Abstract
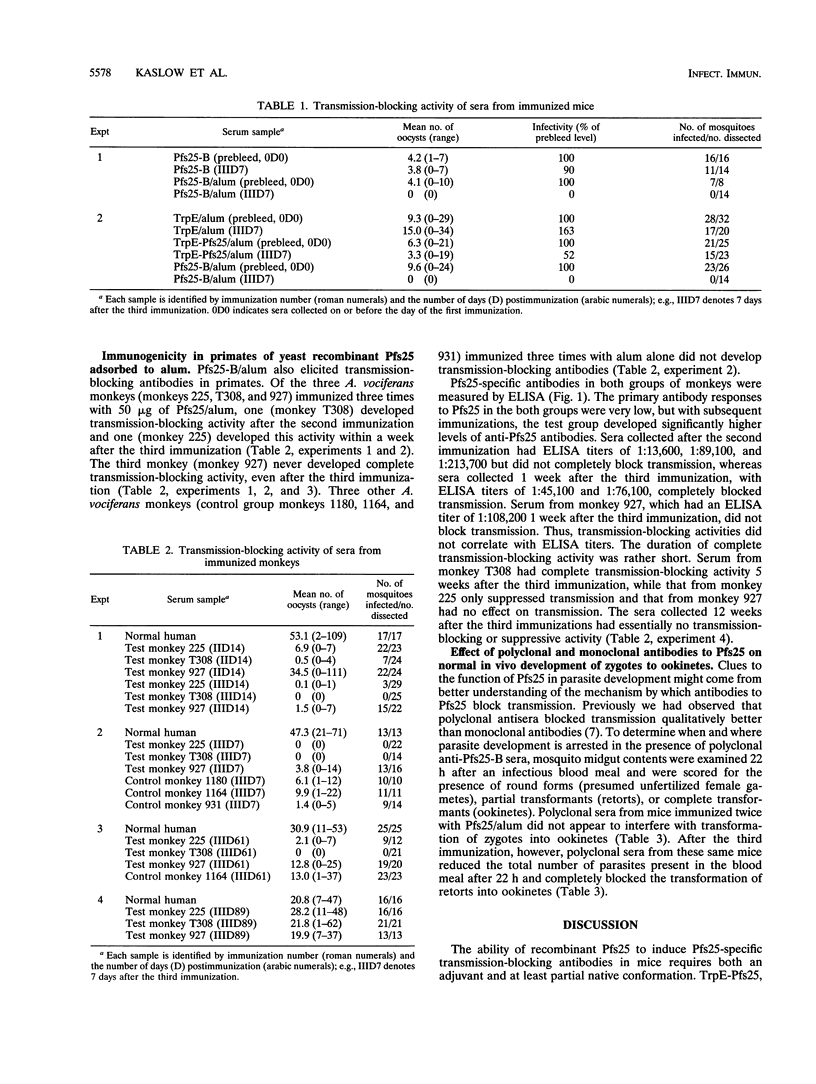
Antibodies to Pfs25, a cysteine-rich 25-kDa protein present on the surface of Plasmodium falciparum zygotes, can completely block the transmission of malaria parasites when mixed with infectious blood and fed to mosquitoes through a membrane feeding apparatus. Recently, a polypeptide analog, Pfs25-B, secreted from recombinant Saccharomyces cerevisiae was found to react with conformation-dependent, transmission-blocking monoclonal antibodies and to elicit transmission-blocking antibodies in experimental animals when emulsified in either Freund's or muramyl tripeptide adjuvant. In this study, Pfs25-B adsorbed to alum induced transmission-blocking antibodies in both rodents and primates. Bacterially produced Pfs25, however, did not elicit complete transmission-blocking antibodies in rodents. Furthermore, unlike monoclonal antibodies to Pfs25, which block transmission only after ookinete development, antisera to Pfs25-B adsorbed to alum appeared to block the in vivo development of zygotes to ookinetes as well.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr P. J., Green K. M., Gibson H. L., Bathurst I. C., Quakyi I. A., Kaslow D. C. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991 Nov 1;174(5):1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkot T. R., Williams J. L., Schneider I. Infectivity to mosquitoes of Plasmodium falciparum clones grown in vitro from the same isolate. Trans R Soc Trop Med Hyg. 1984;78(3):339–341. doi: 10.1016/0035-9203(84)90114-7. [DOI] [PubMed] [Google Scholar]
- Duffy P. E., Pimenta P., Kaslow D. C. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1993 Feb 1;177(2):505–510. doi: 10.1084/jem.177.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst C. A., Kumar N., Carter R., Kaushal D. C. A surface protein expressed during the transformation of zygotes of Plasmodium gallinaceum is a target of transmission-blocking antibodies. Infect Immun. 1984 Sep;45(3):775–777. doi: 10.1128/iai.45.3.775-777.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow D. C., Isaacs S. N., Quakyi I. A., Gwadz R. W., Moss B., Keister D. B. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant vaccinia virus. Science. 1991 May 31;252(5010):1310–1313. doi: 10.1126/science.1925544. [DOI] [PubMed] [Google Scholar]
- Kaslow D. C., Quakyi I. A., Syin C., Raum M. G., Keister D. B., Coligan J. E., McCutchan T. F., Miller L. H. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988 May 5;333(6168):74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- Kaslow D. C. Transmission-blocking immunity against malaria and other vector-borne diseases. Curr Opin Immunol. 1993 Aug;5(4):557–565. doi: 10.1016/0952-7915(93)90037-s. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Howard R. J., Carter R., Good M. F., Nussenzweig V., Nussenzweig R. S. Research toward malaria vaccines. Science. 1986 Dec 12;234(4782):1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Paton M. G., Barker G. C., Matsuoka H., Ramesar J., Janse C. J., Waters A. P., Sinden R. E. Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol Biochem Parasitol. 1993 Jun;59(2):263–275. doi: 10.1016/0166-6851(93)90224-l. [DOI] [PubMed] [Google Scholar]
- Ponnudurai T., Lensen A. H., Van Gemert G. J., Bensink M. P., Bolmer M., Meuwissen J. H. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology. 1989 Apr;98(Pt 2):165–173. doi: 10.1017/s0031182000062065. [DOI] [PubMed] [Google Scholar]
- Sieber K. P., Huber M., Kaslow D., Banks S. M., Torii M., Aikawa M., Miller L. H. The peritrophic membrane as a barrier: its penetration by Plasmodium gallinaceum and the effect of a monoclonal antibody to ookinetes. Exp Parasitol. 1991 Feb;72(2):145–156. doi: 10.1016/0014-4894(91)90132-g. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A. N., Ponnudurai T., Beckers P. J., Verhave J. P., Smits M. A., Meuwissen J. H. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985 Nov 1;162(5):1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A. N., van Deursen J., Brakenhoff R. H., Lensen T. H., Ponnudurai T., Meuwissen J. H. Characterization of Plasmodium falciparum sexual stage antigens and their biosynthesis in synchronised gametocyte cultures. Mol Biochem Parasitol. 1986 Aug;20(2):155–163. doi: 10.1016/0166-6851(86)90027-7. [DOI] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]



