Abstract
The pituitary gland contains six distinct hormone-secreting cell types that are essential for basic physiological processes including fertility and responding to stress. Formation of hormone-secreting cells during development relies on Notch signaling to prevent progenitors from prematurely differentiating. The nature of the signal curtailing Notch signaling in the pituitary is unknown, but a good candidate is the endocytic adaptor protein NUMB. NUMB targets Notch for proteolytic degradation, but it also has a broad range of actions, including stabilizing adherens junctions through interactions with cadherins and influencing cell proliferation by stabilizing expression of the tumor suppressor protein p53. Here, we show that NUMB and its closely related homolog, NUMBLIKE, are expressed in undifferentiated cells during development and later in gonadotropes in the anterior lobe and melanotropes of the intermediate lobe. All four isoforms of NUMB, are detectable in the pituitary, with the shorter forms becoming more prominent after adolescence. Conditionally deleting Numb and Numblike in the intermediate lobe melanotropes with Pomc Cre mice dramatically alters the morphology of cells in the intermediate lobe, coincident with impaired localization of adherens junctions proteins including E-CADHERIN, N-CADHERIN, β-CATENIN, and α-CATENIN. Strikingly, the border between posterior and intermediate lobes is also disrupted. These mice also have disorganized progenitor cells, marked by SOX2, but proliferation is unaffected. Unexpectedly, Notch activity appears normal in conditional knockout mice. Thus, Numb is critical for maintaining cell-cell interactions in the pituitary intermediate lobe that are essential for proper cell placement.
Conditional knockout of Numb and Numblike in the mouse pituitary intermediate lobe disrupts adherens junctions proteins, and alters Sox2 positive progenitor cell localization.
The pituitary is considered the master endocrine gland and is a convenient system with which to study cell fate determination due to the temporal and spatial separation of hormone producing cell differentiation during development. In the adult, pituitary hormone cell number can be altered in response to physiological need, suggesting a tight control of progenitor/stem cell maintenance, and mobilization is necessary to be maintained throughout the life of the animal (1). The five hormone-secreting cell types in the anterior pituitary include the corticotropes, thyrotropes, somatotropes, lactotropes and gonadotropes. These cells secrete ACTH, TSH, GH, prolactin (PRL) and LH and FSH, respectively. In mice, an additional intermediate lobe (IL) exists, and the hormone-secreting cell type is the melanotrope, which produces αMSH (2,3). ACTH and αMSH are alternate cleavage products of the Pomc gene. All cell types in the anterior lobe (AL) and IL develop from a common precursor primordium within the oral ectoderm called Rathke’s pouch (RP), whereas the posterior lobe (PL) is derived from neural tissue and contains glial cells known as pituicytes and axonal projections from the hypothalamus, which secrete oxytocin and arginine vasopressin (AVP).
In mice, RP begins to invaginate, and early differentiation markers are detectable at embryonic d 9.5 (e9.5), including LIM homeodomain transcription factors LHX3, LHX4, and ISL1, among others (4,5,6). Subsequent development of the pituitary involves an orchestration of many major signaling pathways important in other tissues during embryogenesis, including fibroblast growth factors, bone morphogenic proteins, Wnt proteins, Hedgehog factors, and Notch receptors and ligands (7,8,9,10,11,12,13,14,15). These pathways are all critical for induction of the pituitary from the oral ectoderm as well as proper gland structure and size; however, coordination of lineage determination remains largely unknown. Presently, one of the best-characterized cell lineage determinants includes the transcription factor Prophet of Pit1 (Prop1). Mutations in Prop1 cause a postnatal dwarf phenotype (16,17,18) and a reduction in the PIT1 lineage hormone-producing cells: thyrotropes, somatotropes, and lactotropes. Interestingly, NOTCH2 expression is dramatically reduced in the pituitary of Prop1 mouse mutants (12). Notch signaling also appears to directly regulate Prop1 expression. Loss of the essential Notch cofactor Rbp-J (CBF1) impairs PIT1 lineage development, due to loss of Prop1 and results in a premature appearance of the corticotrope lineage (13). A similar acceleration of corticotrope development is observed only when both Prop1 and the prototypical Notch target gene Hes1 are lost (19). Interestingly, complete Hes1 knockouts have a replacement of melanotropes in the IL with somatotropes, suggesting proper IL development is also Notch dependent (15). Finally, a reduction in Notch signaling is necessary for final differentiation of postmitotic PIT1 cells and gonadotropes (13,14). Notch signaling, therefore, cooperates with Prop1 and modifies its expression level. This ultimately influences pituitary cell differentiation in the PIT1 lineage, gonadotrope development timing, and corticotrope and melanotrope identity.
Notch-signaling pathway components expressed in the developing pituitary include the receptors NOTCH 2 and 3, the delta-like 1 ligand, and downstream targets Hes and Hey genes and are detectable from early pituitary formation in the mouse at e9.5 but diminish by e13.5–e14.5 (12,13). Recent data suggest that Notch signaling becomes active again in the adult pituitary and may be present in an adult pituitary stem cell side population (20,21). This population of multipotent cells are SOX2 positive and can differentiate into several hormone-producing cells in culture, which represent a putative source by which hormone-producing cells can be generated in the adult during times of physiological stress (22,23,24). Tight regulation of Notch activity, then, is important for cell fate determination in progenitor cells, including, potentially, stem cells, and improper Notch activation has been implicated in tumor formation (25,26). The protein NUMB is a Notch antagonist and represents a strong candidate for controlling Notch activity in the pituitary.
The adaptor protein NUMB was first identified as an important mediator of asymmetric cell division in Drosophila. Through interactions with the partition-defective (Par) complex including PAR3, PAR6, and atypical protein kinase C, d-NUMB is preferentially segregated to, and necessary for, the differentiation of one of two sensory organ precursor daughter cells (27,28). There is also evidence to suggest asymmetric NUMB segregation after mitosis in mammals. During neurogenesis in mice, asymmetric divisions producing a neuron and progenitor cell showed asymmetric localization of NUMB to the differentiating cell. However, during symmetric divisions producing two neuron daughter cells, NUMB was segregated to both daughter cells (29). One hypothesis is that cell fate is altered in progeny inheriting NUMB. NUMB induces degradation of the Notch receptor, thereby inhibiting Notch signaling and promoting cell differentiation. When Numb was conditionally deleted in developing neurons, however, roles for promoting as well as inhibiting progenitor cell maintenance were uncovered (30,31,32). It has been suggested these different roles of NUMB may also be due, in part, to the expression of multiple isoforms, two of which differ in sequence in the phosphotyrosine-binding (PTB) domain, and two that have alternate sequences in a proline-rich region (PRR). The functions of these regions are only beginning to be understood, but evidence suggests the longer PTB form is important in degradation of the Notch intracellular domain through interactions with ubiquitin ligase ITCH (33,34), as well as localizing NUMB to the plasma membrane (35) and interactions with integrin and cadherin proteins (36,37). Additional progenitor maintenance properties of NUMB may be directed by the longer PRR isoforms. During early mouse pancreas development before endocrine cell specification, both long and short forms of PRR are present; however, after the onset of endocrine cell specification, expression of the long PRR isoform is drastically reduced (38). Similarly, in mouse male germ cells, the longer PRR form is highly expressed compared with a germ cell-depleted testis (39). Lastly, in mouse cortical neuron cultures, overexpression of NUMB lacking the PRR region correlated with higher levels of differentiation transcription factor MASH1 and also enhanced neurite outgrowth compared with forms containing PRR (40). Together, this suggests the PTB region is important for cell adhesion and membrane localization whereas the PRR region, by some yet undetermined mechanism, influences progenitor maintenance and differentiation.
