Abstract
Muscarinic acetylcholine receptors in the nucleus accumbens play an important role in mediating the reinforcing effects of cocaine. However, there is a paucity of data regarding the role of accumbal muscarinic acetylcholine receptors in the reinstatement of cocaine-seeking behavior. The goal of these experiments was to assess the role of muscarinic acetylcholine receptors in the nucleus accumbens core and shell in cocaine and sucrose priming-induced reinstatement. Rats were initially trained to self-administer cocaine or sucrose on a fixed-ratio schedule of reinforcement. Lever-pressing behavior was then extinguished and followed by a subsequent reinstatement phase during which operant responding was induced by either a systemic injection of cocaine in cocaine-experienced rats or non-contingent delivery of sucrose pellets in subjects with a history of sucrose self-administration. Results indicated that systemic administration of the muscarinic acetylcholine receptor antagonist scopolamine (5.0 mg/kg, i.p.) dose-dependently attenuated cocaine, but not sucrose, reinstatement. Furthermore, administration of scopolamine (36.0 μg) directly into the nucleus accumbens shell or core attenuated cocaine-priming induced reinstatement. In contrast, infusion of scopolamine (36.0 μg) directly into the accumbens core, but not shell, attenuated sucrose reinstatement, which suggests that muscarinic acetylcholine receptors in these two subregions of the nucleus accumbens have differential roles in sucrose seeking. Taken together, these results indicate that cocaine-priming induced reinstatement is mediated, in part, by increased signaling through muscarinic acetylcholine receptors in the shell subregion of the nucleus accumbens. Muscarinic acetylcholine receptors in the core of the accumbens, in contrast, appear to play a more general (i.e. not cocaine specific) role in motivated behaviors.
Keywords: relapse, addiction, psychostimulant, sucrose seeking, acetylcholine, scopolamine
1. INTRODUCTION
Cocaine-induced behavioral plasticity is mediated, in part, by the mesocorticolimbic dopaminergic system, which arises from the ventral tegmental area and terminates in forebrain regions including the nucleus accumbens (Schmidt et al., 2005). However, it is increasingly accepted that other neurotransmitter systems in the nucleus accumbens mediate cocaine-induced behavioral plasticity. The nucleus accumbens has relatively high extracellular levels of acetylcholine, which is released from interneurons (Calabresi et al., 2000; Pisani et al., 2001; Zhou et al., 2002). Although cholinergic interneurons represent a small (<5%) proportion of accumbens neurons, these cells are characterized by large dendritic fields and dense dendritic arborizations (Contant et al., 1996; Ligorio et al., 2009; Wilson et al., 1990). Recent evidence implicated nucleus accumbens acetylcholine neurotransmission in the acquisition and maintenance of cocaine self-administration (Crespo et al., 2006; Mark et al., 2006). Cholinergic interneurons in the accumbens shell subregion are activated indirectly by both acute and chronic self-administration of cocaine, with the amount of activation proportional to the amount of self-administered cocaine (Berlanga et al., 2003). In addition, acetylcholine efflux in the accumbens shell was greater in rats self-administering cocaine when compared to animals receiving non-contingent (yoked) administration of cocaine (Mark et al., 1999). Acquisition of cocaine-reinforced behavior was also accompanied by an increase in acetylcholine release in the accumbens core subregion and increased stimulation of muscarinic acetylcholine receptors (Crespo et al., 2006). Moreover, repeated cocaine exposure produces neuroadaptations in muscarinic acetylcholine receptor systems within the nucleus accumbens that may influence cocaine-seeking behavior (for review see, Williams and Adinoff, 2008).
There are five distinct muscarinic acetylcholine receptor subtypes that have been cloned and classified as M1-like (M1, M3, and M5) or M2-like (M2 and M4) (Bonner, 1989; Bonner et al., 1987). Striatal/accumbal cholinergic interneurons express mainly M2, and to a lesser degree M1and M4, receptors (Alcantara et al., 2001; Bernard et al., 1992; Hersch et al., 1994; Levey et al., 1991; Vilaro et al., 1992; Weiner et al., 1990), whereas M5 receptors are expressed on dopamine neurons in the midbrain including the ventral tegmental area (Weiner et al., 1990) as well as presynaptic dopaminergic terminals in the accumbens (Zhang et al., 2002). A growing literature demonstrates that muscarinic acetylcholine receptors play an important role in cocaine-induced behavioral plasticity. Constitutive M5 muscarinic receptor deletion diminishes the reinforcing potency of low doses of cocaine (Thomsen et al., 2005) and decreases striatal dopamine release (Forster et al., 2002; Schmidt et al., 2009). Moreover, cocaine-conditioned place preference was attenuated in mice lacking the M1 muscarinic receptor and in wild-type mice treated with the M1 muscarinic acetylcholine receptor antagonist pirenzepine (Carrigan and Dykstra, 2007). Collectively, these recent studies suggest that M1 and M5 muscarinic acetylcholine receptors play critical roles in cocaine addiction. Behavioral pharmacology studies indicate that laboratory animals self-administer the muscarinic acetylcholine receptor agonist carbachol directly into the nucleus accumbens, which suggests that muscarinic acetylcholine receptor agonists are reinforcing (Ikemoto et al., 1998). In addition, systemic administration of scopolamine, a muscarinic acetylcholine receptor antagonist, completely blocked cocaine conditioned reward, as measured with conditioned place preference (Itzhak and Martin, 2000). However, oxotremorine, a non-selective muscarinic acetylcholine receptor agonist, has been shown to reduce cocaine self-administration when injected into the accumbens shell (Mark et al., 2006). Despite this discrepancy in the literature, which may be due to poorly selective muscarinic acetylcholine receptor ligands, these data collectively suggest that muscarinic acetylcholine receptors, particularly in the nucleus accumbens, play an important role in the reinforcing effects of cocaine.
The present study was designed to investigate the role of accumbens muscarinic acetylcholine receptors in cocaine-primed reinstatement of drug seeking, which has not been assessed previously to our knowledge. We hypothesized that muscarinic acetylcholine receptor antagonism in the nucleus accumbens would attenuate cocaine priming-induced reinstatement of drug-seeking behavior.
