Summary
Melanocortin-4-receptor (MC4R) mutations cause dysregulation of energy balance and hyperinsulinemia. We have used mouse models to study the physiological roles of extrahypothalamic MC4Rs. Re-expression of MC4Rs in cholinergic neurons (ChAT-Cre, loxTB MC4R mice) modestly reduced body weight gain without altering food intake and was sufficient to normalize energy expenditure and attenuate hyperglycemia and hyperinsulinemia. In contrast, restoration of MC4R expression in brainstem neurons including those in the dorsal motor nucleus of the vagus (Phox2b-Cre, loxTB MC4R mice) was sufficient to attenuate hyperinsulinemia, while the hyperglycemia and energy balance were not normalized. Additionally, hepatic insulin action and insulin mediated-suppression of hepatic glucose production were improved in ChAT-Cre, loxTB MC4R mice. These findings suggest that MC4Rs expressed by cholinergic neurons regulate energy expenditure and hepatic glucose production. Our results also provide further evidence of the dissociation in pathways mediating the effects of melanocortins on energy balance and glucose homeostasis.
Introduction
Increased energy intake and decreased energy expenditure contribute to the development of obesity and its co-morbidities, such as type 2 diabetes, making this problem one of the most topical health issues facing western society. Alteration in energy balance is the main determinant of body composition and body weight, and is coordinated by central nervous system (CNS) pathways, including the central melanocortin system (Cone, 2005; Cone, 2006; Morton et al., 2006). An important component of this system is the melanocortin-4 receptor (MC4R), which is expressed by a number of neurons in the CNS (Mountjoy et al., 1994; Kishi et al., 2003; Liu et al., 2003). The role of MC4Rs in regulation of energy balance is demonstrated by severe obesity in MC4R knockout mice, in which obesity is caused by the combined effects of increased food intake and decreased energy expenditure (Huszar et al., 1997; Chen et al., 2000; Ste Marie et al., 2000). Importantly, this obesity syndrome also occurs in humans with MC4R mutations (Vaisse et al., 1998; Yeo et al., 1998).
The role of melanocortins in regulating energy balance has also been demonstrated through pharmacologic experiments. For example, microinjection of non-selective MC3R/MC4R agonist (MTII) and antagonist (SHU9119) into the fourth ventricle near the nucleus of the solitary tract (NTS) and dorsal motor nucleus of the vagus (DMV) or intraparenchymal injection to dorsal vagal complex (including the DMV and NTS) results in an alteration in food intake (Grill et al., 1998; Williams et al., 2000; Zheng et al., 2005). Moreover, recent experiments using these pharmacologic compounds suggest that the feeding and the thermogenic effects of MC3R/MC4R stimulation can be mediated by multiple, anatomically distributed CNS nuclei (Skibicka and Grill, 2009).
While the importance of MC4R signaling in the regulation of energy homeostasis is clear, the specific neural populations responsible for MC4R-mediated regulation of food intake and energy expenditure are at present only partially identified. Restoration of MC4R expression in specific forebrain areas, such as the paraventricular nucleus of the hypothalamus (PVH) and subpopulations of medial amygdala neurons using Sim1-Cre transgenic mice, significantly reduces (~60%) the obesity characteristic of MC4R deficiency (Balthasar et al., 2005). In this model, the increased food intake, typical for MC4R mutations is completely rescued while reduced energy expenditure is unaffected. Similarly, these mice still display impaired glucose homeostasis despite marked improvements in body weight and adiposity. These findings suggest that MC4Rs in the PVH and/or the amygdala participate in the regulation food intake but MC4Rs expressed elsewhere in the CNS might control energy expenditure (Balthasar et al., 2005).
Extrahypothalamic sites where prominent MC4R expression is observed include the cholinergic parasympathetic preganglionic neurons of the DMV (Mountjoy et al., 1994; Kishi et al., 2003; Liu et al., 2003). Similarly, afferent neurons of the nodose ganglion that terminate in the NTS express MC4R (Wan et al., 2008; Gautron et al., 2010). However, the neuronal cell types expressing MC4R in cell bodies of the NTS (Kishi et al., 2003; Liu et al., 2003) are currently unknown.
The neurons of the DMV send visceral efferent output to several internal organs including the pancreas and digestive tract. In contrast, the NTS receives afferent sensory information from the viscera through chemosensory neurons and integrates this with information from descending inputs from the CNS, providing overall coordination of several digestive and neuroendocrine functions (Berthoud and Powley, 1990; Rogers et al., 1996). Notably, our recent data have shown that several peripheral tissues including the liver, stomach and the duodenum are innervated by MC4R expressing vagal afferents and efferents (Gautron et al., 2010).
Another example of extra-hypothalamic MC4R expression are the sympathetic preganglionic neurons of the intermediolateral nucleus of the thoracic spinal cord (IML), which co-express MC4Rs and choline acetyltransferase (ChAT) mRNA (Kishi et al., 2003). Importantly, IML neurons are also directly innervated by a subset of leptin-activated POMC neurons residing in the arcuate nucleus (Elias et al., 1998; Swanson and Kuypers, 1980). Postganglionic neurons innervating brown adipose tissue receive projections from IML, thus providing a pathway to regulate energy expenditure and diet-induced thermogenesis (Bamshad et al., 1999; Rothwell and Stock, 1984; Lowell and Flier, 1997). Taken together, it is possible that MC4Rs in both cholinergic preganglionic sympathetic and parasympathetic neurons may participate in the regulation of energy balance and glucose/insulin homeostasis, but the physiological significance of these pathways has remained difficult to establish.
To directly assess the role of MC4Rs in these extrahypothalamic cholinergic sites, we have generated two mouse models where MC4R expression is restored specifically in both cholinergic preganglionic parasympathetic (DMV) and sympathetic (IML) neurons (ChAT-Cre, loxTB MC4R mice) or only in cholinergic preganglionic parasympathetic neurons (Phox2b-Cre, loxTB MC4R mice). By using these unique genetic tools and parallel approaches we disassociated the contributions of MC4-Rs expressed by sympathetic vs. parasympathetic neurons in the regulation of food intake, energy expenditure and glucose/insulin homeostasis.