In part due to recent developments of identifying protein-binding domains and isoform variants, it has become clearer that NUMB has a broad range of functions that extends beyond restraining Notch signaling during asymmetric division, which may help to explain its diverse roles in many developmental and adult contexts. NUMB interacts with other proteins in addition to Notch to regulate their ubiquitination status. Importantly, loss of NUMB is associated with cancers of salivary gland, lung, and breast, many of which are due to increased p53 degradation (41,42,43,44). NUMB also interacts with cadherins and is necessary for proper cadherin membrane localization and cell adhesion (45), and evidence suggests NUMB partially controls epithelial- to mesenchymal-like transitions and alters cell migration as well (37). Therefore, delineating the functions of NUMB is important, not only for understanding progenitor cell maintenance during development but also adhesion and cell cycle control in adulthood.
The present study examines NUMB protein expression during mouse pituitary development and in adulthood. We show that NUMB and NUMBLIKE are expressed early in pituitary development before hormone cell specification, and that all isoforms are detectable before birth. These isoforms also exhibit dynamic expression patterns such that the shorter PTB and PRR forms are more prevalent than longer forms into adulthood. Interestingly, conditionally deleting Numb and Numblike from the developing corticotropes and melanotropes has identified a seemingly Notch-independent function for these proteins in the IL. Specifically, loss of Numb and Numblike shows the importance of these genes in maintaining cell adhesion proteins, facilitating proper localization of SOX2-positive progenitor cells, and establishing the posterior-intermediate lobe boundary.
Results
NUMB expression patterns in the developing pituitary
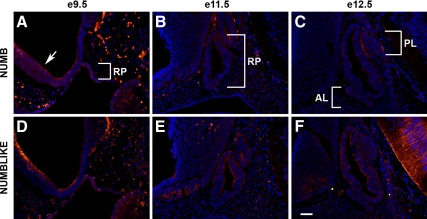
To visualize the expression of NUMB during the course of pituitary development and localize its expression, whole embryos were sectioned and stained using immunohistochemistry. NUMB and NUMBLIKE immunoreactivity was examined beginning from e9.5, where strong staining could be detected throughout RP (Fig. 1, A and D). Positive staining is readily observed in the diencephalon as well, particularly in the ventricular zone (arrow, panel A). The veracity of NUMB immunoreactivity was confirmed by a second NUMB antibody as well as in situ hybridization. NUMB protein (Supplemental Fig. 1A published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org) and mRNA (Supplemental Fig. 1C) appear sporadically expressed throughout RP in midsagittal sections at e10.5, whereas in more peripheral sections, NUMB protein (Supplemental Fig. 1B) and mRNA (Supplemental Fig. 1D) are more limited to the outer border of the gland. At e11.5, NUMB and NUMBLIKE expression appear reduced in RP, and only a few positive cells are observed in the diencephalon (Fig. 1, B and E). NUMBLIKE expression is still observed at the outer border and luminal side of RP and less so in the area to become the future AL. One day later, at e12.5, NUMB staining is similar with the exception of some positive cells at the border between the future IL and PL (Fig. 1C). NUMBLIKE staining is also similar at e12.5 with positive staining at luminal and outer borders and very little around the developing AL (Fig. 1F).
Figure 1.
NUMB and NUMBLIKE are preferentially expressed early in pituitary development. Sagittal sections at e9.5 (A and D), e11.5 (B and E), and e12.5 (C and F) immunostained with NUMB (A–C) and NUMBLIKE (D–F), counterstained with DAPI. Both NUMB and NUMBLIKE are enriched in RP at e9.5. Arrow, ventricular zone. Pictures taken at ×200. Scale bar, 50 μm. RP, Rathke’s pouch; AL, anterior lobe; PL, posterior lobe.
NUMB isoform switch during development
There are at least four isoforms of NUMB, each displaying a dynamic expression pattern in tissues during development and in tumor formation. These isoforms are distinguished by the presence or absence of a 48-amino acid sequence in the PRR and the presence or absence of an 11-amino acid sequence in the PTB region (Fig. 2). Due to the fact the antibodies used in this study recognize sequences outside of the PTB and PRR, to investigate isoform expression during pituitary development, we performed RT-PCR on cDNA prepared from samples from e16.5, postnatal d 1 (P1) and P9 and finally the adult pituitary.
Figure 2.
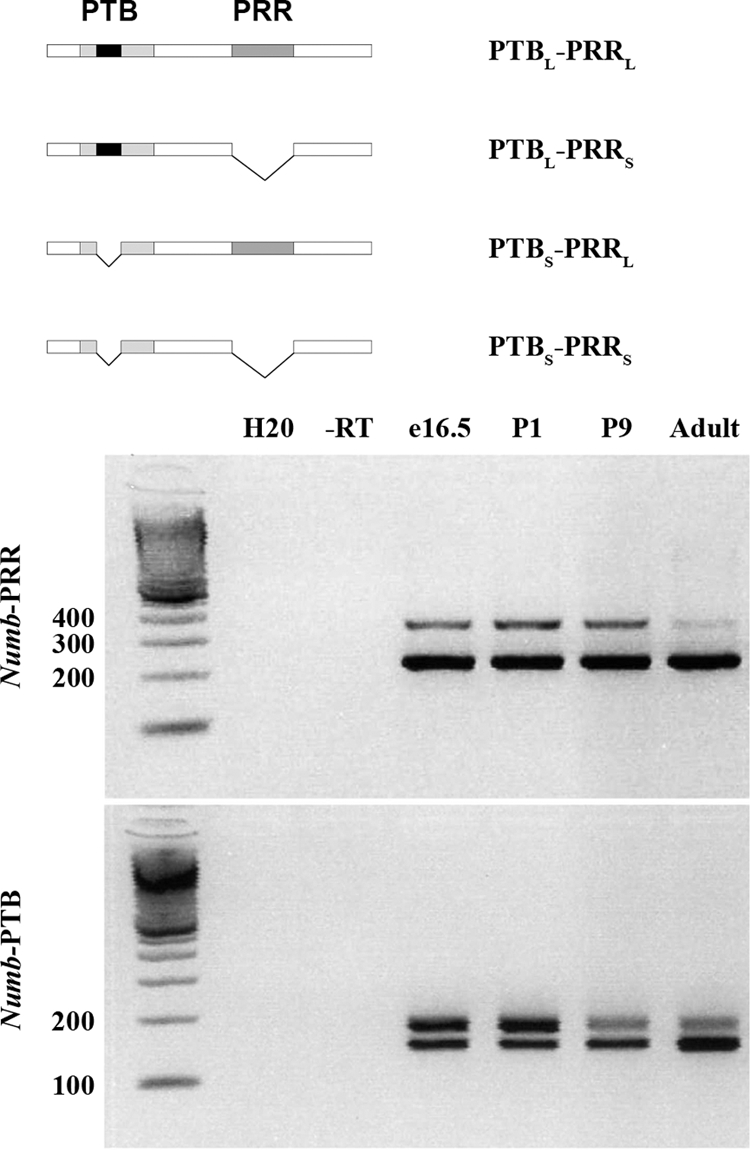
Shorter PRR and PTB Numb isoforms become dominant in adulthood. Diagram of alternatively spliced isoforms for Numb. RT-PCR using primers spanning the PRR and PTB. e16.5, P1, P9, and adult pituitary were analyzed. DNA standard measured in base pairs. −RT, −reverse transcriptase.
