Abstract
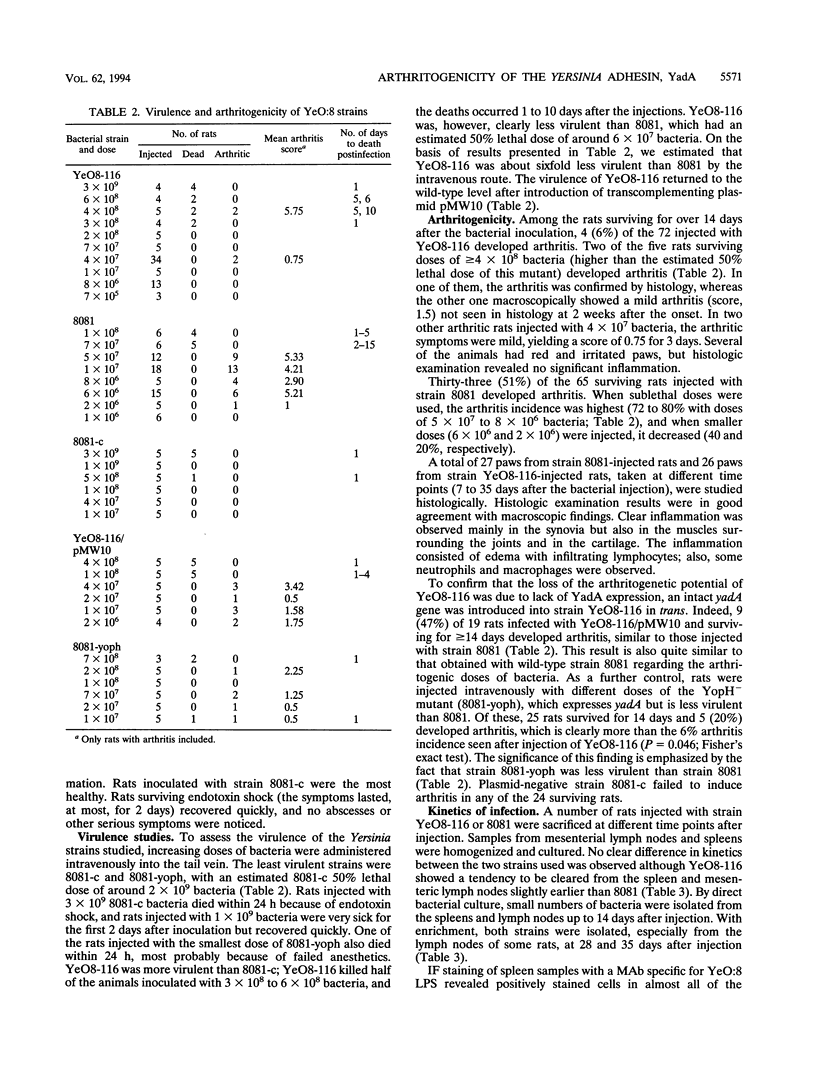
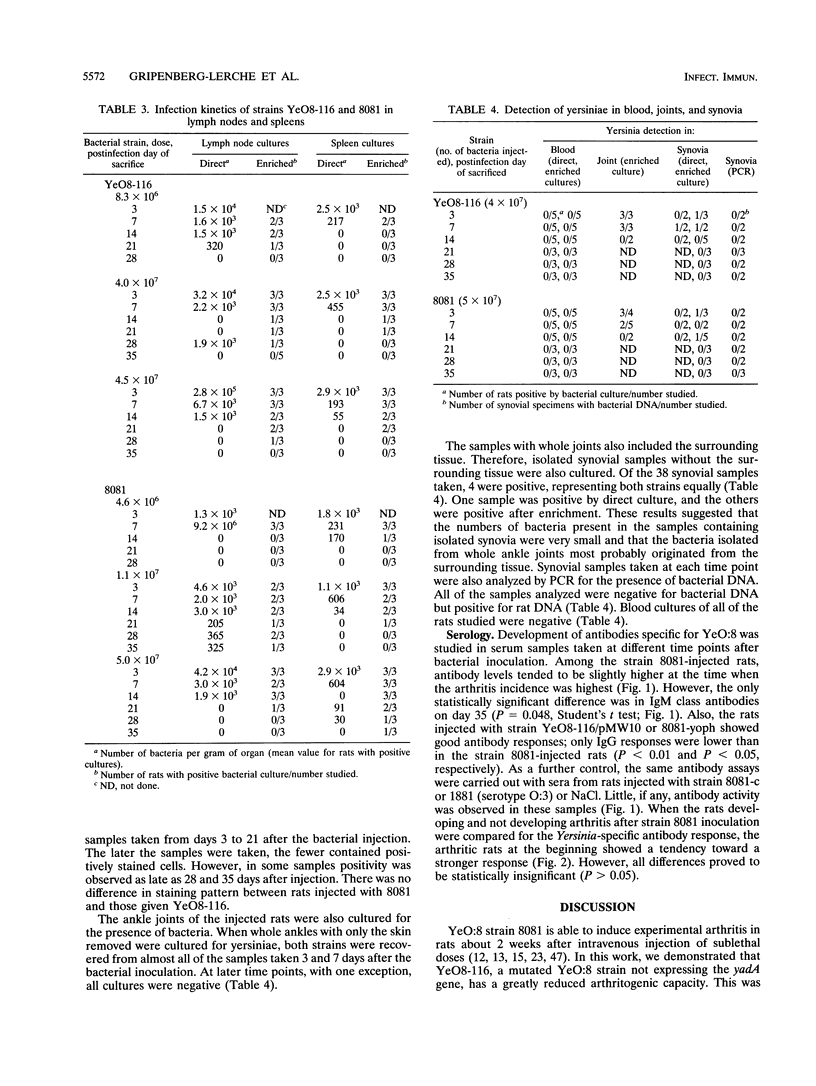
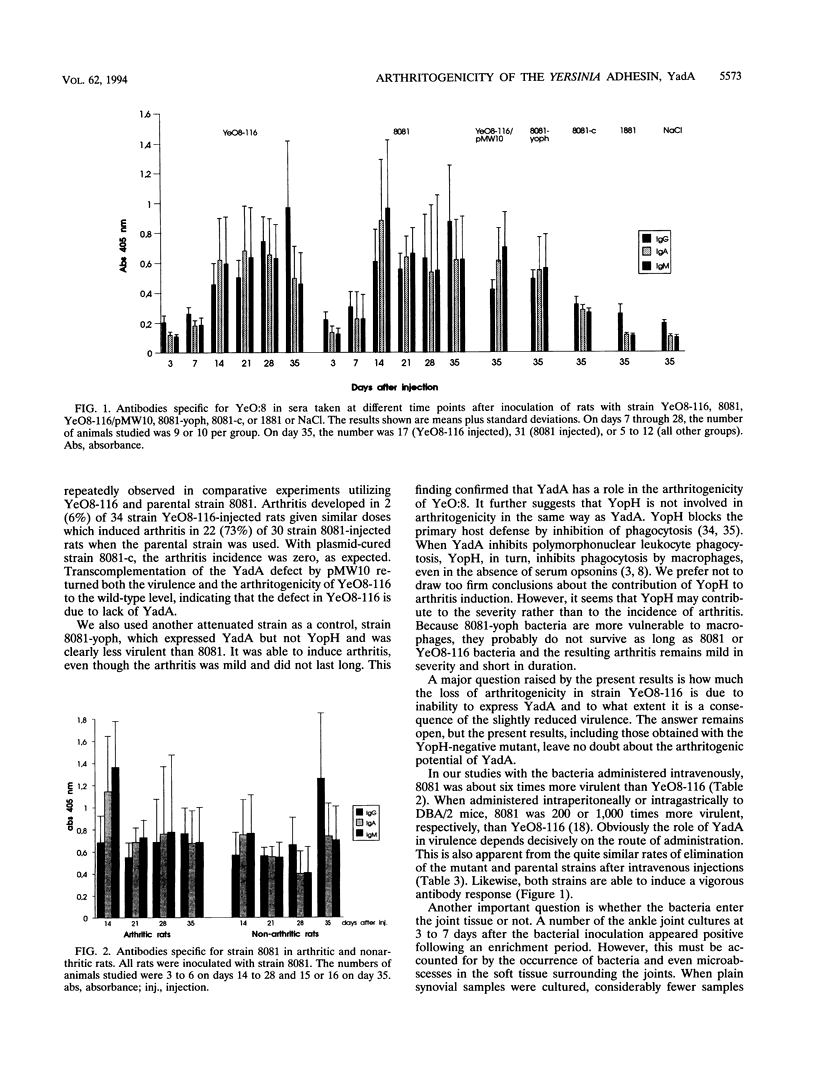
Outer membrane protein YadA, the Yersinia adhesin, is one of the plasmid-encoded virulence factors of yersiniae. To evaluate the role of YadA in the pathogenesis of reactive arthritis experimentally, we used YadA- strain YeO8-116, a kanamycin GenBlock insertion mutant derived from Yersinia enterocolitica O:8 wild-type strain 8081. As control strains, a plasmid-cured derivative (8081-c) of 8081 and a YopH- mutant (8081-yoph) were used. In addition, YeO8-116, with the yadA mutation transcomplemented with plasmid pMW10, was used. YeO8-116 induced arthritis to a considerably lesser extent than did wild-type strain 8081 when inoculated intravenously into Lewis rats. In rats surviving for over 14 days after the bacterial inoculation, the arthritis incidences were 6% (4 of 72) among those inoculated with the yadA mutant and 51% (33 of 65) among those inoculated with wild-type strain 8081. When the yadA gene was transcomplemented back to YeO8-116, YeO8-116/pMW10 induced arthritis in 47% (9 of 19) of the inoculated rats. Plasmid-cured strain 8081-c did not induce arthritis in any of the 24 inoculated rats, whereas YopH- mutant 8081-yoph induced arthritis in 20% (5 of 25) of the rats inoculated. Although the 50% lethal dose of YeO8-116 was about sixfold higher than that of 8081, the kinetics of bacterial elimination from the spleen and mesenteric lymph nodes were about the same with both strains. Antibody responses in rats infected with the two strains were also indistinguishable. Our results indicate that YadA contributes to the arthritogenicity of Y. enterocolitica in the rat model.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balligand G., Laroche Y., Cornelis G. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect Immun. 1985 Jun;48(3):782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Clemens J. C., Dixon J. E., Falkow S. The Yersinia tyrosine phosphatase: specificity of a bacterial virulence determinant for phosphoproteins in the J774A.1 macrophage. J Exp Med. 1992 Dec 1;176(6):1625–1630. doi: 10.1084/jem.176.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Guan K. L., Dixon J. E., Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bukholm G., Kapperud G., Skurnik M. Genetic evidence that the yopA gene-encoded Yersinia outer membrane protein Yop1 mediates inhibition of the anti-invasive effect of interferon. Infect Immun. 