Results
Generation of ChAT-IRES-Cre and Phox2b-Cre BAC transgenic mice
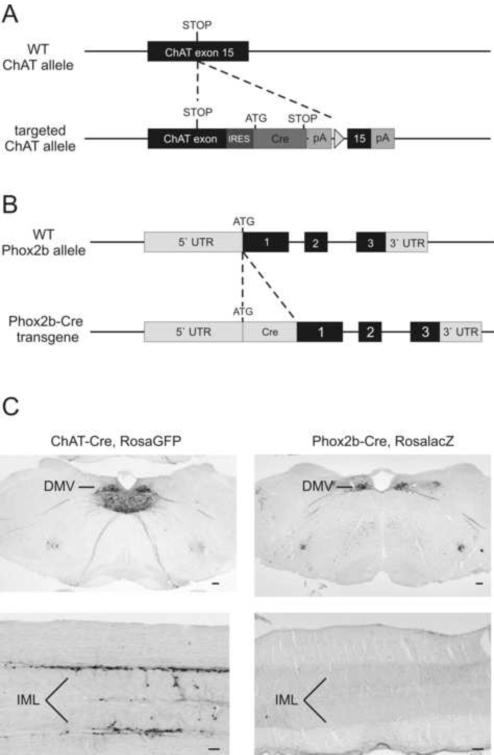
To target Cre expression to cholinergic preganglionic sympathetic and parasympathetic neurons (ChAT-IRES-Cre mice, Jackson Laboratories stock #006410), an optimized internal ribosome entry sequence (IRES) fused to Cre recombinase was inserted after the stop codon of the ChAT gene (Fig 1A). To assess the expression pattern of Cre activity, chimeric ChAT-IRES-Cre mice were crossed with RosaGFP reporter mice, which express GFP after Cre-mediated deletion of a loxP-flanked transcriptional blocker (Jackson Laboratories stock # 004077). Strong GFP activity was found in the preganglionic parasympathetic neurons of the DMV, and in the sympathetic preganglionic neurons in the IML of the thoracic spinal cord (Figure 1C), sites which endogenously express ChAT mRNA (Kishi et al., 2003). Expectedly, we also observed GFP expression in other known cholinergic CNS sites including the nucleus basalis, medial septum, vertical diagonal band of broca, laterodorsal tegmental area (LDT), peduncular pontine tegmental nucleus (PPT), nucleus ambiguous, and cranial nerve nuclei (Armstrong et al., 1983).
Figure 1.
Generation of ChAT-IRES-Cre and Phox2b-Cre BAC Transgenic Mice
(A) To generate mice expressing Cre-recombinase specifically in preganglionic sympathetic and parasympathetic neurons, an optimized internal ribosome entry sequence (IRES) fused to Cre recombinase was inserted after the stop codon of the ChAT gene using homologous recombination techniques. (B) To target Cre-expression in preganglionic parasympathetic neurons, a Cre-recombinase cassette was introduced into the ATG translation start codon of the Phox2b BAC, using bacteria dependent recombination. (C, left column) GFP immunohistochemistry in ChAT-Cre, Rosa GFP mice. Prominent GFP activity was observed in dorsal motor nucleus of vagus (DMV) and in the intermediolateral cell column (IML). (C, right column) Beta-galactosidase immunohistochemistry in Phox2b-Cre, RosalacZ mice. Strong Cre mediated lacZ expression was observed in DMV. No immunoreactivity is observed in IML. Scale bar=100μm.
To target Cre expression specifically to brainstem autonomic control neurons (Pattyn et al., 1997; Brunet and Pattyn, 2002) including cholinergic parasympathetic preganglionic neurons, a BAC clone containing the Phox2b locus flanked by >75Kb of genomic DNA at both the 3` and 5` ends of the gene was used to drive Cre recombinase expression transgenically (Figure 1B). Phox2b-Cre BAC transgenic mice were crossed with a R26R beta-galactosidase (LacZ) reporter mouse line (Jackson Laboratories stock #003309) to map the expression pattern of Cre-recombinase. Cre activated lacZ expression was detected in parasympathetic preganglionic neurons of the DMV as expected (Figure 1C) and in other brachial and visceral motor neurons (not shown). Importantly, expression of lacZ was not observed in preganglionic sympathetic neurons of the truncal spinal cord (Figure 1C). Similarly, Cre activated lacZ expression in other central nervous system sites was clearly below the level required for the recombination (not shown). Furthermore, although Phox2b is expressed by several subtypes of neurons during development (Pattyn et al., 1997), we did not detect Phox2b-Cre activated reporter expression in peripheral ganglia (such as postganglionic sympathetic- and parasympathetic ganglia, dorsal root ganglia, and enteric nervous system) or peripheral tissues such as liver, pancreas, muscle or white- and brown adipose tissue (not shown).
Double immunohistochemistry for ChAT and LacZ revealed strong Cre-activated reporter expression specifically in cholinergic preganglionic neurons of the DMV (Supplemental Figure 1). In contrast, only a few lacZ labeled cells were seen in the NTS and area postrema. Importantly, cholinergic preganglionic sympathetic neurons of the IML were devoid of Cre-immunoreactivity (Supplemental Figure 1).
Re-expression of Mc4r mRNA in cholinergic preganglionic sympathetic vs. parasympathetic neurons
We have previously shown that both cholinergic sympathetic and parasympathetic preganglionic neurons express MC4-Rs, suggesting that both of these neuronal populations might mediate neuroendocrine, autonomic, and behavioural effects of melanocortins (Kishi et al., 2003). To study the role of MC4R signaling in cholinergic neurons, we have used mice with a loxP-modified, null Mc4r allele (loxTB MC4R mice), an allele which can be re-activated by Cre-recombinase (Balthasar et al., 2005). To dissociate the roles of MC4Rs on sympathetic vs. parasympathetic preganglionic neurons, loxTB MC4R mice were crossed with ChAT-IRES-Cre (ChAT-Cre, loxTB MC4R) and Phox2b-Cre BAC transgenic (Phox2b-Cre, loxTB MC4R) mice.