We observed that during development there is a switch in Numb isoform expression. For the PRR isoforms, the PRR short (PRRS) is present in the pituitary at all ages examined (Fig. 2). The PRR long (PRRL) isoform is most prevalent at e16.5 and P1. We begin to see a decline in PRR-long at P9, and it is detectable only at low levels in the adult pituitary. For the PTB isoforms, at e16.5 and P1, the PTBS and PTBL isoforms appear expressed at approximately equal levels (Fig. 2). At P9, the expression pattern shifts so that the upper band, representing PTBL is diminished. In the adult pituitary, there is still the diminished expression of the upper band; however, there also appears to be increased expression of the lower band, representing PTBS. Data from a recent microarray analysis of adult pituitaries identified Numb in a subpopulation of cells and partially supports our findings that Numb is expressed in the adult pituitary (22).
NUMB and hormone colocalization
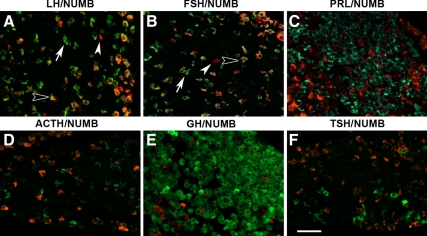
NUMB has been shown to play a role, not only in embryonic development but also in adult tissues; therefore, we examined the expression of NUMB in the adult pituitary. In the mature adult anterior pituitary there are five cell types, each of which secrete a specific hormone: ACTH, GH, TSH, LH, PRL, or FSH. NUMB (red) colocalizes with many, but not all, LHβ- and FSHβ (green)-producing cells (Fig. 3, A and B, open arrowhead). There are some NUMB-negative and LHβ and FSHβ singly-positive cells (Fig. 3, A and B, arrow), as well as some NUMB-positive and LHβ- and FSHβ-negative cells (Fig. 3, A and B, closed arrowhead). NUMB is not detected in any other hormone-producing cell in the AL (Fig. 3, C–F). Costains with α-glycoprotein subunit (dimerization partner for TSHβ, FSHβ, and LHβ) show some colocalization with NUMB and provide further evidence that NUMB is expressed in gonadotropes (data not shown). NUMBLIKE was not detected in hormone-producing cells of the AL by immunostaining (data not shown).
Figure 3.
NUMB is expressed in gonadotropes in the anterior lobe. NUMB (red) is expressed in many but not all LH (LHβ, panel A) and FSH (FSHβ, panel B) cells. NUMB is not expressed in PRL- (C), ACTH- (POMC, D), GH- (E), or TSH- (TSHβ, F) producing cells. All hormones stained in green. Open arrowheads identify colocalizing cells, arrows show hormone-positive, NUMB-negative cells, whereas closed arrowheads show NUMB-positive, hormone-negative cells. Pictures taken at ×400. Scale bar, 50 μm.
Pomc Cre-specific deletion of Numb and Numblike alters adhesion and boundary formation
From the hormone colocalization data, we were able to identify that NUMB (red) is present in many proopiomelanocortin (POMC)-expressing cells (green) of the IL (Fig. 4A), despite a lack of NUMB staining in POMC cells of the anterior lobe. We did not observe any NUMBLIKE immunostaining in the IL. Using this information we employed a Pomc Cre to selectively knock out Numb and Numblike in these POMC-expressing melanotropes in the pituitary (46,47). In addition to the IL, Numb and Numblike will also be eliminated from the Pomc-containing neurons in the hypothalamus. However, POMC neurons are not known to innervate or directly affect IL cells, which are instead innervated by dopaminergic, serotonergic, and γ-aminobutyric acid-containing neurons (48,49,50,51,52). Further, POMC neurons of the arcuate nucleus are of different developmental origin and can function independently from the pituitary. Mice deficient in Mash1 show a lack of POMC neuron differentiation despite normal POMC expression in the pituitary (53). Also, loss of glucose sensing in POMC neurons results in impaired glucose tolerance, yet appears to have little consequence on pituitary function as shown by normal corticosterone levels (54). These data suggest any pituitary effects observed in these Numb and Numblike Pomc Cre conditional knockout mice, should be intrinsic.
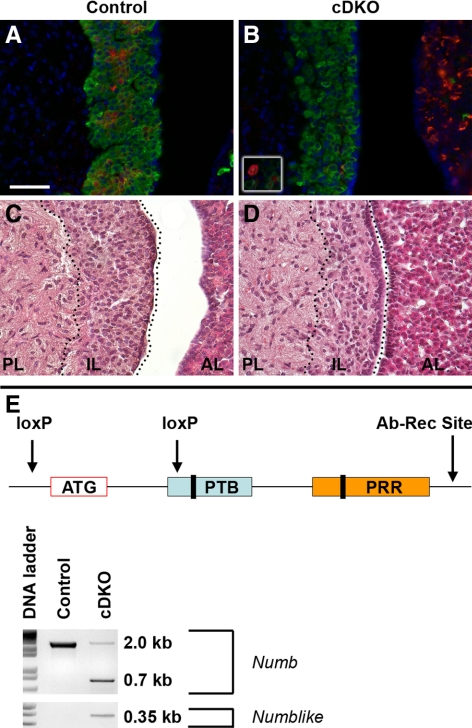
Figure 4.
Numb and Numblike deletion in IL disrupts melanotropes. NUMB immunostaining (red) shows positive cells in IL of control pituitary (A), and conditional knockout (cDKO) has reduced NUMB expression in IL (B) but expression in the AL is unaffected. Occasionally, large NUMB-positive cells are detectable in the cDKO IL (white box, B). POMC immunoreactivity is similar between control (green, A) and cDKO (green, in B), but occasionally POMC cells are seen in the PL of the mutant. Hematoxylin and eosin stain showing distinct structures in the control pituitary (C). In the cDKO, cells in the IL (D) appear larger and disorganized. Schematic showing genomic deletion occurs around ATG start site and where the antibody recognition site (Ab-Rec Site) is (E). The Numb floxed allele that has not been deleted with Cre-recombinase produces a 2-kb band after PCR amplification of pituitary DNA. Fragments of 0.7 and 0.35 kb correspond to Numb and Numblike, respectively, are generated by Cre-mediated deletion. Black bars within PTB and PRR regions represent alternative isoforms and antibodies used in this study recognize NUMB C-terminal to PRR region. Pictures taken at ×400. Scale bar, 50 μm. RP, Rathke’s pouch; AL, anterior lobe; PL, posterior lobe.