2. MATERIALS and METHODS
2.1 Animals and housing
Male Sprague-Dawley rats weighing 250-300 g were obtained from Taconic Laboratories (Germantown, NY). Animals were individually housed, with sucrose and water available ad libitum in their home cage (rats undergoing sucrose reinstatement were placed on restricted diets, as outlined below). A 12/12 hr light/dark cycle was used throughout these experiments with the lights on at 7:00 a.m. All experimental procedures were performed during the light cycle. The experimental protocols were all consistent with the guidelines issued by the U.S. National Institutes of Health and were approved by the Boston University School of Medicine and the University of Pennsylvania School of Medicine Institutional Animal Care and Use Committee Institutional Animal Care and Use Committees.
2.2 Materials
All experiments used Med-Associates (Georgia, VT) modular testing instrumentation enclosed within ventilated, sound-attenuating chambers (22”W × 22”H × 16”D). Each operant box had the following interior dimensions: 12.0”L × 9.5”W × 8.25”H. The operant boxes were equipped with response levers, sucrose pellet dispensers, and injection pumps for infusing drugs intravenously. Each operant chamber had two response levers, only one of which was active or paired with delivery of a reinforcer.
2.3 Drugs
Cocaine was obtained from the National Institute on Drug Abuse (Rockville, MD) and dissolved in bacteriostatic 0.9% saline. Scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile 0.9% saline. Doses of scopolamine were selected based on systemic doses known to activate striatal dopamine levels (Chapman et al., 1997; Forster and Blaha, 2000) and intracranial doses used to assess cocaine reinforcement (Ikemoto and Goeders, 2000) or food intake (Pratt and Kelley, 2005).
2.4 Surgery
Prior to surgery, the rats were anesthetized with ketamine (80 mg/kg, i.p.; Sigma-Aldrich, St. Louis, MO) and xylazine (12 mg/kg, i.p.; Sigma-Aldrich, St. Louis, MO). An indwelling silastic catheter (inner diameter 0.33 mm, outer diameter 0.64 mm) was inserted into the right jugular vein and sutured in place. The catheter was then threaded subcutaneously over the shoulder blade, and was routed to a mesh backmount platform (CamCath, Cambridge, UK) that secured the placement. Catheters were flushed daily with 0.3 ml of the antibiotic Timentin (ticarcillin disodium/potassium clavulanate, 0.93 mg/ml; Henry Schein, Melville, NY) dissolved in heparinized saline. The catheters were sealed with plastic obturators when not in use.
Following catheter insertion, animals were mounted in a stereotaxic apparatus and four stainless steel guide cannulae (14 mm, 22 gauge) for microinjections were implanted bilaterally 2 mm dorsal to the nucleus accumbens core and shell in each animal. Cannulae were cemented in place by affixing dental acrylic to stainless steel screws secured in the skull. The coordinates for the ventral ends of the guide cannulae, relative to bregma according to the atlas of Paxinos and Watson (1997) were as follows: nucleus accumbens shell: +1.0 mm A/P, ±1.0 mm M/L, −5.0 mm D/V; nucleus accumbens core: +1.0 mm A/P, ±2.5 mm M/L, −5.0 mm D/V. An obturator (14 mm, 33 gauge stainless steal wire) was inserted into each guide cannula in order to prevent occlusion.
2.5 Cocaine self-administration and extinction training
After surgery, rats were allowed seven days to recover before behavioral testing commenced. Initially, rats were placed in operant chambers and allowed to lever press for intravenous infusions of cocaine (0.25 mg cocaine/59 μl saline, infused over a 5 sec period) on a fixed-ratio 1 (FR1) schedule of reinforcement. Each session began with the i.v. administration of 59 μl cocaine (0.25 mg) to fill the catheter (i.e. little or none of this non-contingent injection reached the systemic circulation). Rats were allowed to self-administer a maximum of 30 injections per 120-minute operant session under the FR1 schedule. Once an animal achieved at least 20 infusions of cocaine in a single daily session under the FR1 schedule, the subject was switched to a fixed-ratio 5 (FR5) schedule of reinforcement. The maximum number of injections was again limited to 30 per daily self-administration session under the FR5 schedule. For both the FR1 and FR5 schedules, a 20 second time-out period followed each cocaine infusion, during which time active lever responses were tabulated but had no scheduled consequences. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during both the FR1 and FR5 training sessions.
Daily operant sessions (5 days/week) under the FR1 and FR5 schedules of reinforcement were continued for a total of 21 days. Following cocaine self-administration, drug-seeking behavior was extinguished by replacing the cocaine with 0.9% saline. The extinction phase continued until responding on the active lever was <15% of the response rate maintained by cocaine self-administration under the FR5 schedule of reinforcement. Typically, it took approximately 8 days for rats to meet this criterion.
2.6 Cocaine Priming-Induced Reinstatement
Following extinction, animals entered the reinstatement phase of the experiment. During reinstatement, satisfaction of the response requirements for each ratio resulted in saline rather than cocaine infusion. Each reinstatement session was followed by extinction sessions until responding was again less than 15% of the response rate maintained by cocaine self-administration. Generally, only a single day of extinction was necessary in order to reach extinction criterion between reinstatement test sessions. The FR5 schedule was used throughout the extinction and reinstatement phases of these experiments.
2.7 Microinjection procedures
Prior to a reinstatement test session, obturators were removed from the guide cannulae and 33 gauge stainless steel microinjectors (Small Parts Inc.) were inserted. These microinjectors were cut to a length that extended 2 mm below the ventral end of the guide cannulae and into the nucleus accumbens core and shell. Bilateral infusions were performed simultaneously over a 120 second time period in a total volume of 0.5 μl per hemisphere. Following microinfusion of scopolamine or vehicle, the microinjectors were left in place for 60 seconds in order to allow the solution to diffuse away from the tips of the microinjectors before they were removed. A systemic priming injection of cocaine (10 mg/kg, i.p.) was administered 10 min following microinjections and animals were placed in the operant chambers and the reinstatement session began immediately. The goal of the experimental design was to have each animal served as its own control and received up to three microinjections per brain region (i.e. two doses of scopolamine plus vehicle for a maximum of three microinjections per brain region). However, we were frequently forced to deviate from this experimental design when technical difficulties (i.e. blocked microinjection cannula or loss of catheter patency) made it impossible to test all doses of the muscarinic acetylcholine receptor antagonist plus vehicle in an entire cohort of subjects. Therefore, a mixed-factors design was used in these behavioral experiments. In every case, however, an animal received at a minimum, treatment of one drug dose and its vehicle. In order to control for potential order effects of drug and vehicle administrations, all drug and vehicle treatments were counterbalanced across reinstatement sessions.