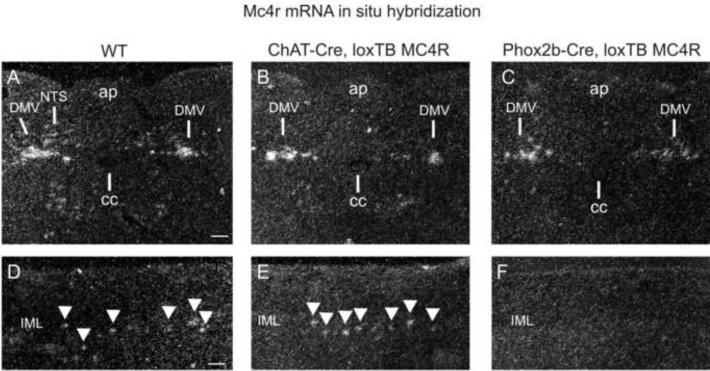
To analyze Mc4r mRNA re-expression, in situ hybridization was performed on ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R mice (Figure 2). In ChAT-Cre, loxTB MC4R mice, Mc4r mRNA expression was restored in the full rostrocaudal axis of DMV and in the thoracic spinal cord, including the IML, the areas which endogenously express MC4Rs in wild-type mice (Fig. 2B, E).
Figure 2.
Expression of Mc4r mRNA in ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R Mice
(A) Mc4r mRNA in situ hybridization in hindbrain of wild-type mouse. Note Mc4r mRNA expression in both DMV and NTS. (B, C) In ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R mice Mc4r mRNA is re-expressed specifically in the DMV. (D) Mc4r mRNA in situ hybridization in wild-type mice IML of the thoracic spinal cord. (E) In ChAT-Cre, loxTB MC4R mice Mc4r mRNA is re-expressed in rostral-caudal axis of longitudinal section of thoracic IML (arrowheads), comparably to wild-type mice (D). (C) In Phox2b-Cre mice, Mc4r mRNA is not re-expressed in IML. Scale bar=100μm.
Consistently with limited NTS Cre-expression in Phox2b-Cre, loxTB MC4R mice (Supplemental Figure 1), Mc4r mRNA re-expression was seen mainly in preganglionic parasympathetic neurons of the DMV, and with low levels in NTS (Figure 2C). Importantly, Mc4r mRNA was not re-expressed in sympathetic preganglionic neurons in the IML (Figure 2F).
MC4R-mediated effects in cholinergic neurons on body weight and composition
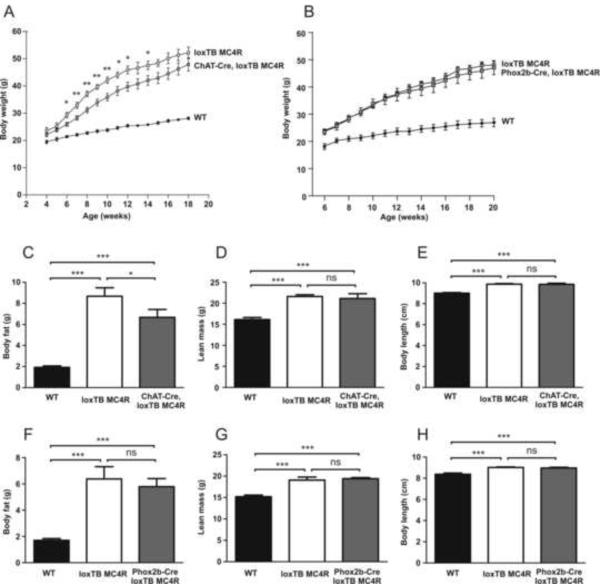
Re-expression of MC4Rs in cholinergic neurons had a small, but significant effect on body weight (Figure 3A). Starting from 6–8 weeks of age, ChAT-Cre, loxTB MC4R mice were approximately 10% leaner compared to homozygous loxTB MC4 mice, but were still markedly obese compared to wild type control mice. Wild-type mice containing a ChAT-Cre transgene did not show this body weight phenotype, indicating that the transgene itself does not negatively affect body weight gain (not shown). To determine the origin of increased body weight, body composition was studied in more detail using NMR in 7–8-week-old WT, loxTB MC4R and ChAT-Cre, loxTB MC4R mice (Figure 3C and 3D). As shown previously, the increased body weight in loxTB MC4R mice arose both from increased body fat and lean body mass (Balthasar et al., 2005). Notably, although ChAT-Cre, loxTB MC4R mice had markedly higher body fat content in contrast to wild-type mice, they had significantly reduced adiposity in comparison to loxTB MC4R mice (Figure 3C). In contrast to ChAT-Cre, loxTB MC4R mice, reactivation of MC4R signaling in preganglionic parasympathetic neurons in Phox2b-Cre, loxTB mice was not sufficient to affect body weight or composition (Figure 3B, 3F and 3G).
Figure 3.
Body weight curve and body composition in ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R mice.
(A) Body weight curve in ChAT-Cre, loxTB MC4R male mice (n=7–11/group, mean±SEM, *p<0.05, **p<0.01). (B) Body weight curve in Phox2b-Cre, loxTB MC4R mice (n=6–12/group, mean±SEM) (C–E) Body composition and body length in 7–8-week old ChAT-Cre, loxTB MC4R male mice (8–14/group, mean±SEM, *p<0.05, ***p<0.001). (F–H) Body composition and body length in 7–8-week old Phox2b-Cre, loxTB MC4R mice (n=7–10/group, mean±SEM, ***p<0.001).
Since MC4Rs are known to regulate body length (Huszar et al., 1997; Balthasar et al., 2005), snout-anus length was measured in 7–8-week-old male mice (Figure 3E and 3H). The body length of loxTB MC4R, ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R mice was comparable, and significantly higher in comparison to WT mice. Thus, reactivation of MC4R receptors in cholinergic sympathetic or parasympathetic preganglionic neurons is not sufficient to suppress linear growth characteristic of MC4R deficiency.
In summary, reactivation of melanocortin-4 signaling in sympathetic preganglionic, but not in parasympathetic neurons plays a small, but significant role in regulation of body weight and body composition.