We examined the adult pituitary of conditional double-knockout (cDKO) (Numb fl/fl Numblike fl/fl Pomc Cre) or control (Numbfl/fl Numblikefl/fl) littermate mice for hormone staining as well as morphological differences. In cDKO ILs at P30, we find that the melanotropes are specified in the absence of Numb (Fig. 4B, green) and that NUMB expression is absent from almost all melanotropes (Fig. 4B, red). On occasion, a large NUMB/POMC double-labeled cell is observed in the cDKO ILs (box, Fig. 4B). The cDKO melanotropes also appear disorganized as confirmed by hematoxylin and eosin stain (Fig. 4, C and D) and are less dense. Manual cell counts of 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei reinforced the observation that the cDKO ILs had significantly fewer cells per area (42 ± 2.8 and 34 ± 2.1 cells per square inch for control and cDKO, respectively; P < 0.05). As expected, NUMB expression is maintained in the AL of cDKO animals (Fig. 4B), because NUMB does not colocalize with ACTH-producing cells. The conditional knockout strategy includes one loxP site upstream of the ATG initiation codon and one loxP site within part of the PTB sequence (Fig. 4E). The antibodies used in this study recognize NUMB peptides downstream of the PRR. Also shown is confirmation of deletion of Numb and Numblike at the genomic level by PCR from DNA isolated from whole pituitaries (Fig. 4E).
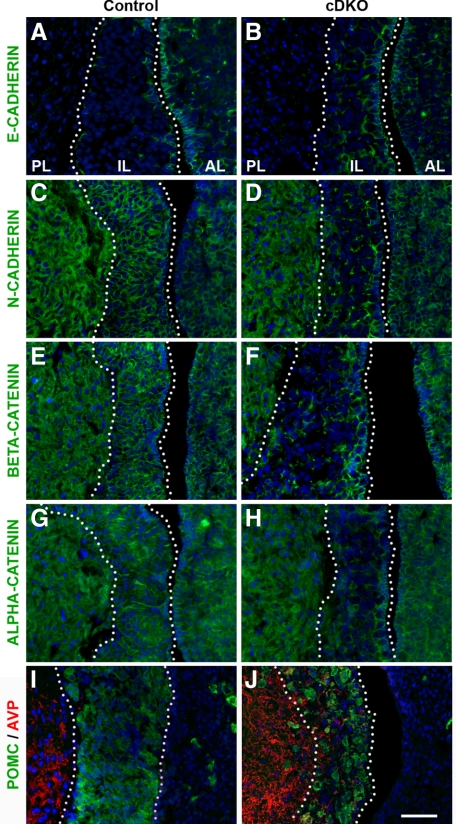
Knowing that NUMB is important for maintaining adherens junctions in radial glial cells (45), several adhesion markers were investigated by immunohistochemistry in the cDKO pituitaries. Compared with control samples (Fig. 5A), cDKO ILs have dysregulated E-CADHERIN expression (Fig. 5B). Notably, there appears to be increased E-CADHERIN expression throughout the IL, but it is more diffuse and less localized to the membrane than the restricted border of E-CADHERIN-positive cells in the control. In contrast to up-regulated E-CADHERIN, other components of adherens junctions appear dramatically down-regulated. In the control, N-CADHERIN is present on the membrane of virtually all cells in the IL, as well as the PL and AL (Fig. 5C). In contrast, the cDKO has a loss of membrane N-CADHERIN expression exclusively in the IL cells (Fig. 5D). A similar pattern is seen for β-CATENIN, localized to the cell membrane of control IL cells (Fig. 5E), compared with a selective loss in the IL of the cDKO (Fig. 5F). Finally, α-CATENIN is similarly lost in the IL cells of the cDKO (Fig. 5H), compared with the uniform expression seen in the control (Fig. 5G). To investigate the impact of the loss of adherens junction proteins on the integrity of the posterior-intermediate lobe boundary, the location of the neuron terminals containing AVP was examined. AVP neurons (red) exclusively terminate in the PL of the control mice (Fig. 5I) and are distinct from the POMC cells (green) in the IL. In contrast, cDKO pituitaries contain AVP-immunoreactive axon terminals (red) extending into the IL, intermixed with POMC cells, and occasional large POMC cells in the PL (Fig. 5J). Mice were also analyzed at P90 and P180, and adhesion phenotypes were similar to P30 (data not shown). Also, no differences between male and female mice were observed. Numb, then, appears critical to maintain cell-cell adhesion and proper separation of the PL from IL.
Figure 5.
Conditional Numb and Numblike knockout in IL disrupts adherens junctions protein expression and alters the posterior IL border. E-CADHERIN immunostaining shows limited expression in control IL (A) but enhanced and highly disordered expression in the cDKO (B) ILs. N-CADHERIN (C and D), β-CATENIN (E and F), and α-E-CATENIN (G and H) all show disrupted expression in the cDKO (D, F, and H) compared with control (C, E, and G). AVP (red) positive axons located within cDKO IL (J) unlike the control (I). Pictures taken at ×400. Scale bar, 50 μm. RP, Rathke’s pouch; AL, anterior lobe; PL, posterior lobe.
Numb affects pituitary progenitor cell organization
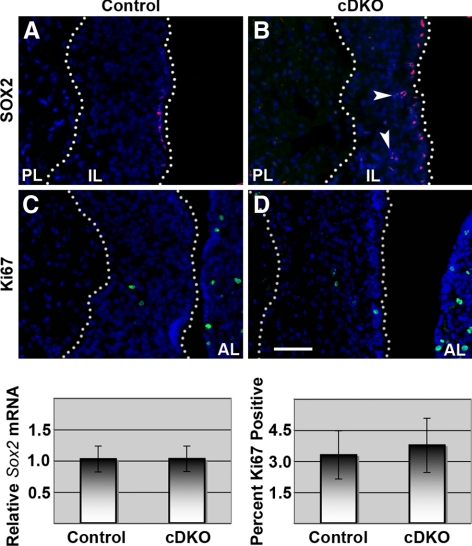
Given that Numb affects progenitor and differentiated cell distribution during neurogenesis, and that we see reduced membrane localization of adhesion molecules in the cDKO pituitary, we investigated the distribution of SOX2-positive stem-like progenitor cells in the IL (22,24). SOX2 progenitor cells in the pituitary can also be E-CADHERIN positive (24) so we speculated that dysregulated E-CADHERIN in the cDKO pituitaries also affects the progenitor cell niche. It has been recently demonstrated that SOX2-positive cells in the IL are found at the cleft border (22). We confirm this result in control animals by SOX2 immunostaining (Fig. 6A); however, in cDKO ILs, SOX2-positive cells are often observed throughout the IL rather than confined to the lumen border (Fig. 6B, arrowheads). The level of Sox2 mRNA from the whole pituitary, however, appears unchanged (Fig. 6, graph). Finding disorganized progenitor cells and improper E-CADHERIN expression, it was logical to speculate the niche of the SOX2-containing progenitor cells is disrupted, which might have downstream consequences on aspects of their behavior, such as proliferation. At P30, Ki67-positive cells are scattered throughout the IL, not confined to the SOX2-positive cells, and are approximately equal in location and number between control and cDKO animals (Fig. 6, C and D, graph). These data indicate that although Numb is necessary for IL architecture and cell placement, cell proliferation is not controlled by these elements.
Figure 6.
NUMB affects pituitary progenitor cell organization but there is no change in progenitor cell number or proliferation. SOX2 immunostaining (red) in control pituitaries is limited to IL border (A) but is observed throughout cDKO IL (B). Ki67 immunostaining (green) in cDKO IL (D) does not differ visually compared with control (C). Real-time PCR of whole pituitaries shows no Sox2 mRNA differences between control and cDKO mice (n = 4) after normalization to Gapdh. Cell counts of Ki67-positive cells compared with total number of IL cells shows proliferation is similar between control and cDKO (n = 3). Pictures taken at ×400. Scale bar, 50 μm.
