actuator. Nano Lett. 6, 689–693 10.1021/nl052435w (doi:10.1021/nl052435w) [DOI] [PubMed] [Google Scholar]
- 178.Hu S. H., Liu T. Y., Huang H. Y., Liu D. M., Chen S. Y. 2008. Magnetic-sensitive silica nanospheres for controlled drug release. Langmuir 24, 239–244 10.1021/la701570z (doi:10.1021/la701570z) [DOI] [PubMed] [Google Scholar]
- 179.Kloxin A. M., Kasko A. M., Salinas C. N., Anseth K. S. 2009. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 10.1126/science.1169494 (doi:10.1126/science.1169494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee K. Y., Peters M. C., Anderson K. W., Mooney D. J. 2000. Controlled growth factor release from synthetic extracellular matrices. Nature 408, 998–1000 10.1038/35044106 (doi:10.1038/35044106) [DOI] [PubMed] [Google Scholar]
- 181.Laham R. J., Sellke F. W., Edelman E. R., Pearlman J. D., Ware J. A., Brown D. L., Gold J. P., Simons M. 1999. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery: results of a phase I randomized, double-blind, placebo-controlled trial. Circulation 100, 1865–1871 [DOI] [PubMed] [Google Scholar]
- 182.Ruel M., Laham R. J., Parker J. A., Post M. J., Ware J. A., Simons M., Sellke F. W. 2002. Long-term effects of surgical angiogenic therapy with fibroblast growth factor 2 protein. J. Thorac. Cardiovasc. Surg. 124, 28–34 10.1067/mtc.2002.121974 (doi:10.1067/mtc.2002.121974) [DOI] [PubMed] [Google Scholar]
- 183.Govender S., et al. 2002. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Joint Surg. Am. 84-A, 2123–2134 [DOI] [PubMed] [Google Scholar]
- 184.Jones A. L., Bucholz R. W., Bosse M. J., Mirza S. K., Lyon T. R., Webb L. X., Pollak A. N., Golden J. D., Valentin-Opran A. 2006. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J. Bone Joint Surg. Am. 88, 1431–1441 10.2106/JBJS.E.00381 (doi:10.2106/JBJS.E.00381) [DOI] [PubMed] [Google Scholar]
- 185.Vaccaro A. R., Lawrence J. P., Patel T., Katz L. D., Anderson D. G., Fischgrund J. S., Krop J., Fehlings M. G., Wong D. 2008. The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis: a long-term (>4 years) pivotal study. Spine 33, 2850–2862 10.1097/BRS.0b013e31818a314d (doi:10.1097/BRS.0b013e31818a314d) [DOI] [PubMed] [Google Scholar]
- 186.Vaccaro A. R., et al. 2008. The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft for posterolateral lumbar arthrodesis: minimum 4-year follow-up of a pilot study. Spine J. 8, 457–465 10.1016/j.spinee.2007.03.012 (doi:10.1016/j.spinee.2007.03.012) [DOI] [PubMed] [Google Scholar]
- 187.Mooney D. J., Kaufmann P. M., Sano K., Mcnamara K. M., Vacanti J. P., Langer R. 1994. Transplantation of hepatocytes using porous, biodegradable sponges. Transplant Proc. 26, 3425–3426 [PubMed] [Google Scholar]
- 188.Hsu Y. Y., Gresser J. D., Trantolo D. J., Lyons C. M., Gangadharam P. R., Wise D. L. 1997. Effect of polymer foam morphology and density on kinetics of in vitro controlled release of isoniazid from compressed foam matrices. J. Biomed. Mater. Res. 35, 107–116 (doi:10.1002/(SICI)1097-4636(199704)35:1<107::AID-JBM11>3.0.CO;2-G) [DOI] [PubMed] [Google Scholar]
- 189.Whang K., Thomas C. H., Healy K. E., Nuber G. 1995. A novel method to fabricate bioabsorbable scaffolds. Polymer 36, 837–842 10.1016/0032-3861(95)93115-3 (doi:10.1016/0032-3861(95)93115-3) [DOI] [Google Scholar]
- 190.Hua F. J., Kim G. E., Lee J. D., Son Y. K., Lee D. S. 2002. Macroporous poly(L-lactide) scaffold. 1. Preparation of a macroporous scaffold by liquid–liquid phase separation of a PLLA–dioxane–water system. J. Biomed. Mater. Res. 63, 161–167 10.1002/jbm.10121 (doi:10.1002/jbm.10121) [DOI] [PubMed] [Google Scholar]
- 191.Lo H., Ponticiello M. S., Leong K. W. 1995. Fabrication of controlled release biodegradable foams by phase separation. Tissue Eng. 1, 15–28 10.1089/ten.1995.1.15 (doi:10.1089/ten.1995.1.15) [DOI] [PubMed] [Google Scholar]
- 192.Thomson R. C., Yaszemski M. J., Powers J. M., Mikos A. G. 1995. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J. Biomater. Sci. Polym. Ed. 7, 23–38 10.1163/156856295X00805 (doi:10.1163/156856295X00805) [DOI] [PubMed] [Google Scholar]
- 193.Busby W., Cameron N. R., Jahoda C. A. 2001. Emulsion-derived foams (PolyHIPEs) containing poly(epsilon-caprolactone) as matrixes for tissue engineering. Biomacromolecules 2, 154–164 10.1021/bm0000889 (doi:10.1021/bm0000889) [DOI] [PubMed] [Google Scholar]
- 194.Bryant S. J., Anseth K. S. 2001. The effects of scaffold thickness on tissue engineered cartilage in photocrosslinked poly(ethylene oxide) hydrogels. Biomaterials 22, 619–626 10.1016/S0142-9612(00)00225-8 (doi:10.1016/S0142-9612(00)00225-8) [DOI] [PubMed] [Google Scholar]
- 195.Temenoff J. S., Park H., Jabbari E., Conway D. E., Sheffield T. L., Ambrose C. G., Mikos A. G. 2004. Thermally cross-linked oligo(poly(ethylene glycol) fumarate) hydrogels support osteogenic differentiation of encapsulated marrow stromal cells in vitro. Biomacromolecules 5, 5–10 10.1021/bm030067p (doi:10.1021/bm030067p) [DOI] [PubMed] [Google Scholar]