Using this experimental design, subjects underwent a series of alternating extinction and reinstatement sessions that lasted approximately 16 days. During this period, extinction of the ability of priming injections of cocaine to induced reinstatement is a concern. However, we have previously shown that reinstatement of cocaine seeking persists for at least 20 days after the initial extinction of cocaine self-administration behavior (Anderson et al., 2008; Famous et al., 2008). Moreover, since the scopolamine treatments were counterbalanced across reinstatement days, we were able to assess the magnitude of reinstatement across sessions in the current experiments. All subjects demonstrated stable drug seeking throughout the reinstatement phase of these experiments.
2.8 Sucrose self-administration and reinstatement
Potential nonspecific rate-suppressing effects of systemic or intra-accumbens infusions of the muscarinic acetylcholine receptor antagonist scopolamine were evaluated by assessing the influence of scopolamine on the reinstatement of sucrose-reinforced responding. Rats were trained initially to press a lever under an FR1 schedule of reinforcement for sucrose pellet (Research Diets, Inc, New Brunswick, NJ) delivery in daily 1-hour sessions. The animals were restricted to 4 pieces of lab chow (Harlan Teklad, Wilmington, DE) per day for the duration of these experiments. Each piece of lab chow weighed on average 5.41±0.16 g. Once animals had acquired self-administration on the FR1 schedule (less than 15% variation in responding on 2 consecutive days), the rats were switched to an FR5 schedule of reinforcement. Sucrose reinforced subjects spent an average of 5.31±0.30 days training on the FR1 schedule before transitioning to an FR5 schedule of reinforcement. Animals were limited to 30 sucrose pellets within a 1-hour session.
After 3 weeks of sucrose-maintained responding on the FR5 schedule (21 total days of responding under FR1 and FR5 schedules of reinforcement), rats underwent an extinction phase wherein the sucrose pellets were removed from the dispenser, and responding no longer resulted in sucrose delivery. After lever pressing decreased to 10% or less of the responding maintained by contingent sucrose reinforcement, animals began reinstatement testing the following day. Each reinstatement session began with the issue of a non-contingent sucrose pellet prime. The experimenter remotely administered one sucrose pellet every 2 min thereafter for the first 10 min of the reinstatement session. Similar to the extinction phase of the experiment, lever responses were recorded but did not results in delivery of sucrose pellets. Each sucrose reinstatement session was followed by extinction sessions until responding was again less than 10% of the response rate maintained by sucrose.
2.9 Behavioral Experiment 1
The effect of systemic administration of the muscarinic acetylcholine receptor antagonist scopolamine (Sigma-Aldrich, St. Louis, MO) on cocaine priming-induced reinstatement of drug seeking was assessed in 11 subjects (4 out of an initial 15 subjects lost catheter patency during the experiment and were subsequently dropped from the study). Scopolamine (0.5 [n=10] or 5.0 [n=7] mg/kg, i.p.) or vehicle (n=11) was administered 10 min prior to a 10 mg/kg (i.p.) cocaine priming injection. In all of the following behavioral experiments, the animals were placed in the modular testing chambers immediately after the systemic priming injection of cocaine. Also, in all experiments, the administration of vehicle and all doses of scopolamine were counterbalanced across reinstatement sessions in order to avoid any potential rank-order effects of drug administration.
2.10 Behavioral Experiment 2
The effect of systemic administration of muscarinic acetylcholine receptor antagonist scopolamine on sucrose priming-induced reinstatement was assessed in 7 rats. Scopolamine (5.0 mg/kg, i.p.; n=5) or vehicle (n=7) was administered 10 min prior to a sucrose reinstatement session. Reinstatement sessions began with delivery of a non-contingent sucrose pellet prime. Subsequent priming pellets were delivered remotely every 2 min thereafter for the first 10 min of the reinstatement session. Scopolamine data points were excluded from two subjects because one did not reinstate to sucrose pellets in a subsequent test of sucrose reinstatement and another subject was lethargic on the scopolamine test day. Therefore, due to behavioral and health confounds, scopolamine-induced attenuation data were excluded from this experiment for these two animals.
2.11 Behavioral Experiment 3
A total of 26 rats were used for this experiment However, catheter patency was lost in 3 subjects who were ultimately dropped from this study. The effect of intracranial administration of scopolamine on systemic priming injections of cocaine was assessed in 22 animals (data from 2 subjects were excluded due to misplaced guide cannulae in the accumbens shell or core and 3 animals were excluded due to significant tissue damage around the injection sites). Scopolamine (3.6 [n=6] and 36.0 [n=6] μg/0.5 μl) or vehicle (n=9) was injected into either the nucleus accumbens core or shell 10 min prior to a 10 mg/kg (i.p.) priming injection of cocaine. Animals were placed immediately into the operant chambers following a priming injection of cocaine and the reinstatement session ensued.
2.12 Behavioral Experiment 4
The effect of intracranial administration of scopolamine on sucrose reinstatement was assessed in 18 subjects (data from 2 subjects were excluded due to misplaced guide cannulae in either the accumbens shell or core). Animals received 3.6 or 36.0 μg/0.5 μl scopolamine or vehicle injections into either the nucleus accumbens core or shell. Ten minutes following drug treatment, the animals were placed in the operant chambers and the session began with the issue of a non-contingent sucrose pellet prime. Over the first 10 min of the reinstatement session, the experimenter remotely administered a non-contingent sucrose pellet every two minutes. Each reinstatement session was followed by extinction sessions until responding was again less than 10% of the response rate maintained by sucrose self-administration.
2.13 Verification of cannulae placements
Following the completion of all microinjection experiments, the animals were given an overdose of pentobarbital (100 mg/kg, i.p.) and perfused intracardially with 0.9% saline followed by 10% formalin. The brain was removed and coronal sections (100 μm) were taken at the level of the nucleus accumbens with a Vibratome (Technical Products International; St. Louis, MO). The sections were mounted on gelatin-coated slides and stained with Cresyl violet. Using a light microscope, an individual blinded to the animals’ behavioral response identified microinjection sites as well as potential drug- or cannula-induced neuronal damage. Cell death and associated gliosis also were assessed in these sections. Animals with cannula placements outside of the accumbens core or shell subregions, or with excessive mechanical damage, were excluded from subsequent data analyses.