1990 Jul;58(7):2245–2251. doi: 10.1128/iai.58.7.2245-2251.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Varga C. F., Keet E. E. New strain of Yersinia enterocolitica pathogenic for rodents. Appl Microbiol. 1973 Dec;26(6):1016–1018. doi: 10.1128/am.26.6.1016-1018.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China B., N'Guyen B. T., de Bruyere M., Cornelis G. R. Role of YadA in resistance of Yersinia enterocolitica to phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1994 Apr;62(4):1275–1281. doi: 10.1128/iai.62.4.1275-1281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China B., Sory M. P., N'Guyen B. T., De Bruyere M., Cornelis G. R. Role of the YadA protein in prevention of opsonization of Yersinia enterocolitica by C3b molecules. Infect Immun. 1993 Aug;61(8):3129–3136. doi: 10.1128/iai.61.8.3129-3136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delor I., Cornelis G. R. Role of Yersinia enterocolitica Yst toxin in experimental infection of young rabbits. Infect Immun. 1992 Oct;60(10):4269–4277. doi: 10.1128/iai.60.10.4269-4277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emödy L., Heesemann J., Wolf-Watz H., Skurnik M., Kapperud G., O'Toole P., Wadström T. Binding to collagen by Yersinia enterocolitica and Yersinia pseudotuberculosis: evidence for yopA-mediated and chromosomally encoded mechanisms. J Bacteriol. 1989 Dec;171(12):6674–6679. doi: 10.1128/jb.171.12.6674-6679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripenberg-Lerche C., Toivanen P. Variability in the induction of experimental arthritis: Yersinia associated arthritis in Lewis rats. Scand J Rheumatol. 1994;23(3):124–127. doi: 10.3109/03009749409103043. [DOI] [PubMed] [Google Scholar]
- Gripenberg-Lerche C., Toivanen P. Yersinia associated arthritis in SHR rats: effect of the microbial status of the host. Ann Rheum Dis. 1993 Mar;52(3):223–228. doi: 10.1136/ard.52.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. L., Yu D. T. Development of an experimental animal model for reactive arthritis induced by Yersinia enterocolitica infection. Infect Immun. 1987 Mar;55(3):721–726. doi: 10.1128/iai.55.3.721-726.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skurnik M., Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987 Sep;55(9):2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W. J., Cavanaugh D. C. Correlation of autoagglutination and virulence of yersiniae. J Clin Microbiol. 