MC4R-mediated effects in cholinergic neurons on food intake and energy expenditure
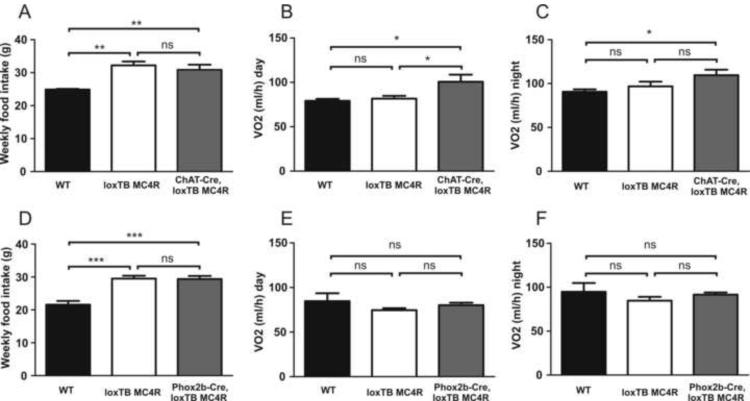
We next monitored feeding behavior and energy expenditure to study the effects of MC4R re-expression on cholinergic preganglionic sympathetic vs. parasympathetic neurons on energy balance. Weekly food intake did not differ between wild type and ChAT-Cre littermates, indicating that the transgene itself does not have an effect on feeding behavior. In contrast to wild-type control mice, loxTB MC4R, ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R mice displayed significant hyperphagia (Figure 4A and 4D). Importantly, increased food intake typical for loxTB MC4R mice was not altered in ChAT-Cre, loxTB MC4R or Phox2b-Cre mice. This suggests that re-expressing MC4Rs solely in cholinergic preganglionic sympathetic or parasympathetic neurons is not sufficient to affect melanocortin-mediated regulation of food intake.
Figure 4.
Weekly food intake and energy expenditure in ChAT-Cre, loxTB MC4R and Phox2bCre, loxTB MC4R mice. (A) Weekly food intake in 8-week-old male ChAT-Cre, loxTB MC4R mice (n=8–11/group, mean±SEM, **p<0.01). (B and C) Average oxygen consumption was calculated for both light (B) and dark (C) periods in 6–7-week old ChAT-Cre, loxTB MC4R mice (n=8–9/group, mean±SEM, *p< 0.05). (D) Weekly food intake in 8-week-old male Phox2bCre, loxTB MC4R mice (n=6–12/group, mean±SEM, ***p<0.001). Energy expenditure in 6–7-week-old Phox2b-Cre, loxTB MC4R during light (E) or dark (F) cycle (n=6/group, mean±SEM).
Since reactivation of MC4Rs in cholinergic neurons had a small effect on body weight without changes in food intake, we also analyzed energy expenditure. As an assessment of energy expenditure, oxygen consumption was measured in 6–7-week-old males using integrated indirect calorimetry (TSE Systems®) (Figure 4B, C, E and F). To exclude the possibility that changes in body composition would affect the differences in metabolic rate, energy expenditure measurements were also analyzed from weight matched loxTB MC4R, ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R mice (Supplemental Figure 2).
Notably, ChAT-Cre, loxTB MC4R mice (Figure 4B and 4C), but not Phox2b-Cre, loxTB MC4R mice (Figure 4E and 4F) displayed significantly increased oxygen consumption during the light and dark period. Similar results were obtained when the oxygen consumption was expressed per gram of lean body mass (Supplemental Figure 2) or when the data was expressed on a total body weight basis (not shown). Respiratory exchange ratio was similar between groups. Similarly, locomotor activity did not differ between genotypes (Supplemental Figure 2).
Taken together, re-expression of Mc4r mRNA in cholinergic preganglionic (sympathetic and parasympathetic) neurons does not affect feeding behavior. Furthermore, MC4Rs specifically on cholinergic sympathetic preganglionic, but not brainstem parasympathetic preganglionic neurons, mediate the effects of melanocortins on energy expenditure.
MC4R-mediated effects in cholinergic neurons on glucose homeostasis
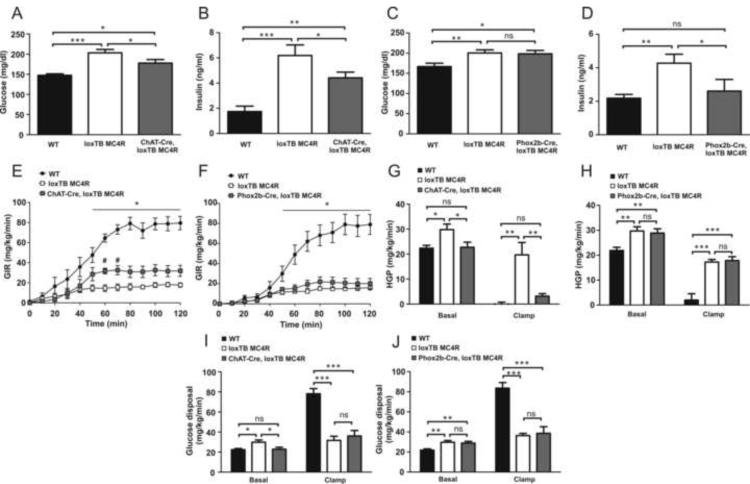
To investigate whether re-expression of MC4Rs in cholinergic preganglionic sympathetic or parasympathetic neurons affects glucose homeostasis, we monitored blood glucose and insulin levels in 5–6-week-old male mice (Figure 5 A–D). We found no difference in either glucose or insulin levels between wild type, ChAT-Cre or Phox2b-Cre control littermates, indicating that transgene itself does not have an effect on glucose homeostasis (not shown). Expectedly (Huszar et al., 1997; Balthasar et al., 2005) loxTB MC4R animals demonstrated marked hyperglycemia and hyperinsulinemia in comparison to wild-type mice (Figure 5A–D). We found that reactivation of MC4R signaling in cholinergic neurons ameliorated both hyperglycemia and −insulinemia (Figure 5A and 5B). Interestingly, while hyperinsulinemia was clearly attenuated in Phox2b-Cre, loxTB MC4R mice, hyperglycemia was not affected (Figure 5C and 5D). To exclude the possibility that observed changes in insulin levels could be due to altered pancreatic insulin content, we determined the total pancreatic insulin pool. Total pancreatic insulin content was similar between the genotypes (Supplemental Figure 3).
Figure 5.