Numb actions on Notch activity
One of the best-characterized actions of NUMB is to inhibit Notch signaling; therefore, we questioned whether NUMB normally suppresses Notch receptor actions and expression of downstream Notch targets in the pituitary. NOTCH2 is important for early pituitary development, but becomes undetectable after e14.5 (12,14). Therefore, NUMB expression in the adult IL may act, in part, to reduce expression of Notch receptors. We found no differences in Notch2 or downstream targets Hes1, HeyL, Hey1, and Hey2 mRNA between control and cDKO pituitaries isolated at P30 (Supplemental Fig. 2), or by immunostaining for NOTCH2 in POMC-expressing cells (data not shown). Numb, then, does not appear to regulate Notch activity in the differentiated melanotropes.
Discussion
NUMB is a complex evolutionarily conserved adaptor protein with important actions during embryonic development. This includes facilitating asymmetric cell division, cell adhesion and migration, endocytosis, and ubiquitination (27,28,29,33,34,36,37,45). NUMB does this through interactions with several pathways including Notch, Hedgehog, cadherins, and p53 (33,34,36,37,41,42,43,44,45,55). Although the function of NUMB during embryogenesis in other tissues has been well characterized, its role in differentiated cells is less well understood. The present study represents, to the best of our knowledge, the first characterization of NUMB expression and function in the pituitary. In the postnatal pituitary, we observe NUMB staining in gonadotropes as well as sporadically in the IL. We show that loss of NUMB in the mouse IL melanotropes dramatically alters cell adhesion and progenitor cell localization and results in PL-IL cell intermixing.
NUMB contains multiple protein-interaction domains, allowing for a diverse range of functions. A domain that mediates many actions of NUMB is the PTB region. This area is best known to be critical for inducing Notch degradation, although it is also involved with localizing NUMB to the plasma membrane and interactions with integrin and cadherin proteins (33,34,35,36,37). The PTB region can be subject to alternative splicing, and the longer form is required for Itch-mediated ubiquitination and endosome trafficking of NOTCH1 for degradation (33,34). We find strongest expression of the longer isoform (PTBL) before and at birth, with a decline, but not complete loss, into adulthood. It is known that the Notch-signaling pathway is critical for early pituitary formation (12,13,14), but becomes largely undetectable after e14.5, and can be found in isolated pituitary stem cells (20,21,22). Although NUMB and Notch signaling components appear to be expressed in the same location of the developing pituitary, it is unknown whether NUMB affects Notch signaling at that time. It may be that NUMB controls two distinct but related functions of Notch during embryogenesis and adulthood via PTBL: hormone cell specification and stem cell maintenance, respectively. Based on our data, if the PTB is important for modulating Notch activity and transcriptional activation in adult pituitaries, it is likely occurring in a population of cells not expressing POMC. However, we cannot exclude the possibility of other unknown proteins restoring Notch activity and compensating for the loss of Numb, or of changes in RNA expression undetectable by our whole pituitary RT-PCR analysis.
As previously mentioned, the PTB region is not only involved with NOTCH degradation, but can also bind cadherins (37). After deletion of Numb in the IL, we find altered E-CADHERIN with a loss of additional adhesion molecules including N-CADHERIN, β-CATENIN, and α-CATENIN. Based on our isoform analysis, it is possible that the residual PTBL form detectable in adulthood is required for proper localization of these adhesion molecules. Likely as a result of these disrupted adhesion proteins, cellular organization in these cDKO ILs is highly disordered and the cells are significantly less dense. This corroborates with previous studies showing that NUMB is required for proper cadherin protein function and localization (37,45). Strikingly, cDKO pituitaries show extensive mixing of POMC cells with PL projections expressing AVP, and many large irregularly shaped POMC cells are evident in the PL region. One possible explanation might be some PL axon terminals receive improper guidance cues from pituicytes, which could be mislocalized to the IL of cDKO mice. In another study, mice deficient in Munc18-1, a protein necessary for docking and fusion of secretory vesicles, had initial establishment of the hypothalamo-neurohypophysial system. However, before birth, PL axon terminals failed to reach their proper targets (56). This demonstrates that communication between PL axon terminals and supporting glial pituicytes is necessary for proper gland formation (56). These results all suggest that NUMB is important for maintaining cell adhesion and pituitary morphology and it may have a similar function in other endocrine tissues in which it is expressed, such as the pancreas.
Another region of NUMB that mediates distinct interactions and is subject to alternative splicing is the PRR. We find PRRL is most prevalent before and shortly after birth with a decline at P9, and it is detectable at low levels in the adult pituitary. Previous studies show longer isoforms of PRR occur predominantly in progenitor cells, and this expression profile has been supported by experiments in mouse pancreas, testes, and neurons (38,39,40). It is not well understood why PRR is preferentially localized to progenitor cells or how it may promote the maintenance of these cells. However, experiments in neuronal progenitors show that Numb knockdown followed by reexpression of PRRL isoforms in embryonic cortical progenitors at e13.5 and from P19 neurons caused an increased number of neuronal progenitors expressing NESTIN, and these effects are Notch independent (40). Pituitary progenitor cell maintenance and early expansion then may be mediated by PRRL isoforms, and continued expression into adulthood may be related to preservation of the small population of Sox2- and Sox9-positive adult pituitary stem-like cells (22). Recently, it has been shown these SOX2-positive cells in the adult pituitary can differentiate into several hormone-producing cells in culture and are likely important for proliferating and up-regulating hormone-producing cells during times of physiological stress (22,23,24). We found a disruption in the distribution of SOX2-positive cells, but analysis of whole pituitary samples showed no difference in the amount of Sox2 transcription. Thus, NUMB is important for maintaining the proper SOX2-positive cell niche. This aberrant localization of SOX2-positive cells could be due, in part, to loss of important NUMB-protein interactions in melanotropes, or from indirect effects of adhesion impairment by loss of adherens junction proteins.
One prediction, based on the known functions of NUMB, is that IL hyperplasia or tumors would result when Numb and Numblike are lost. The melanotropes are a relatively defined population and serve as a good model to investigate tumor formation because many mouse models lacking tumor suppressors are susceptible to IL tumors (57,58,59,60,61,62,63). Recent studies have shown loss of NUMB has been implicated in invasive cancers (44,64). Also there is evidence that NUMB normally inhibits cell cycle progression through promoting p53 stability (43), and mutations in p53 are found in approximately 50% of all human malignant tumors (65). Given the role of Numb in maintenance of cellular quiescence in other tissues, we looked for differences in proliferation in the cDKO pituitaries. Proliferation, marked by Ki67, is unchanged between control and cDKO pituitaries. Additionally, no tumor formation is found, at least until 6 months of age. Although we did not examine p53 expression, it is known that loss of p53 in mice does not cause pituitary tumors; however, loss of one or two copies of p53 can enhance tumor development in mice also heterozygous for the Retinoblastoma gene (Rb+/−) (66). Although proliferation remains the same between control and cDKO mice, the altered cadherin-catenin protein complex and mislocalized progenitor cells in the IL parallels the phenotype of loss of Numb in dorsal forebrain radial glial cells. In these mice, neocortex rosettes contain progenitor cells and resemble primitive neuroectodermal human brain tumors (45). This suggests loss of Numb may not affect proliferation directly but can induce clusters of progenitor cells, which may have an increased tendency to proliferate abnormally. The abnormal groupings of Sox2 progenitor cells observed in the ILs of cDKO mice may have an increased likelihood of becoming cancerous if subjected to an additional mutation or environmental insult according to the hit hypothesis in cancer development.