1980 Apr;11(4):430–432. doi: 10.1128/jcm.11.4.430-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Husar S. D. Binding of Yersinia enterocolitica to purified, native small intestinal mucins from rabbits and humans involves interactions with the mucin carbohydrate moiety. Infect Immun. 1994 Apr;62(4):1219–1227. doi: 10.1128/iai.62.4.1219-1227.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R. J. Thermoregulation-dependent expression of Yersinia enterocolitica protein 1 imparts serum resistance to Escherichia coli K-12. J Bacteriol. 1989 Jul;171(7):3732–3739. doi: 10.1128/jb.171.7.3732-3739.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilahti-Palo R., Gripenberg-Lerche C., Söderström K. O., Toivanen P. Long term follow up of SHR rats with experimental yersinia associated arthritis. Ann Rheum Dis. 1992 Jan;51(1):91–96. doi: 10.1136/ard.51.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerregaard A., Espersen F., Skurnik M. Role of the Yersinia outer membrane protein YadA in adhesion to rabbit intestinal tissue and rabbit intestinal brush border membrane vesicles. APMIS. 1991 Mar;99(3):226–232. doi: 10.1111/j.1699-0463.1991.tb05143.x. [DOI] [PubMed] [Google Scholar]
- Pai C. H., Mors V. Production of enterotoxin by Yersinia enterocolitica. Infect Immun. 1978 Mar;19(3):908–911. doi: 10.1128/iai.19.3.908-911.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe J. C., Badger J. L., Miller V. L. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol Microbiol. 1994 Jan;11(1):123–135. doi: 10.1111/j.1365-2958.1994.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Pepe J. C., Miller V. L. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6473–6477. doi: 10.1073/pnas.90.14.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz D., Vocke T., Heesemann J., Brade V. Mechanism of YadA-mediated serum resistance of Yersinia enterocolitica serotype O3. Infect Immun. 1992 Jan;60(1):189–195. doi: 10.1128/iai.60.1.189-195.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen L., Granberg I., Granfors K., Toivanen A. Restriction map of virulence plasmid in Yersinia enterocolitica O:3. Plasmid. 1986 Nov;16(3):225–227. doi: 10.1016/0147-619x(86)90062-4. [DOI] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Forsberg A., Rimpiläinen M., Bergman T., Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990 Apr;4(4):657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schulze-Koops H., Burkhardt H., Heesemann J., Kirsch T., Swoboda B., Bull C., Goodman S., Emmrich F. Outer membrane protein YadA of enteropathogenic yersiniae mediates specific binding to cellular but not plasma fibronectin. Infect Immun. 