Glucose and insulin metabolism in ChAT-Cre, MC4RloxTB and Phox2b-Cre, loxTB MC4R mice. (A and B) Plasma glucose and insulin in fed 5–6 week-old male ChAT-Cre, loxTB MC4R mice (n=8–12/group, mean±SEM, *p<0.05, **p<0.01, ***p<0.001). (C and D) Plasma glucose and insulin in fed 5–6 week-old male Phox2b-Cre, loxTB MC4R mice (n=11–18/group, mean±SEM, *p<0.05, **p<0.01). (E) Glucose infusion rate (GIR) during hyperinsulinemic-euglycemic clamp in chow-fed ChAT-Cre, loxTB MC4R mice (n= 6–7/group, mean±SEM, *p<0.05 between WT vs. loxTB MC4R and ChAT-Cre, loxTB MC4R mice, #p<0.05 between loxTB MC4R vs. ChAT-Cre mice). (F) Glucose infusion rate (GIR) during hyperinsulinemic-euglycemic clamp in chow-fed Phox2b-Cre, loxTB MC4R mice (n= 6–7/group, mean±SEM, *p<0.05 between WT vs. loxTB MC4R and Phox2b-Cre, loxTB MC4R mice). (G and H) Hepatic glucose production (EndoRa) at basal or hyperinsulinemic conditions (n= 6–7/group, mean±SEM, *p<0.05, **p<0.01, ***p<0.001). (I and J) Glucose disposal rate (Rd) at basal and hyperinsulinemic conditions (n= 6–7/group, mean±SEM, *p<0.05, **p<0.01, ***p<0.001).
To further characterize the mechanisms underlying dysregulation of glucose and insulin homeostasis, hyperinsulinemic-euglycemic clamp studies were performed in 3-month-old male mice (Hill et al., 2010). Basal glucose levels were elevated in ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R mice compared to wild-type mice (Supplemental Figure 4A and B). During the clamp plasma insulin levels were similarly elevated in all groups (Supplemental Figure 4C and D). The glucose infusion rate (GIR) required to maintain euglycemia was markedly reduced in loxTB MC4R mice compared to wild-type littermates, indicating impaired whole-body insulin action (Figure 5E and F). Notably, in ChAT-Cre, loxTB MC4R mice, the GIR was increased compared to loxTB MC4R mice, indicating improved insulin sensitivity (Figure 5E). This improvement in insulin sensitivity was not observed in Phox2b-Cre, loxTB MC4R mice (Figure 5F). Both basal hepatic glucose production (HPG) and glucose disposal were increased in loxTB MC4R in comparison to wild-type mice, consistent with observed hyperglycemia (Figure 5G–J).
During the clamp, WT mice displayed clear insulin mediated suppression of HGP and increased glucose disposal (Figure 5G–J). Notably, insulin mediated suppression in HGP was evident in ChAT-Cre, loxTB MC4R mice. This inhibition was not observed in Phox2b-Cre, loxTB MC4R animals (Figure 5G and H). Glucose disposal rates during clamp were comparable and similarly suppressed in ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R in comparison to WT littermates (Figure 5I and J). Our data indicate that MC4R expression specifically in cholinergic sympathetic preganglionic neurons improves hepatic insulin action compared the MC4R null mice. Moreover, our data suggest that MC4R signaling in parasympathetic preganglionic neurons alleviates hyperinsulinemia typical for MC4R deficiency but is not sufficient to normalize hepatic insulin action. Collectively, our results suggest that MC4Rs specifically in cholinergic neurons regulate the ability of insulin to suppress hepatic glucose production.
Discussion
While the multiple CNS sites expressing MC4Rs have been described in detail (Kishi et al., 2003; Mountjoy et al., 1994; Liu et al., 2003) the neuronal populations mediating the anti-obesity and anti-diabetic effects of melanocortin's remain unclear.
To elucidate the physiologically important sites of MC4R expression, we used mice with a loxP-modified, null MC4R allele (loxTB MC4R mice), which can be reactivated by Cre recombinase (Balthasar et al., 2005). A recent study from our group revealed a divergence in the melanocortin pathways in the control of food intake and energy expenditure. We found that MC4R re-expression in SIM1 neurons in the PVH and subpopulations of medial amygdala neurons in a MC4R-null background, completely normalized the increased food intake typical for MC4 null mice. However, this manipulation failed to improve energy expenditure, and only partially improved glucose homeostasis (Balthasar et al., 2005). To determine whether MC4Rs on cholinergic preganglionic sympathetic and/or parasympathetic neurons mediate melanocortin's effects on food intake, energy expenditure and glucose metabolism, we used Cre/loxP system to re-express MC4R in these neurons.
MC4R physiological roles in cholinergic neurons on aspects of body weight homeostasis
Our current data demonstrates that re-expression of MC4Rs in both cholinergic preganglionic sympathetic and parasympathetic neurons is not sufficient to rescue the increased body length typical for MC4R deficiency, but has a small, but significant role in body weight regulation. This reduction in body weight in ChAT-Cre, loxTB MC4R mice in comparison to loxTB MC4R mice was due to reduced body fat content.
To determine the mechanisms that contributed to this reduction in body weight, we measured energy intake and energy expenditure in ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R mice. Reactivation of MC4R receptors in both preganglionic cholinergic sympathetic and parasympathetic neurons in ChAT-Cre, loxTB MC4R mice or only in preganglionic cholinergic parasympathetic neurons in Phox2b-Cre, loxTB MC4R mice did not affect food intake. This finding is very interesting considering a number of studies suggesting a potentially important role for the MC4R-expressing brainstem neurons in the regulation of feeding behavior. For example, both POMC neurons and MC4R are present in the NTS, and animals with NTS lesion have lower food intake and body weight gain (Palkovits et al., 1987; Kishi et al., 2003; Mountjoy et al., 1994; Hyde and Miselis, 1983). Furthermore, the direct action of melanocortins has also been demonstrated through the microinjection of MC3R/MC4R non-selective agonist MTII and antagonist SHU9119 into the fourth ventricle near the NTS and DMV, or intraparenchymal injection to dorsal vagal complex (including the DMV and NTS), which results in an alteration in food intake (Grill et al., 1998; Williams et al., 2000; Zheng et al., 2005). Interestingly, the food intake suppressing effects of hypothalamic POMC activation can be prevented by MC3R/MC4R antagonist administration to NTS and its vicinity, suggesting a functional role of hypothalamic-brainstem neuronal projections in the control of feeding behavior (Zheng et al., 2010). Finally, recent experiments have suggested that activation of MC4Rs modulate glutamatergic inputs to the NTS, via mainly excitatory actions on presynaptic MC4Rs on vagal and nonvagal neurons (Wan et al., 2008). Our current data complements these pharmacologic findings. Since our model does not express Cre in all classes of NTS neurons, it is possible that MC4Rs non-cholinergic NTS neurons regulate food intake. However, it should be noted that MC4R bearing DMV neurons might participate in feeding behavior when MC4R expression is restored in other sites of the CNS. Future studies using mouse models with Cre expression targeting the NTS and vagal afferent pathways are clearly warranted.