This work identifies NUMB and NUMBLIKE as putative regulatory proteins during early pituitary development as well as having critical functions in adulthood in the IL. The actions of NUMB and NUMBLIKE are broad; however, we show these proteins are major components of proper cell adhesion in the pituitary IL. NUMB expression in gonadotropes is intriguing, and future studies will attempt to address whether the function is unique or similar to that in the IL. Given the evidence supporting a role of Notch proteins in cancer and pituitary adult stem cell maintenance, this highlights the importance of future studies to understand mechanisms by which NUMB contributes to normal development and disease in other cell populations of the pituitary.
Materials and Methods
Mice
CD1 mice from e9.5 to e12.5, as well as adult pituitaries, were fixed in 3.8% formaldehyde for 1–24 h, dehydrated in ethanol, and then embedded in paraffin. Sagittal sections of 6 μm were mounted on charged slides and prepared for in situ hybridization or immunohistochemistry.
Pomc Cre mice and Numb and Numblike floxed mice were purchased from The Jackson Laboratory (Bar Harbor, ME) (46,47). A breeding colony was established with mice that contained both alleles of Numb and Numblike floxed (Numbfl/fl, Numblikefl/fl). These mice were bred to mice that had both Numb and Numblike floxed and also contained the Pomc Cre transgene (Pomc Cre Tg; Numbfl/fl, Numblikefl/fl). Numb and Numblike floxed as well as Pomc Cre mice were genotyped according to previously published protocols (46,67). Additionally, deletion of Numb and Numblike from pituitary DNA was confirmed according to previously published protocols (46,67). All mice were maintained according to the University of Illinois Institutional Animal Care and Use Committee.
In situ hybridization
A sense and antisense probe for Numb were prepared from the pCMV-SPORT6 Vector containing a full-length mouse Numb cDNA (MGI: 3991630; purchased from Open Biosystems, Huntsville, AL). The probe was linearized and then transcribed with T7 polymerase in the presence of digoxigenin-labeled nucleotides to create an antisense probe and SP6 polymerase to create the sense probe. The slides were rehydrated with xylene, followed by a gradient of ethanol before equilibrating in PBS. After washing in PBS, the slides were then acetylated, after which a 1:1 solution of 2× hybridization solution (Sigma Chemical Co., St. Louis, MO) and deionized formamide was put on each slide, and the slides were incubated at 57 C. The probes were then denatured for 3 min and then put on the slides under a coverslip overnight at 57 C. For the second day the slides were put in a 50% formamide 0.5 × standard sodium citrate solution at hybridization temperature. The slides were then blocked with 10% heat-inactivated sheep serum in Tris-buffered saline containing 2% BSA and 0.1% Triton X-100, followed by the application of the antidigoxigenin antibody conjugated to alkaline phosphatase (Roche, Indianapolis, IN). NBT-BCIP was added overnight for detection. Controls that were used included one section that no probe was applied to, a section with sense probe, and one with a Mash1 probe that showed a consistent level and pattern of staining as previously described (19).
Immunohistochemistry
Antibodies used in the studies include: goat polyclonal NUMB antibody (1:3000; AbCam, Inc., Cambridge, MA), rabbit polyclonal numb antibody (1:200, Upstate Biotechnology, Lake Placid, NY), rabbit polyclonal NUMBLIKE antibody (1:100; Proteintech Group, Chicago, IL), rat monoclonal Ki67 (1:100; DAKO Corp., Carpinteria, CA), rabbit polyclonal E-cadherin (1:150; Cell Signaling Technology, Inc., Danvers, MA), mouse monoclonal N-cadherin (1:300; Zymed Laboratories, Inc., South San Francisco, CA; Invitrogen, Carlsbad, CA), rabbit polyclonal β-catenin (1:200, Cell Signaling Technology) rabbit polyclonal α-catenin (1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit polyclonal AVP (1:1000; Millipore Corp., Bedford, MA), rabbit polyclonal Sox2 (1:400, Millipore). Hormone antibodies used include rabbit polyclonal GH, LH, PRL, TSH (1:1000; National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, Dr. A. Parlow) and POMC (1:3000, DAKO).
To detect protein localization, slides were deparaffinized in xylene and rehydrated in a gradient of ethanol before equilibrating in PBS. For Ki67, E-CADHERIN, N-CADHERIN, β-CATENIN, α-CATENIN, SOX2 staining procedures, slides were boiled in 0.01 m citric acid for 5–10 min and then allowed to cool for 10 min. All slides were then blocked in a suppressor serum of PBS containing 3% BSA, 0.1% Triton X-100 immunohistochemistry buffer, and 5% normal donkey serum, after which slides were incubated overnight at 4 C with the primary antibody diluted in immunohistochemistry buffer. Primary antibodies were detected with either direct Cy3 or DyLight 488-conjugated (1:300) or biotin-conjugated secondary antibodies, biotin-rabbit (1:250), biotin-goat (1:400), biotin-rat (1:250), biotin-mouse (1:200), and then amplified with either Streptavidin Cy3 or Strepavidin DyLight 488 (1:250). All secondary antibodies were obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). During staining, slides are washed in PBS containing 0.05% Tween 20 and mounted in an aqueous florescence mounting media. These experiments were completed a minimum of three times on independent samples, with two sections per sample being examined. Controls for single-channel labeling involved running a sample in the absence of primary antibody. Controls for the rabbit double labeling included samples without each primary as well as samples lacking both primary antibodies, with a 1-h blocking period between primary and detection antibodies using donkey antirabbit IgG (1:100, Jackson ImmunoResearch).
Experiments were viewed at ×200 or ×400 magnification with a Leica DM2500 microscope (Leica Corp., Deerfield, IL) and photographed with the Retiga 2000R color camera. The pictures were acquired in Q-Capture Pro, after which they were transferred to Adobe Photoshop. Quantification of immunopositive cells was recorded manually relative to total number of nuclei (DAPI) present in a complete ×400 image IL. For cell-density quantification, total number of nuclei present in a ×400 image of IL section was compared with area determined using NIH ImageJ. Statistics were performed using Statistical Analysis Software (SAS).
Reverse transcription-PCR
Manually dissected pituitaries were put in RNAlater (Ambion, Inc., Austin, TX) and stored at −20 C. Pituitaries were then moved to lysis solution and, using a homogenizer, were mechanically dissociated. An RNAqeous kit (Ambion) was then used to isolate the RNA from the homogenized samples. Reverse transcription was then performed to create cDNA from 0.5 μg of isolated mRNA template. After the formation of cDNA a PCR was performed with primers for either PRR or PTB isoform of Numb. The PRR primers were as follows. Numb RT-PRR forward, 5′-CTT GTG TTC CCA GAT CAC CAG-3′; Numb RT-PRR reverse, 5′-CCG CAC ACT CTT TGA CAC TTC-3′; Numb RT-PTB forward, 5′-ATG AGC AAG CAG TGT TGT CCT GG-3′; Numb RT-PTB reverse, 5′-ACA GCC ATG AAA CAA TGA CAG-3′ (40). The PCR conditions for both primer sets used were 92 C for 3 min (one cycle), 92 C for 30 sec, 55 C for 30 sec, 72 C for 30 sec (34 cycles), and 72 C for 10 min. PCR products were visualized on a 2% agarose gel stained with ethidium bromide.