2.14 Behavioral Experiment Data Analyses
In the reinstatement experiments, a mixed-factors design was used in which we aimed to administer each dose of a drug plus the drug vehicle to all subjects in a cohort during the reinstatement phase. However, technical difficulties (loss of catheter patency and clogging of guide cannulae, etc.) regularly resulted in missing cells. We therefore used a statistical model (a mixed-model multivariate analysis of variance or MANOVA) that accommodates missing cells in the within-subjects aspect of an experimental design without assigning place-keeper values (such as the treatment mean) using the residual maximum likelihood approach (Berk, 1987). The systemic and intra-accumbens scopolamine cocaine priming-induced reinstatement data (total lever responses per session) were analyzed with a two-way mixed-model MANOVA. The two factors were lever (active-inactive) and drug treatment. The systemic scopolamine food reinstatement control experiment was analyzed with an unpaired t-test. The intra-accumbens scopolamine food reinstatement control data were analyzed with a one-way mixed-model MANOVA. Time course data were analyzed by mixed-factor MANOVAs with repeated measures over time. Pairwise comparisons for all significant effects as revealed by MANOVAs were made using Fisher’s LSD post hoc test (P<0.05).
3. RESULTS
3.1 Systemic administration of the muscarinic acetylcholine receptor antagonist scopolamine dose-dependently attenuates cocaine priming-induced reinstatement of drug seeking
The muscarinic acetylcholine receptor antagonist scopolamine was administered systemically prior to a priming injection of cocaine. The total number of lever responses (mean±S.E.M.) following a systemic injection of scopolamine 10 min prior to a systemic priming injection of cocaine (10 mg/kg, i.p.) during the reinstatement phase of the experiment are shown in Fig. 1A. Total active and inactive lever responses are displayed for animals receiving systemic injections (i.p.) of vehicle (n=11), 0.5 (n=10) or 5.0 mg/kg scopolamine (n=7) prior to a systemic priming injection of cocaine (10 mg/kg, i.p.). Total lever press data from Fig. 1A were analyzed with a two-way mixed-model MANOVA, which did not reveal a significant main effect of treatment (F2,25=2.68, P<0.088). However, a significant main effect of lever (F1,25=69.03, P<0.0001) as well as a significant lever × treatment interaction (F2,25=3.67, P<0.04) were revealed. Subsequent pairwise analyses showed that the active lever response rate in the 5.0 mg/kg scopolamine treatment was significantly different from the vehicle treatment (Fisher’s LSD, P<0.05). The active lever response rate for each 10-minute component of the reinstatement session for systemic vehicle and 5.0 mg/kg scopolamine treatments is shown in Fig. 1B.
Figure 1.
Systemic injections of scopolamine dose-dependently attenuate cocaine priming-induced reinstatement of drug seeking. (A) Total numbers of responses (mean±S.E.M.) on the active and inactive levers during the reinstatement session following systemic injection of vehicle (n=11), 0.5 (n=10) and 5.0 (n=7) mg/kg scopolamine and a subsequent priming injection of cocaine (10 mg/kg, i.p.). *P<0.05 for active lever responding between vehicle- and 5.0 mg/kg scopolamine-treated animals. (B) The time courses of active lever responses (mean±S.E.M.) for subjects that received either vehicle or 5.0 mg/kg scopolamine prior to a priming injection of cocaine. (C) Total active lever responses (mean±S.E.M.) during a 1-h sucrose reinstatement session for animals treated with vehicle (n=5) or 5.0 μg scopolamine (n=7). There was no significant difference between treatments on sucrose-seeking behavior (un-paired t-test, P<0.23).
In order to evaluate potential nonspecific rate suppressing effects of systemic injections of scopolamine and their effects on a natural reinforcer, animals were administered scopolamine (0 and 5.0 mg/kg, i.p.) prior to a sucrose reinstatement session. Systemic administration of 5.0 mg/kg scopolamine, the same dose that attenuated cocaine priming-induced reinstatement, did not affect sucrose-seeking behavior (Fig. 1C). Vehicle (n=5) or 5.0 mg/kg scopolamine (n=7) was administered 10 min prior to a 1-h sucrose reinstatement session, wherein non-contingent administration of sucrose pellets reinstates responding previously maintained by sucrose reinforcement. Total active lever responses (mean±S.E.M.) following systemic administration of vehicle or 5.0 mg/kg scopolamine are depicted in Fig. 1C. These data were analyzed with an unpaired t-test. Although there was a trend toward a similar decrease as seen in Fig. 1A, this analysis did not reveal a significant difference between treatments on sucrose-seeking behavior (t(10)=1.28, P<0.23). These data suggest that attenuation of cocaine-seeking behavior is not due to generalized motor impairment and that pharmacological blockade of muscarinic acetylcholine receptors plays a critical role in cocaine reinstatement and not a more generalized role in reinforced behaviors.
3.2 Administration of scopolamine directly into the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking
The present results indicate that systemic injections of scopolamine dose-dependently attenuate cocaine priming-induced reinstatement of drug seeking. One limitation of this experiment is that systemic administration of scopolamine crosses the blood-brain barrier and blocks muscarinic acetylcholine receptors throughout the brain. While these data suggest that muscarinic acetylcholine receptors in the brain play a critical role in cocaine-seeking behavior, they do not clarify the precise role of muscarinic acetylcholine receptors in discrete nuclei in cocaine reinstatement. Therefore, scopolamine was directly infused into the nucleus accumbens in order to determine the role of muscarinic acetylcholine receptor-mediated signaling within this nucleus during cocaine seeking.