1993 Jun;61(6):2513–2519. doi: 10.1128/iai.61.6.2513-2519.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Koops H., Burkhardt H., Heesemann J., von der Mark K., Emmrich F. Plasmid-encoded outer membrane protein YadA mediates specific binding of enteropathogenic yersiniae to various types of collagen. Infect Immun. 1992 Jun;60(6):2153–2159. doi: 10.1128/iai.60.6.2153-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M., Batsford S., Mertz A., Schiltz E., Toivanen P. The putative arthritogenic cationic 19-kilodalton antigen of Yersinia enterocolitica is a urease beta-subunit. Infect Immun. 1993 Jun;61(6):2498–2504. doi: 10.1128/iai.61.6.2498-2504.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M., Bölin I., Heikkinen H., Piha S., Wolf-Watz H. Virulence plasmid-associated autoagglutination in Yersinia spp. J Bacteriol. 1984 Jun;158(3):1033–1036. doi: 10.1128/jb.158.3.1033-1036.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Skrzypek E., Plano G. V., Bliska J. B. Yops of Yersinia spp. pathogenic for humans. Infect Immun. 1993 Aug;61(8):3105–3110. doi: 10.1128/iai.61.8.3105-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm A., Tarkkanen A. M., Korhonen T. K., Kuusela P., Toivanen P., Skurnik M. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol Microbiol. 1993 Dec;10(5):995–1011. doi: 10.1111/j.1365-2958.1993.tb00971.x. [DOI] [PubMed] [Google Scholar]
- Tertti R., Skurnik M., Vartio T., Kuusela P. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect Immun. 1992 Jul;60(7):3021–3024. doi: 10.1128/iai.60.7.3021-3024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivanen A., Toivanen P. Epidemiologic aspects, clinical features, and management of ankylosing spondylitis and reactive arthritis. Curr Opin Rheumatol. 1994 Jul;6(4):354–359. doi: 10.1097/00002281-199407000-00002. [DOI] [PubMed] [Google Scholar]
- Veromaa T., Vainio O., Eerola E., Toivanen P. Monoclonal antibodies against chicken Bu-1a and Bu-1b alloantigens. Hybridoma. 1988 Feb;7(1):41–48. doi: 10.1089/hyb.1988.7.41. [DOI] [PubMed] [Google Scholar]
- al-Hendy A., Toivanen P., Skurnik M. Lipopolysaccharide O side chain of Yersinia enterocolitica O:3 is an essential virulence factor in an orally infected murine model. Infect Immun. 1992 Mar;60(3):870–875. doi: 10.1128/iai.60.3.870-875.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