Real-time PCR
RNA and cDNA were prepared from whole pituitaries as described above, and all pituitaries used for real-time analysis came from mice at P27. Samples were run and analyzed on Bio-Rad iCycler IQ (Bio-Rad Laboratories, Hercules, CA). Primer sequences were developed on Beacon Designer 7.0. Primer sequences are as follows. Sox2 forward, 5′-GGA GAA AGA AGA GGA GAG AG-3′; Sox2 reverse, 5′-CTG GCG GAG AAT AGT TGG-3′; GAPDH forward, 5′-GGT GAG GCC GGT GCT GAG TAT G-3′; GAPDH reverse, 5′-GAC CCG TTT GGC TCC ACC CTT C-3′; Notch2 forward, 5′-TGC CAA TAC TCC ACC TCT C-3′; Notch2 reverse, 5′-TCC ACT GAC ACT GCT TCC-3′; Hes1 forward, 5′-CTC GCT CAC TTC GGA CTC-3′; Hes1 reverse, 5′-GTG GGC TAG GGA CTT TAC G-3′; HeyL forward, 5′-GGA ACA ACA GAG AAT GAA C-3′; HeyL reverse, 5′-CAG CAG TAG TGA GTA ACC-3′; Hey1 forward, 5′-CAC GCC ACT ATG CTC AAT G-3′; Hey1 reverse, 5′-CCT TCA CCT CAC TGC TCT G-3′; Hey2 forward, 5′-GAT TCC GAG AGT GCT TGA C-3′; Hey2 reverse, 5′-AGG TGC TGA GAT GAG AGA C-3′. The PCR conditions for all primer sets used were 95 C for 20 sec, 55 C for 30 sec, and 72 C for 30 sec (40 cycles).
Supplementary Material
Acknowledgments
We thank members of the Raetzman laboratory for discussions; Paven Aujla (University of Illinois at Urbana-Champaign, Urbana, IL) for comments on AVP staining; and Katherine Brannick (University of Illinois at Urbana-Champaign, Urbana, IL) for mouse breeding and genotyping.
Footnotes
This work was supported by National Institutes of Health Grant R01DK076647 from National Institute of Diabetes and Digestive and Kidney Diseases (to L.T.R.).
Disclosure Summary: The authors have nothing to declare.
Abbreviations: AL, Anterior lobe; AVP, arginine vasopressin; cDKO, conditional double-knockout; DAPI, 4′,6-diamidino-2-phenylindole; e9.5, embryonic d 9.5; P1, postnatal d 1; PL, posterior lobe; POMC, proopiomelanocortin; PRL, prolactin; PRR, proline-rich region; PTB, phosphotyrosine binding; PRRL, long PRR isoform; PRRS, short PRR isoform; PTBL, long PTB isoform; PTBS short PTB isoform; RP, Rathke’s pouch.
First Published Online November 17, 2010
References
- Vankelecom H, Gremeaux L 2010 Stem cells in the pituitary gland: a burgeoning field. Gen Comp Endocrinol 166:478–488 [DOI] [PubMed] [Google Scholar]
- Japón MA, Rubinstein M, Low MJ 1994 In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem 42:1117–1125 [DOI] [PubMed] [Google Scholar]
- Kawamura K, Kouki T, Kawahara G, Kikuyama S 2002 Hypophyseal development in vertebrates from amphibians to mammals. Gen Comp Endocrinol 126:130–135 [DOI] [PubMed] [Google Scholar]
- Thor S, Ericson J, Brännström T, Edlund T 1991 The homeodomain LIM protein Isl-1 is expressed in subsets of neurons and endocrine cells in the adult rat. Neuron 7:881–889 [DOI] [PubMed] [Google Scholar]
- Roberson MS, Schoderbek WE, Tremml G, Maurer RA 1994 Activation of the glycoprotein hormone α-subunit promoter by a LIM-homeodomain transcription factor. Mol Cell Biol 14:2985–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng HZ, Moriyama K, Yamashita T, Li H, Potter SS, Mahon KA, Westphal H 1997 Multistep control of pituitary organogenesis. Science 278:1809–1812 [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T 1998 Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development 125:1005–1015 [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C 2000 An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127:483–492 [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N 2000 FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 277:643–649 [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG 1998 Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev 12:1691–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, O'Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang PT, McMahon AP, Rosenfeld MG 2001 Hedgehog signaling is required for pituitary gland development. Development 128:377–386 [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ 2004 Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol 265:329–340 [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG 2006 Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev 20:2739–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA 2006 Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol 20:2898–2908 [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Cai JX, Camper SA 2007 Hes1 is required for pituitary growth and melanotrope specification. Dev Biol 304:455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Crenshaw III EB, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG 1990 Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347:528–533 [DOI] [PubMed] [Google Scholar]
- Camper SA, Saunders TL, Katz RW, Reeves RH 1990 The Pit-1 transcription factor gene is a candidate for the murine Snell dwarf mutation. Genomics 8:586–590 [DOI] [PubMed] [Google Scholar]
- Nasonkin IO, Ward RD, Raetzman LT, Seasholtz AF, Saunders TL, Gillespie PJ, Camper SA 2004 Pituitary hypoplasia and respiratory distress syndrome in Prop1 knockout mice. Hum Mol Genet 13:2727–2735 [DOI] [PubMed] [Google Scholar]
- Himes AD, Raetzman LT 2009 Premature differentiation and aberrant movement of pituitary cells lacking both Hes1 and Prop1. Dev Biol 325:151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H 2005 The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 146:3985–3998 [DOI] [PubMed] [Google Scholar]
- Chen J, Crabbe A, Van Duppen V, Vankelecom H 2006 The notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Mol Endocrinol 20:3293–3307 [DOI] [PubMed] [Google Scholar]
- Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere S, Vankelecom H 2009 Pituitary progenitor cells tracked down by side population dissection. Stem Cells 27:1182–1195 [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Michurina T, Encinas JM, Roig JL, Krasnov P, Balordi F, Fishell G, Rosenfeld MG, Enikolopov G 2008 Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci USA 105:6332–6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC 2008 SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 105:2907–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CO, Moreno CS, Zhan X, McCabe MT, Vertino PM, Desiderio DM, Oyesiku NM 2008 Molecular pathogenesis of human prolactinomas identified by gene expression profiling, RT-qPCR, and proteomic analyses. Pituitary 11:231–245 [DOI] [PubMed] [Google Scholar]
- Moreno CS, Evans CO, Zhan X, Okor M, Desiderio DM, Oyesiku NM 2005 Novel molecular signaling and classification of human clinically nonfunctional pituitary adenomas identified by gene expression profiling and proteomic analyses. Cancer Res 65:10214–10222 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Kuchinke U, Knust E 1999 Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature 402:544–547 [DOI] [PubMed] [Google Scholar]
- Rhyu MS, Jan LY, Jan YN 1994 Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76:477–491 [DOI] [PubMed] [Google Scholar]
- Shen Q, Zhong W, Jan YN, Temple S 2002 Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development 129:4843–4853 [DOI] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W 2002 Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature 419:929–934 [DOI] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Krauss S, Zhong W 2004 Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nat Neurosci 7:803–811 [DOI] [PubMed] [Google Scholar]