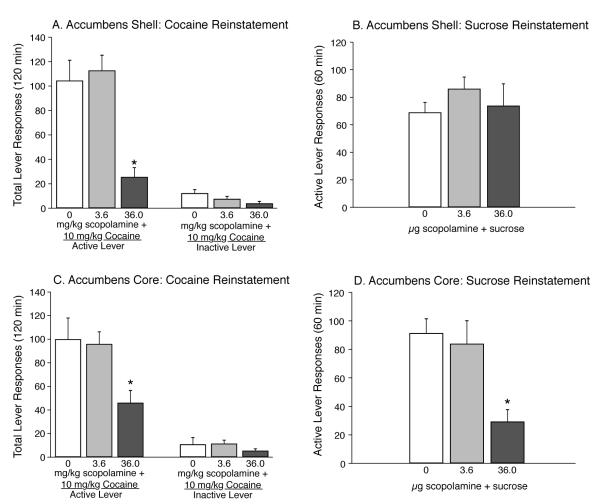
The total number of lever responses (mean±S.E.M.) following microinjection of scopolamine (0, 3.6 μg and 36.0 μg) into the nucleus accumbens shell 10 min prior to a systemic priming injection of cocaine (10 mg/kg, i.p.) during the reinstatement phase of the experiment are shown in Fig. 2A. Total lever data in Fig. 2A were analyzed with a two-way MANOVA, which revealed significant main effects of treatment (F2,18=11.32, P<0.001) and lever (F1,18=74.54, P<0.0001) as well as a significant lever × treatment interaction (F2,18=10.49, P=0.001). Post-hoc analyses revealed a significant difference in responding on the active lever between subjects pretreated with vehicle (n=9) or 3.6 μg scopolamine (n=6) and 36.0 μg scopolamine (n=6) (Fisher’s LSD, P<0.05). Total inactive lever responding (mean±S.E.M.) for animals receiving microinjections of vehicle (n=9), 3.6 (n=6) or 36.0 (n=6) μg scopolamine into the accumbens shell prior to a systemic priming injection of cocaine are plotted in Fig. 2A. No significant differences were found between treatments on inactive lever responding.
Figure 2.
Microinjection of scopolamine into the core and shell subregions of the nucleus accumbens dose-dependently attenuates cocaine priming-induced reinstatement of drug seeking. (A) Total number of responses (mean±S.E.M.) on the active and inactive levers during the reinstatement session following intra-accumbens shell infusion of vehicle (n=9), 3.6 (n=6) and 36.0 (n=6) μg scopolamine 10-min prior to a systemic priming injection of cocaine (10 mg/kg, i.p.). *P<0.05 for active lever responding between vehicle- and 36.0 μg scopolamine-treated animals. (B) Total number of lever responses completed during sucrose reinstatement. Microinfusion of vehicle (n=9), 3.6 (n=5) and 36.0 (n=7) μg scopolamine directly into the accumbens shell 10 min prior to a 1-h sucrose reinstatement session. No significant differences in sucrose-seeking behavior were found between treatments. (C) Total number of responses (mean±S.E.M.) on the active and inactive levers during 2-h cocaine priming-induced reinstatement sessions following intra-accumbens core injections of vehicle (n=8), 3.6 (n=6) and 36.0 (n=8) μg scopolamine prior to a subsequent priming injection of cocaine (10 mg/kg, i.p.). Active lever responding was significantly different between subjects treated with vehicle or 3.6 μg scopolamine and animals receiving 36.0 μg scopolamine (Fisher’s LSD, *P<0.05). No significant differences were found between treatments in responding on the inactive lever. (D) Total number of operant responses (mean±S.E.M.) completed during the 1-h sucrose reinstatement session for animals receiving intra-accumbens core infusions of vehicle (n=8), 3.6 (n=6) or 36.0 (n=8) μg scopolamine. A significant difference in responding was found between animals pretreated with vehicle and 36.0 μg scopolamine in the accumbens core 10-min prior to the sucrose reinstatement session (Fisher’s LSD, *P<0.05).
In order to evaluate whether attenuation of cocaine priming-induced reinstatement of drug seeking following intra-cranial administration of scopolamine was due to general behavioral suppression, the ability of intra-accumbens shell microinjections of scopolamine to alter sucrose reinstatement was assessed. Subjects received microinfusion of vehicle (n=9), 3.6 (n=5) or 36.0 (n=7) μg scopolamine directly into the accumbens shell 10-min prior to a sucrose reinstatement session, wherein non-contingent administration of sucrose pellets reinstates responding previously maintained by sucrose reinforcement. Total active lever responses (mean±S.E.M.) following intra-accumbens shell administration of vehicle, 3.6 or 36.0 μg scopolamine are depicted in Fig. 2B. These data were analyzed with a one-way MANOVA, which did not reveal a significant main effect of treatment on sucrose-seeking behavior (F2,3=2.76, P<0.21). These data suggest that attenuation of cocaine-seeking behavior is not due to generalized motor impairment and that pharmacological blockade of muscarinic acetylcholine receptors in the accumbens shell plays a selective role in cocaine reinstatement.
3.3 Administration of scopolamine directly into the nucleus accumbens core attenuates cocaine priming-induced reinstatement of drug seeking
Total lever responses (mean±S.E.M.) following intra-accumbens core injection of the competitive muscarinic acetylcholine receptor antagonist scopolamine 10-min prior to a systemic priming injection of cocaine (10 mg/kg, i.p.) during the reinstatement phase of the experiment are shown in Fig. 2C. Total lever responses (mean±S.E.M.) from Fig. 2C were analyzed with a two-way MANOVA, which revealed significant main effects of treatment (F2,19=4.72, P<0.023) and lever (F1,19=74.96, P<0.0001) as well as a significant treatment × lever interaction (F2,19=3.77, P<0.042). Subsequent post-hoc analysis using Fisher’s LSD test revealed a significant difference in responding on the active lever between subjects treated with vehicle (n=8) or 3.6 (n=6) and 36 (n=8) μg scopolamine (P<0.05). No significant differences in total inactive lever responding between treatments were found.
In order to examine the selectivity of pharmacological blockade of muscarinic acetylcholine receptors in the accumbens core on reinforced behaviors, animals received vehicle (n=8), 3.6 (n=6) or 36.0 (n=8) μg scopolamine directly into the accumbens core 10-min prior to a sucrose reinstatement session. Total active lever responses (mean±S.E.M.) following intra-accumbens core administration of vehicle, 3.6 or 36.0 μg scopolamine are depicted in Fig. 2D. These data were analyzed with a one-way MANOVA, which revealed a significant main effect of treatment (F2,4=8.49, P<0.036). Follow-up analysis demonstrated a significant difference in active lever responding between animals treated with vehicle and 36.0 μg scopolamine (Fisher’s LSD, P<0.05).
3.4 Histology
The cannulae placement from the aforementioned experiments included the medial nucleus accumbens shell or the nucleus accumbens core. The locations of the cannulae placements in the accumbens core and shell are detailed in Fig. 3. The medial shell placements also included the anatomical border between the shell and core subregions of the nucleus accumbens. Injections into the nucleus accumbens core were aimed at the anterior commissure. Gliosis or necrosis around the infusion sites in the accumbens shell or core was not evident following administration of scopolamine. Furthermore, there was no excessive mechanical damage observed in either of these brain regions following repeated microinfusions.