A key finding of our study is that re-expression of MC4Rs in autonomic preganglionic neurons in young ChAT-Cre, loxTB MC4R mice improves the energy expenditure defect typical of MC4R deficiency. The most likely neuronal population involved in MC4R mediated regulation of energy expenditure are the sympathetic preganglionic neurons in IML. These cells express MC4Rs and are directly innervated by a subset of leptin-activated POMC neurons in the Arc (Elias et al., 1998). Furthermore, postganglionic neurons innervating brown adipose tissue receive projections from the IML, thus providing a pathway to regulate energy expenditure and diet-induced thermogenesis (Bamshad et al., 1999; Rothwell and Stock, 1984; Lowell and Flier, 1997). In line with these findings, rescued energy expenditure in ChAT-Cre, loxTB MC4R mice, but not with DMV specific MC4R reactivation in Phox2b-Cre, loxTB MC4R mice indicates that MC4Rs expressed specifically in the sympathetic preganglionic neurons of the spinal cord contribute to melanocortin's effects to regulate energy expenditure.
According to our current results, the observation that body weight and body composition was partially improved in ChAT-Cre, loxTB MC4R, but not in Phox2b-Cre, loxTB MC4R mice suggest that MC4Rs in sympathetic preganglionic neurons play a modest role through yet undefined effect on energy expenditure. The observed improvement in energy expenditure may be mediated by several potential mechanisms, such as Ca2+ cycling in muscle and brown adipose tissue, induction of brown adipocytes in white fat and enhanced proton leaks in interscapular BAT and muscle mitochondria (Kozak, 2010). Similarly, other mechanisms, such as the recently described direct melanocortinergic control of peripheral lipid metabolism via the sympathetic nervous system might contribute to reduced fat content in ChAT-Cre, loxTB MC4R mice (Nogueiras et al., 2007).
MC4R-mediated regulation of glucose homeostasis in cholinergic neurons
Several functional studies have suggested that the central melanocortin system regulates insulin secretion, glucose utilization and production. First, MC4R null mice display hyperinsulinemia prior to onset of obesity, and intracerebroventricular (icv) administration of melanocortin agonist MTII has been shown to decrease plasma insulin levels in ob/ob and lean mice (Huszar et al., 1997; Fan et al., 1997). Second, central administration of melanocortin agonists enhances the actions of insulin on both glucose uptake and production, while antagonism results in the opposite effect (Obici et al., 2001). These effects on hepatic and peripheral insulin action are likely driven by the activation of central melanocortinergic neurons and are presumably mediated through the autonomic nervous system (Obici et al., 2001). Finally, mutations in the human MC4R gene leads to hyperinsulinemia in comparison to sex and age matched obese control subjects who do not have MC4R mutations (Farooqi et al., 2003).
In the present study, we found that the selective re-expression of MC4Rs in both cholinergic preganglionic sympathetic and parasympathetic neurons reduces hyperglycemia and hyperinsulinemia, without changes in the feeding behavior. While hyperglycemia was clearly improved in ChAT-Cre, loxTB MC4R mice, this was not observed in Phox2b-Cre, loxTB mice. Thus, our data suggests that the effect of melanocortins to suppress blood glucose levels may be at least in part mediated through MC4Rs expressed in preganglionic sympathetic neurons. However, we cannot exclude the possibility that the improvements in hyperglycemia seen in ChAT-Cre, loxTB MC4R mice might be partially indirect, and mediated by improved body composition.
Selective re-expression of MC4Rs in DMV resulted in a significant reduction in plasma insulin levels. This result was unexpected considering the classically described role of vagal efferent stimulation of the pancreas as a stimulatory signal for insulin secretion (Ahren, 2000). Since DMV neurons are known to be spontaneously active (Travagli et al., 2006), it is tempting to speculate that re-expression of MC4Rs in cholinergic preganglionic neurons in Phox2b-Cre, loxTB MC4R mice might be sufficient to suppress this tonic activity through melanocortin-induced inhibition of DMV neurons, resulting in amelioration of hyperinsulinemia. Although our results indicate that MC4Rs signaling in preganglionic parasympathetic neurons is sufficient to suppress pancreatic insulin levels typical of MC4R deficiency, the possible contribution of MC4Rs in preganglionic sympathetic neurons of the IML in the suppression of insulin levels cannot be ruled out. Importantly, in both ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R mice the total pancreatic insulin content was comparable to that of wild type mice, indicating that the changes in blood insulin are not mediated by altered pancreatic insulin production.
Furthermore, our results confirm that central melanocortin neurons play an important role in regulation of glucose and insulin homeostasis. For example, the suppression of insulin mediated hepatic glucose production was observed only in ChAT-Cre, loxTB MC4R mice, indicating that MC4Rs in cholinergic preganglionic sympathetic neurons participate in the regulation of glucose homeostasis by suppressing hepatic glucose production. These findings agree with recent observations showing that hepatic muscarinic acetylcholine receptors, and parasympathetic cholinergic outflow is not critically involved in maintaining glucose homeostasis in mice (Li et al., 2009). Thus, in line with recent observations underlining the importance of sympathetic outflow on centrally mediated regulation of hepatic glucose production (van den Hoek et al., 2008), our data suggests that central melanocortin circuits contribute to the regulation of insulin action and hepatic glucose production through the sympathetic nervous system. Future studies to define the specific hepatic insulin signaling pathways are clearly warranted.