- Li HS, Wang D, Shen Q, Schonemann MD, Gorski JA, Jones KR, Temple S, Jan LY, Jan YN 2003 Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron 40:1105–1118 [DOI] [PubMed] [Google Scholar]
- McGill MA, Dho SE, Weinmaster G, McGlade CJ 2009 Numb regulates post-endocytic trafficking and degradation of Notch1. J Biol Chem 284:26427–26438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MA, McGlade CJ 2003 Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem 278:23196–23203 [DOI] [PubMed] [Google Scholar]
- Dho SE, French MB, Woods SA, McGlade CJ 1999 Characterization of four mammalian numb protein isoforms. Identification of cytoplasmic and membrane-associated variants of the phosphotyrosine binding domain. J Biol Chem 274:33097–33104 [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kaibuchi K 2007 Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell 13:15–28 [DOI] [PubMed] [Google Scholar]
- Wang Z, Sandiford S, Wu C, Li SS 2009 Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J 28:2360–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Tokunaga A, Nakao K, Okano H 2003 Distinct expression patterns of splicing isoforms of mNumb in the endocrine lineage of developing pancreas. Differentiation 71:486–495 [DOI] [PubMed] [Google Scholar]
- Corallini S, Fera S, Grisanti L, Falciatori I, Muciaccia B, Stefanini M, Vicini E 2006 Expression of the adaptor protein m-Numb in mouse male germ cells. Reproduction 132:887–897 [DOI] [PubMed] [Google Scholar]
- Bani-Yaghoub M, Kubu CJ, Cowling R, Rochira J, Nikopoulos GN, Bellum S, Verdi JM 2007 A switch in numb isoforms is a critical step in cortical development. Dev Dyn 236:696–705 [DOI] [PubMed] [Google Scholar]
- Westhoff B, Colaluca IN, D'Ario G, Donzelli M, Tosoni D, Volorio S, Pelosi G, Spaggiari L, Mazzarol G, Viale G, Pece S, Di Fiore PP 2009 Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci USA 106:22293–22298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorano E, Favia G, Pece S, Resta L, Maisonneuve P, Di Fiore PP, Capodiferro S, Urbani U, Viale G 2007 Prognostic implications of NUMB immunoreactivity in salivary gland carcinomas. Int J Immunopathol Pharmacol 20:779–789 [DOI] [PubMed] [Google Scholar]
- Colaluca IN, Tosoni D, Nuciforo P, Senic-Matuglia F, Galimberti V, Viale G, Pece S, Di Fiore PP 2008 NUMB controls p53 tumour suppressor activity. Nature 451:76–80 [DOI] [PubMed] [Google Scholar]
- Pece S, Serresi M, Santolini E, Capra M, Hulleman E, Galimberti V, Zurrida S, Maisonneuve P, Viale G, Di Fiore PP 2004 Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol 167:215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasin MR, Gazula VR, Breunig JJ, Kwan KY, Johnson MB, Liu-Chen S, Li HS, Jan LY, Jan YN, Rakic P, Sestan N 2007 Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci 10:819–827 [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua Jr SC, Elmquist JK, Lowell BB 2004 Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42:983–991 [DOI] [PubMed] [Google Scholar]
- Zilian O, Saner C, Haged[swl]orn L, Lee HY, Säuberli E, Suter U, Sommer L, Aguet M 2001 Multiple roles of mouse Numb in tuning developmental cell fates. Curr Biol 11:494–501 [DOI] [PubMed] [Google Scholar]
- Goudreau JL, Falls WM, Lookingland KJ, Moore KE 1995 Periventricular-hypophysial dopaminergic neurons innervate the intermediate but not the neural lobe of the rat pituitary gland. Neuroendocrinology 62:147–154 [DOI] [PubMed] [Google Scholar]
- Kawano H, Daikoku S 1987 Functional topography of the rat hypothalamic dopamine neuron systems: retrograde tracing and immunohistochemical study. J Comp Neurol 265:242–253 [DOI] [PubMed] [Google Scholar]
- Léránth C, Palkovits M, Krieger DT 1983 Serotonin immunoreactive nerve fibers and terminals in the rat pituitary–light- and electron-microscopic studies. Neuroscience 9:289–296 [DOI] [PubMed] [Google Scholar]
- Mezey E, Léránth C, Brownstein MJ, Friedman E, Krieger DT, Palkovits M 1984 On the origin of the serotonergic input to the intermediate lobe of the rat pituitary. Brain Res 294:231–237 [DOI] [PubMed] [Google Scholar]
- Oertel WH, Mugnaini E, Tappaz ML, Weise VK, Dahl AL, Schmechel DE, Kopin IJ 1982 Central GABAergic innervation of neurointermediate pituitary lobe: biochemical and immunocytochemical study in the rat. Proc Natl Acad Sci USA 79:675–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay DE, Pelling M, Claxton S, Guillemot F, Ang SL 2006 Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol Endocrinol 20:1623–1632 [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB 2007 Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449:228–232 [DOI] [PubMed] [Google Scholar]
- Di Marcotullio L, Ferretti E, Greco A, De Smaele E, Po A, Sico MA, Alimandi M, Giannini G, Maroder M, Screpanti I, Gulino A 2006 Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nat Cell Biol 8:1415–1423 [DOI] [PubMed] [Google Scholar]
- Korteweg N, Maia AS, Verhage M, Burbach JP 2004 Development of the mouse hypothalamo-neurohypophysial system in the munc18-1 null mutant that lacks regulated secretion. Eur J Neurosci 19:2944–2952 [DOI] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K 1996 Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707–720 [DOI] [PubMed] [Google Scholar]
- Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM 1996 A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85:733–744 [DOI] [PubMed] [Google Scholar]
- Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A 1996 Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85:721–732 [DOI] [PubMed] [Google Scholar]
- Lee EY, Chang CY, Hu N, Wang YC, Lai CC, Herrup K, Lee WH, Bradley A 1992 Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359:288–294 [DOI] [PubMed] [Google Scholar]
- Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA 1992 Effects of an Rb mutation in the mouse. Nature 359:295–300 [DOI] [PubMed] [Google Scholar]
- Hu N, Gutsmann A, Herbert DC, Bradley A, Lee WH, Lee EY 1994 Heterozygous Rb-1 δ 20/+mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene 9:1021–1027 [PubMed] [Google Scholar]
- Nikitin AYu, Lee WH 1996 Early loss of the retinoblastoma gene is associated with impaired growth inhibitory innervation during melanotroph carcinogenesis in Rb+/− mice. Genes Dev 10:1870–1879 [DOI] [PubMed] [Google Scholar]
- Stylianou S, Clarke RB, Brennan K 2006 Aberrant activation of notch signaling in human breast cancer. Cancer Res 66:1517–1525 [DOI] [PubMed] [Google Scholar]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC 1991 p53 Mutations in human cancers. Science 253:49–53 [DOI] [PubMed] [Google Scholar]
- Harvey M, Vogel H, Lee EY, Bradley A, Donehower LA 1995 Mice deficient in both p53 and Rb develop tumors primarily of endocrine origin. Cancer Res 55:1146–1151 [PubMed] [Google Scholar]
- Wilson A, Ardiet DL, Saner C, Vilain N, Beermann F, Aguet M, Macdonald HR, Zilian O 2007 Normal hemopoiesis and lymphopoiesis in the combined absence of numb and numblike. J Immunol 178:6746–6751 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.