Figure 3.
Coronal sections at the level of the nucleus accumbens depicting the subregions targeted by microinjections used in the present experiments. Closed circles denote microinjections in the medial limb of the nucleus accumbens shell. Open circles denote infusions into the nucleus accumbens core. Numbers indicate distance from bregma in the anteroposterior direction. Representative microinjection sites are displayed for the accumbens shell (top panel) and core (bottom panel) in the inserts. Black arrows indicate ventral ends of microinjector tips in the accumbens shell and core. The grey arrow in the top panel indicates a guide cannula track aimed at the accumbens shell. Images were visualized with a light microscope using a 4× objective. Data from animals with necrotic or mechanical damage at microinjection sites were excluded from these experiments. AC = anterior commisure.
4. DISCUSSION
While a growing literature indicates that cholinergic transmission in limbic nuclei, including the nucleus accumbens, is involved in the reinforcing effects of cocaine (Williams and Adinoff, 2008), the precise role for accumbens muscarinic acetylcholine receptors in cocaine priming-induced reinstatement of drug-seeking behavior had not been determined previously. The present results include the novel findings that administration of the muscarinic acetylcholine receptor antagonist scopolamine directly into the nucleus accumbens core or shell dose-dependently attenuated the ability of a priming injection of cocaine to reinstate drug seeking. Moreover, pharmacological blockade of muscarinic acetylcholine receptors in the accumbens core, but not shell, attenuated sucrose-seeking behavior. Collectively, these results suggest that muscarinic acetylcholine receptors in the accumbens shell modulate the reinstatement of cocaine seeking selectively, whereas muscarinic acetylcholine receptors in the accumbens core play a more generalized role in motivated behaviors.
4.1 Nucleus accumbens muscarinic acetylcholine receptors and cocaine-seeking behavior
The present data demonstrate, for the first time, that pharmacological antagonism of muscarinic acetylcholine receptors in the nucleus accumbens core and shell subregions attenuates cocaine priming-induced reinstatement of drug seeking. These results add to a growing literature examining the role of acetylcholine transmission in cocaine addiction (Williams and Adinoff, 2008). Extracellular acetylcholine levels are increased in nucleus accumbens subregions of rats during the acquisition (Crespo et al., 2006) and maintenance (Mark et al., 1999) of cocaine self-administration. Moreover, acquisition of cocaine self-administration is associated with stimulation of accumbens muscarinic acetylcholine receptors (Crespo et al., 2006). Furthermore, systemic administration of a partial muscarinic acetylcholine receptor agonist attenuated cocaine self-administration in mice (Rasmussen et al., 2000). Taken together, these results and the present findings suggest that accumbens acetylcholine transmission plays a critical role in the reinforcing effects of cocaine and the reinstatement of cocaine-seeking behavior (however see Ikemoto and Goeders, 2000).
The nucleus accumbens contains a small population of spontaneously active cholinergic interneurons (de Rover et al., 2002; Kawaguchi et al., 1995; Pennartz and Lopes da Silva, 1994; Wilson et al., 1990) that synapse on accumbal GABAergic cells (Pickel and Chan, 1990). Cholinergic interneurons can modulate the excitability of GABAergic medium spiny projection neurons in the accumbens by activating postsynaptic muscarinic acetylcholine receptors (Sugita et al., 1991; Uchimura and North, 1990; Zhang and Warren, 2002). The spontaneous firing frequency of nucleus accumbens cholinergic interneurons is regulated, in part, by endogenous acetylcholine release and activation of muscarinic acetylcholine receptors. For example, pharmacological blockade of muscarinic acetylcholine receptors located on cholinergic interneurons promotes tonic, and not burst, firing of accumbens cholinergic interneurons (de Rover et al., 2002). Therefore, administration of a muscarinic acetylcholine receptor antagonist prior to a priming injection of cocaine may decrease cocaine-induced elevations in accumbens acetylcholine (Imperato et al., 1992) thus preventing cocaine priming-induced reinstatement of drug seeking, although this remains to be determined.
Activation of striatal muscarinic acetylcholine receptors results in both excitatory and inhibitory post-synaptic potentials in the striatum that are mediated, in part, by M4/M5 muscarinic acetylcholine receptor-regulated neurotransmitter release from presynaptic terminals (Zhang et al., 2002). Excitation of accumbal GABAergic projection neurons is inhibited by activation of muscarinic acetylcholine receptors on presynaptic glutamate terminals (de Rover et al., 2002; Pennartz and Lopes da Silva, 1994). Consistent with these findings, stimulation of presynaptic muscarinic acetylcholine receptors in the nucleus accumbens decreases glutamatergic transmission (Zhang and Warren, 2002). In addition to modulating presynaptic glutamate release, muscarinic acetylcholine receptors also play a critical role in regulating presynaptic dopamine release in the nucleus accumbens (Rahman and McBride, 2002). Taken together, these results suggest that stimulation of presynaptic accumbal muscarinic acetylcholine receptors regulates information processing in the nucleus accumbens by gating specific glutamatergic and dopaminergic inputs to medium spiny GABAergic projection neurons.
The present results demonstrate that blocking muscarinic acetylcholine receptors in the nucleus accumbens core and shell attenuates cocaine priming-induced reinstatement of drug seeking. However, the exact contribution of nucleus accumbens pre- and postsynaptic muscarinic acetylcholine receptors in this behavior is not clear. Previous studies have shown that increased glutamate transmission in the accumbens core and shell is sufficient to reinstate cocaine seeking (Famous et al., 2008; Famous et al., 2007; Kalivas, 2009). Thus, pharmacological blockade of muscarinic acetylcholine receptors located on presynaptic glutamate terminals would increase glutamate release and subsequently spontaneous excitatory postsynaptic current frequency and amplitude in accumbens GABAergic medium spiny neurons (de Rover et al., 2002). Increased dopamine transmission in the nucleus accumbens also has been demonstrated to play a critical role in cocaine priming-induced reinstatement (Schmidt et al., 2006; Schmidt and Pierce, 2006a; b). Therefore, antagonism of muscarinic acetylcholine receptors may reduce dopamine and/or glutamate levels in the accumbens and attenuate dopamine and/or glutamate receptor-mediated reinstatement of cocaine seeking. However, the precise role of specific accumbens muscarinic acetylcholine receptor subtypes in cocaine priming-induced reinstatement remains to be determined.