Our data does not exclude the possibility that other efferent, presumably noncholinergic or melanocortin-independent vagal pathways may exist which participate in regulation of glucose homeostasis in liver (Lam et al., 2005; Pocai et al., 2005).
Technical Considerations
In this study, we focused our efforts on examining the role of MC4Rs in extrahypothalamic cholinergic sites, and their involvement on food intake, energy expenditure and glucose metabolism. One consideration of the genetic approach is that reactivation of MC4Rs in the MC4R-null, loxTB model is dependent on both the spatial and temporal expression of Cre recombinase. Thus, we cannot definitively exclude the possibility that developmental compensation for alterations in the timing and pattern of MC4R expression in this model contributes in part to the metabolic phenotypes observed.
In addition, neuronal sub-population-specific reactivation of MC4Rs in the loxTB MC4R model can only determine whether MC4Rs expressed in those sub-populations are sufficient to mediate aspects of the regulation of energy balance. However, it is not possible to determine whether those neurons are the only site mediating these effects of melanocortins. Thus, here, we cannot rule out the possibility that MC4Rs also in noncholinergic neurons participate in the regulation of energy expenditure. Indeed, recent pharmacologic experiments suggest that a certain degree of redundancy exists in melanocortin mediated regulation of energy balance (Skibicka and Grill, 2009; Fan et al., 2007).
As we have reported in the previous publication, reactivation of MC4R expression in PVN/amygdala using Sim-1-Cre, loxTB MC4R mice prevents ~60% of the obesity and 100% of the hyperphagia observed in MC4R null mice (Balthasar et al., 2005). In the current study, while increased food intake typical for MC4R null mice was not rescued, reactivation of MC4R receptors in preganglionic sympathetic neurons only partially reduced body weight and adiposity. The difference in body weight between MC4R null mice and ChAT-Cre, loxTB MC4R mice was ~10%. Our data collectively indicate that MC4Rs expressed on PVH neurons do not regulate energy expenditure (Balthasar et al., 2005) and MC4Rs on ChAT neurons do not regulate food intake (this study). However, we do not believe in simple additivity of PVH and ChAT- neuron melanocortin-mediated effects due to the potential existence of aforementioned redundant pathways.
Other sites where MC4Rs are possibly reactivated include LDT and PPT where we have found co-expression of ChAT immunoreactivity and MC4R expression (Kishi et al., 2003; Liu et al., 2003). These sites are thought to be key components of the CNS arousal system and play an important role in the regulation of locomotor activity (Saper et al., 2001; Winn, 2006). Although we cannot exclude the possibility that MC4R reactivation in these areas may have effects on energy balance in our models, we favor the role of sympathetic preganglionic neurons in IML as mediators of MC4R action on energy expenditure, also since we did not see significant alterations in locomotor activity.
In summary, the present study shows that reactivation of melanocortin signaling in cholinergic sympathetic preganglionic neurons is sufficient to increase energy expenditure and attenuate hyperglycemia, hyperinsulinemia and hepatic glucose production without drastically affecting the hyperphagia or obesity typical of MC4R deficiency. In contrast, re-expression of MC4Rs in cholinergic preganglionic parasympathetic neurons has no effect on food intake, energy expenditure, and hepatic glucose production. Current findings emphasize the importance of MC4R signaling for glucose and insulin homeostasis and provide further evidence of the dissociation in pathways mediating the effects of melanocortin on energy balance. Moreover, our observations provide information by which the dysregulation of these autonomic pathways could contribute to the pathophysiology of type II diabetes.
Experimental Procedures
Generation of ChAT-IRES-Cre and Phox2b-Cre BAC Transgenic Mice
For detailed description, see Supplemental Experimental Procedures. Briefly, to target Cre expression specifically to cholinergic neurons (ChAT-IRES-Cre mice), an optimized internal ribosome entry sequence (IRES) fused to Cre recombinase followed by a neomycin resistance cassette that is flanked by Flp recombinase recognition sites (FRT sites) was inserted after the ChAT stop codon. Generation of genetically modified mice and removal of Flp-flanked neomycin cassette was as previously described (Tong et al., 2007).
To target Cre-expression in preganglionic parasympathetic neurons, a BAC clone containing the Phox2b locus flanked by >75Kb of genomic DNA at both the 3` and 5` ends of the gene, was used to drive Cre recombinase expression. A Cre recombinase cassette was introduced into the ATG translation start codon of the Phox2b BAC, using bacteria dependent recombination. Briefly, a Cre expression cassette was coupled to 90bp of DNA homologous to the Phox2b locus 5` of the Phox2b ATG and to DNA directly 3` of the ATG by PCR. This targeting fragment was then electroporated into recombination competent bacteria harboring the Phox2b BAC. Recombinants were identified by positive antibiotic selection and injected into C57Bl/6J mouse pronuclei to produce independent founder lines. All animal procedures were conducted in accordance with UTSW Southwestern Institutional Animal Care and Use Committee guidelines and those of the Association for Assessment and Accreditation of Laboratory Animal Care.
In Situ Hydridization
In situ hybridization for mouse Mc4r mRNA was performed as previously described (Balthasar et al., 2005). The following primers were used to generate mouse specific Mc4r cDNA probe: F 5'-ATTACCTTGACCATCCTGAT-3' and R 5'-ATGTCAATTCATAACGCCCA-3'.
Immunohistochemistry
ChAT-IRES-Cre and Phox2b-Cre BAC transgenic mice were crossed with Rosa-GFP (Jackson Laboratories, stock #004077) and R26R LacZ (Jackson Laboratories stock #003309) reporter mouse lines, respectively. Douple transgenic offspring were perfused transcardially with 10% formalin and the brains and spinal cord were sectioned on a sliding microtome and immunohistochemistry was performed using same protocols and antibodies as previously described (Balthasar et al., 2005).
Energy expenditure
For detailed description, see Supplemental Experimental Procedures. Briefly, energy expenditure (O2 consumption) was measured by indirect calorimetry using TSE Labmaster monitoring system (TSE Systems GmbH, Bad Homburg, Germany). For metabolic measurements, 6–7-week old male mice were used. Average oxygen consumption was calculated for both light and dark periods and expressed per animal.