4.2 Nucleus accumbens muscarinic acetylcholine receptors and food-seeking behavior
Nucleus accumbens muscarinic acetylcholine receptors play a critical role in coordinating food-directed behavior (Pratt and Kelley, 2004). Selective lesions of accumbens cholinergic interneurons alter food intake patterns and impair reward-related learning and memory processes (Hajnal et al., 2000; Kitabatake et al., 2003). Furthermore, administration of the muscarinic acetylcholine receptor antagonist scopolamine into the accumbens core or shell prevented the acquisition of sucrose self-administration, reduced the progressive ratio breakpoint required for obtaining sucrose reinforcement and decreased daily food intake (Pratt and Kelley, 2004; 2005). Microinjection of scopolamine directly into the accumbens core also decreased the salience of sucrose-associated contextual or visual stimuli (Pratt et al., 2007). In contrast, Crespo et al. (2008) found that unilateral administration of scopolamine to the core via a microdialysis probe did not impair acquisition of food-reinforced behavior. It seems likely that methodological differences in scopolamine administration account for these differential results. Moreover, administration of a non-selective muscarinic acetylcholine receptor agonist directly into the accumbens shell had no effect on food self-administration (Mark et al., 2006). Taken together, these studies suggest that muscarinic acetylcholine receptors in the accumbens core and shell may promote food-directed behaviors. However, the role of muscarinic acetylcholine receptor subtypes in food-reinforced behavior remains to be determined. The present findings are the first to demonstrate that pharmacological antagonism of muscarinic acetylcholine receptors in the accumbens core, but not shell, attenuates the reinstatement of sucrose-seeking behavior. Therefore, muscarinic acetylcholine receptors in the accumbens core seem to play a more generalized role in acquisition of sucrose self-administration, motivation to self-administer food (Pratt and Kelley, 2004), and reinstatement of sucrose-seeking behavior (present findings). Interestingly, muscarinic acetylcholine receptors in the accumbens shell may have a more specific role in mediating the acquisition of food-mediated behaviors but not sucrose reinstatement.
4.3 Summary/Conclusions
The present findings indicate that muscarinic acetylcholine receptors in the accumbens core and shell play prominent roles in cocaine priming-induced reinstatement of drug-seeking behavior. However, muscarinic acetylcholine receptors in the accumbens core, but not the shell, also regulate sucrose-seeking behavior, which suggests that stimulation of accumbens core muscarinic acetylcholine receptors plays a more generalized role in the reinstatement of previously reinforced behavior. Elucidating the role of nucleus accumbens muscarinic acetylcholine receptor subtypes in cocaine-seeking behavior will require the development of receptor subtype-selective agonists and antagonists and/or transgenic animals with impaired receptor subtype signaling. The present results suggest that pharmacological treatments that modulate muscarinic acetylcholine receptor signaling may prevent cocaine craving and relapse in abstinent human addicts. One limitation of current muscarinic acetylcholine receptor antagonists is their ability to produce memory deficits (Deutsch and Rocklin, 1967). Therefore, development of novel, more selective muscarinic acetylcholine receptor subtype antagonists that modulate muscarinic acetylcholine receptor tone in the brain may attenuate cocaine craving and relapse by regulating neurotransmitter systems, including dopamine and glutamate, known to mediate cocaine-seeking behavior (present results; Schmidt et al., 2005; Schmidt and Pierce, 2010).
5. Acknowledgements
This research was supported by grants from the National Institutes of Health (NIH) to RCP (R01 DA15214 and K02 DA18678). KRF was partially supported by a National Research Service Award (NRSA) from the NIH (F30 DA19304), as well as an NIH training grant (T32 GM008541-7). HDS was also partially supported by an NRSA from the NIH (DA16824). The authors thank Audrey Pierce for her administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- Alcantara AA, Mrzljak L, Jakab RL, Levey AI, Hersch SM, Goldman-Rakic PS. Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol. 2001;434:445–460. doi: 10.1002/cne.1186. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Berk K. Computing for incomplete repeated measures. Biometrics. 1987;43:385–398. [PubMed] [Google Scholar]
- Berlanga ML, Olsen CM, Chen V, Ikegami A, Herring BE, Duvauchelle CL, Alcantara AA. Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine. Neuroscience. 2003;120:1149–1156. doi: 10.1016/s0306-4522(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner TI. The molecular basis of muscarinic receptor diversity. Trends Neurosci. 1989;12:148–151. doi: 10.1016/0166-2236(89)90054-4. [DOI] [PubMed] [Google Scholar]
- Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- Carrigan KA, Dykstra LA. Behavioral effects of morphine and cocaine in M1 muscarinic acetylcholine receptor-deficient mice. Psychopharmacology (Berl) 2007;191:985–993. doi: 10.1007/s00213-006-0671-1. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Yeomans JS, Blaha CD, Blackburn JR. Increased striatal dopamine efflux follows scopolamine administered systemically or to the tegmental pedunculopontine nucleus. Neuroscience. 1997;76:177–186. doi: 10.1016/s0306-4522(96)00358-2. [DOI] [PubMed] [Google Scholar]
- Contant C, Umbriaco D, Garcia S, Watkins KC, Descarries L. Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience. 1996;71:937–947. doi: 10.1016/0306-4522(95)00507-2. [DOI] [PubMed] [Google Scholar]
- Crespo JA, Stockl P, Zorn K, Saria A, Zernig G. Nucleus accumbens core acetylcholine is preferentially activated during acquisition of drug- vs food-reinforced behavior. Neuropsychopharmacology. 2008;33:3213–3220. doi: 10.1038/npp.2008.48. [DOI] [PubMed] [Google Scholar]
- Crespo JA, Sturm K, Saria A, Zernig G. Activation of muscarinic and nicotinic acetylcholine receptors in the nucleus accumbens core is necessary for the acquisition of drug reinforcement. J Neurosci. 2006;26:6004–6010. doi: 10.1523/JNEUROSCI.4494-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rover M, Lodder JC, Kits KS, Schoffelmeer AN, Brussaard AB. Cholinergic modulation of nucleus accumbens medium spiny neurons. Eur J Neurosci. 2002;16:2279–2290. doi: 10.1046/j.1460-9568.2002.02289.x. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Rocklin KW. Amnesia induced by scopolamine and its temporal variations. Nature. 1967;216:89–90. doi: 10.1038/216089b0. [DOI] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famous KR, Schmidt HD, Pierce RC. When administered into the nucleus accumbens core or shell, the NMDA receptor antagonist AP-5 reinstates cocaine-seeking behavior in the rat. Neurosci Lett. 2007;420:169–173. doi: 10.1016/j.neulet.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- Forster GL, Yeomans JS, Takeuchi J, Blaha CD. M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J Neurosci. 2002;22:RC190. doi: 10.1523/JNEUROSCI.22-01-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Szekely M, Galosi R, Lenard L. Accumbens cholinergic interneurons play a role in the regulation of body weight and metabolism. Physiol Behav. 2000;70:95–103. doi: 10.1016/s0031-9384(00)00236-5. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Rats self-administer carbachol directly into the nucleus accumbens. Physiol Behav. 1998;63:811–814. doi: 10.1016/s0031-9384(98)00007-9. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Goeders NE. Intra-medial prefrontal cortex injections of scopolamine increase instrumental responses for cocaine: an intravenous self-administration study in rats. Brain Res Bull. 2000;51:151–158. doi: 10.1016/s0361-9230(99)00214-2. [DOI] [PubMed] [Google Scholar]
- Imperato A, Obinu MC, Demontis MV, Gessa GL. Cocaine releases limbic acetylcholine through endogenous dopamine action on D1 receptors. Eur J Pharmacol. 1992;229:265–267. doi: 10.1016/0014-2999(92)90565-l. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Scopolamine inhibits cocaine-conditioned but not unconditioned stimulant effects in mice. Psychopharmacology (Berl) 2000;152:216–223. doi: 10.1007/s002130000537. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kitabatake Y, Hikida T, Watanabe D, Pastan I, Nakanishi S. Impairment of reward-related learning by cholinergic cell ablation in the striatum. Proc Natl Acad Sci U S A. 2003;100:7965–7970. doi: 10.1073/pnas.1032899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligorio M, Descarries L, Warren RA. Cholinergic innervation and thalamic input in rat nucleus accumbens. J Chem Neuroanat. 2009;37:33–45. doi: 10.1016/j.jchemneu.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- Mark GP, Kinney AE, Grubb MC, Zhu X, Finn DA, Mader SL, Berger SP, Bechtholt AJ. Injection of oxotremorine in nucleus accumbens shell reduces cocaine but not food self-administration in rats. Brain Res. 2006;1123:51–59. doi: 10.1016/j.brainres.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Lopes da Silva FH. Muscarinic modulation of synaptic transmission in slices of the rat ventral striatum is dependent on the frequency of afferent stimulation. Brain Res. 1994;645:231–239. doi: 10.1016/0006-8993(94)91656-x. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J. Spiny neurons lacking choline acetyltransferase immunoreactivity are major targets of cholinergic and catecholaminergic terminals in rat striatum. J Neurosci Res. 1990;25:263–280. doi: 10.1002/jnr.490250302. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Picconi B, Tolu M, Giacomini P, Scarnati E. Role of tonically-active neurons in the control of striatal function: cellular mechanisms and behavioral correlates. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:211–230. doi: 10.1016/s0278-5846(00)00153-6. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Kelley AE. Nucleus accumbens acetylcholine regulates appetitive learning and motivation for food via activation of muscarinic receptors. Behav Neurosci. 2004;118:730–739. doi: 10.1037/0735-7044.118.4.730. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Kelley AE. Striatal muscarinic receptor antagonism reduces 24-h food intake in association with decreased preproenkephalin gene expression. Eur J Neurosci. 2005;22:3229–3240. doi: 10.1111/j.1460-9568.2005.04489.x. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Spencer RC, Kelley AE. Muscarinic receptor antagonism of the nucleus accumbens core causes avoidance to flavor and spatial cues. Behav Neurosci. 2007;121:1215–1223. doi: 10.1037/0735-7044.121.6.1215. [DOI] [PubMed] [Google Scholar]
- Rahman S, McBride WJ. Involvement of GABA and cholinergic receptors in the nucleus accumbens on feedback control of somatodendritic dopamine release in the ventral tegmental area. J Neurochem. 2002;80:646–654. doi: 10.1046/j.0022-3042.2001.00739.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Sauerberg P, Nielsen EB, Swedberg MD, Thomsen C, Sheardown MJ, Jeppesen L, Calligaro DO, DeLapp NW, Whitesitt C, Ward JS, Shannon HE, Bymaster FP, Fink-Jensen A. Muscarinic receptor agonists decrease cocaine self-administration rates in drug-naive mice. Eur J Pharmacol. 2000;402:241–246. doi: 10.1016/s0014-2999(00)00442-8. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006a;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Systemic administration of a dopamine, but not a serotonin or norepinephrine, transporter inhibitor reinstates cocaine seeking in the rat. Behav Brain Res. 2006b;175:189–194. doi: 10.1016/j.bbr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LS, Miller AD, Lester DB, Bay-Richter C, Schulein C, Frikke-Schmidt H, Wess J, Blaha CD, Woldbye DP, Fink-Jensen A, Wortwein G. Increased amphetamine-induced locomotor activity, sensitization, and accumbal dopamine release in M(5) muscarinic receptor knockout mice. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Uchimura N, Jiang ZG, North RA. Distinct muscarinic receptors inhibit release of gamma-aminobutyric acid and excitatory amino acids in mammalian brain. Proc Natl Acad Sci U S A. 1991;88:2608–2611. doi: 10.1073/pnas.88.6.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci. 2005;25:8141–8149. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura N, North RA. Muscarine reduces inwardly rectifying potassium conductance in rat nucleus accumbens neurones. J Physiol. 1990;422:369–380. doi: 10.1113/jphysiol.1990.sp017989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaro MT, Wiederhold KH, Palacios JM, Mengod G. Muscarinic M2 receptor mRNA expression and receptor binding in cholinergic and non-cholinergic cells in the rat brain: a correlative study using in situ hybridization histochemistry and receptor autoradiography. Neuroscience. 1992;47:367–393. doi: 10.1016/0306-4522(92)90253-x. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–1797. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Warren RA. Muscarinic and nicotinic presynaptic modulation of EPSCs in the nucleus accumbens during postnatal development. J Neurophysiol. 2002;88:3315–3330. doi: 10.1152/jn.01025.2001. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 2002;22:6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]