Body Weight, Composition, Length and Blood Samples
Body weight gain was measured starting 4–5-weeks of age. Body composition of 7–8-week-old ad libitum fed male mice was assessed using nuclear magnetic resonance spectroscopy using Bruker Minispec q10 (Bruker Optics, Billerica, MA). For nose-anus body length measurements, 7–8-week-old male mice were manually immobilized and gently extended to their full length. All body length measurements were performed by the same investigator. Tail vein blood was collected during morning hours (9–10am) and assayed for glucose (One Touch Ultra, Lifescan, Milpitas, California), and insulin levels (Ultra Sensitive Rat Insulin ELISA-kit, Crystal Chem. Inc., Downers Growe, Illinois) from ad libitum fed 5–6-week-old male mice.
Hyperinsulinemic-euglycemic clamp
Hyperinsulinemic-euglycemic clamps were performed as previously described (Hill et al., 2010). Age matched, and similar body weight MC4R null and reactivated ChAT-Cre, loxTB MC4R and Phox2b-Cre, loxTB MC4R mice were used to avoid the confounding influence of body weight on results. For detailed description, see Supplemental Experimental procedures.
Statistical Methods
Statistical analysis was carried out using GraphPad5 (GraphPad, San Diego, California) software. Statistical analysis for glucose infusion rate during hyperinsulinemiceuglycemic clamp was carried out by using two-way, repeated measures ANOVA, followed by Bonferroni post test to compare replicate means. All other data sets were analyzed by one-way ANOVA, followed by post hoc Student-Newman-Keuls test. All data are presented as mean ± SEM. P-value less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
We would like to thank the Mouse Metabolic Phenotyping Cores at University of Texas Southwestern Medical Center at Dallas (supported by PL1 DK081182-01 and UL1RR024923) and Beth Israel Deaconess Transgenic Core (supported by BNORC P30 DK46200 and BADERC P30 DK057521). We thank Dr. Jen-Chieh Chuang for providing technical advice in total pancreatic insulin measurements, Dr. Liz Robertson for providing the IRES-Cre fusion plasmid, and Dr. Laurent Gautron for the discussion of our work. This work was supported by grants from: J.R. (Sigrid Jusélius Foundation, Academy of Finland, and Finnish Cultural Foundation), N.B. (Wellcome Trust, ADAEASD Transatlantic Fellowship, BONRC P30DK046200), D.O. (K08DK071561), M.S. (K99DK024719-02) E.B. (TORS-Interdisciplinary Research Training Program 5TL1DK081181), B.B.L. (RO1DK075632) and J. K. E. (R01DK53301, DK071320 and RL1DK081185) and support from the American Diabetes Association and a Smith Family Foundation Pinnacle Program Project Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216:53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Identification of vagal preganglionics that mediate cephalic phase insulin response. Am. J. Physiol. 1990;258:R523–R530. doi: 10.1152/ajpregu.1990.258.2.R523. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A. Phox2 genes - from patterning to connectivity. Curr. Opin. Genet. Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, Marsh DJ, Forrest MJ, Gopal-Truter S, Fisher J, Camacho RE, Strack AM, Mellin TN, MacIntyre DE, Chen HY, Van der Ploeg LH. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr. Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Fan W, Morrison SF, Cao WH, Yu P. Thermogenesis activated by central melanocortin signaling is dependent on neurons in the rostral raphe pallidus (rRPa) area. Brain Res. 2007;1179:61–69. doi: 10.1016/j.brainres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J Med. 2003;20(348):1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol. 2010;518:6–24. doi: 10.1002/cne.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci. 1998;18:10128–10135. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Bruning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Miselis RR. Effects of area postrema/caudal medial nucleus of solitary tract lesions on food intake and body weight. Am. J Physiol. 1983;244:R577–R587. doi: 10.1152/ajpregu.1983.244.4.R577. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11:263–267. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat. Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- Li JH, Gautam D, Han SJ, Guettier JM, Cui Y, Lu H, Deng C, O'Hare J, Jou W, Gavrilova O, Buettner C, Wess J. Hepatic muscarinic acetylcholine receptors are not critically involved in maintaining glucose homeostasis in mice. Diabetes. 2009;58:2776–2787. doi: 10.2337/db09-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Flier JS. Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Annu. Rev. Med. 1997;48(307–16):307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schurmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O'Rahilly S, Rohner-Jeanrenaud F, Tschop MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin. Invest. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin. Invest. 2001;108:1079–1085. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M, Mezey E, Eskay RL. Pro-opiomelanocortin-derived peptides (ACTH/beta-endorphin/alpha-MSH) in brainstem baroreceptor areas of the rat. Brain Res. 1987;436:323–338. doi: 10.1016/0006-8993(87)91676-3. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Rogers RC, McTigue DM, Hermann GE. Vagal control of digestion: modulation by central neural and peripheral endocrine factors. Neurosci Biobehav Rev. 1996;20:57–66. doi: 10.1016/0149-7634(95)00040-l. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. Effects of denervating brown adipose tissue on the responses to cold, hyperphagia and noradrenaline treatment in the rat. J Physiol. 1984;355(457–63):457–463. doi: 10.1113/jphysiol.1984.sp015431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology. 2009;150:5351–5361. doi: 10.1210/en.2009-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. A direct projection from the ventromedial nucleus and retrochiasmatic area of the hypothalamus to the medulla and spinal cord of the rat. Neurosci. Lett. 1980;17:307–312. doi: 10.1016/0304-3940(80)90041-5. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu. Rev. Physiol. 2006;68(279–305):279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat. Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- van den Hoek AM, Van Heijningen C, Schroder-van der Elst JP, Ouwens DM, Havekes LM, Romijn JA, Kalsbeek A, Pijl H. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes. 2008;57:2304–2310. doi: 10.2337/db07-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud HR, Travagli RA. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci. 2008;28:4957–4966. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141:1332–1337. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- Winn P. How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol. Sci. 2006;248:234–250. doi: 10.1016/j.jns.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am. J Physiol Regul. Integr. Comp Physiol. 2005;289:R247–R258. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Rhodes CJ, Louis GW, Skibicka KP, Grill HJ, Myers MG, Jr., Berthoud HR. A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake. Am. J Physiol Regul. Integr. Comp Physiol. 2010;298:R720–R728. doi: 10.1152/ajpregu.00619.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.