Abstract
The prevailing view of sexual differentiation of mammalian brain is that androgen synthesized in the fetal and neonatal testis and aromatized centrally during a perinatal sensitive period is the sole source of brain estradiol and the primary determinant of sex differences. Subregions of the diencephalon are among the most sexually dimorphic in the brain, and there are well-established sex differences in the amount of testosterone and estradiol measured in the hypothalamus and preoptic area during the perinatal period. We previously reported unexpectedly high estradiol in the hippocampus and cortex of both male and female newborn rat. This prompted a thorough investigation of the developmental profile of steroids in the rat brain using RIA to quantify the level of estradiol, testosterone, and dihydrotestosterone in discrete subregions of the brain from embryonic d 19 to adulthood. Plasma estradiol levels from individual animals were assessed when sufficient sample was available. A significant sex difference in hypothalamic testosterone prior to birth was consistent with previous findings. Postnatally, there was a distinct pattern of changing steroid concentrations in each brain region, and these were unrelated to circulating steroid. Removal of the gonads and adrenals at birth did not significantly reduce steroids in any brain region assayed 3 d later. Aromatase activity was detectable in all brain areas at birth, and the difference in activity level paralleled the observed regional differences in estradiol content. Based on these findings, we propose that steroidogenesis in the brain, independent of peripherally derived precursors, may play a critical role in mammalian brain development of both sexes, beyond the establishment of sex differences.
We present a distinct developmental profile of estradiol, testosterone, and dihydrotestosterone in the hippocampus, frontal cortex, and hypothalamus of the male and female rat.
The importance of perinatal hormones to sexual differentiation of physiology and behavior in rodents has been established for over 50 yr (1,2). Developing male brains are exposed to high levels of testosterone from their own testis, with peak levels toward the end of gestation (3) and again approximately 2 h after birth (4,5). By contrast, circulating testosterone is consistently low in the perinatal female rat (6). In the newborn, plasma estradiol is relatively low, but tissue levels in the male diencephalon are elevated (5,6,7) due to aromatization in neurons of peripherally derived testosterone (8). Although conversion of androgens to estrogens in the brain is common at this age, the plasma glycoprotein, α-fetoprotein, binds circulating estradiol of maternal or placental origin to protect the developing female brain from the masculinizing effects of this steroid (9). Cellular changes initiated by neuronally derived estradiol in the neonatal rat permanently masculinize the hypothalamus and preoptic area by organizing the neural networks controlling male sexual behavior and gonadotropin secretion in adulthood (10,11,12,13). A scenario in which peripherally derived steroids are the drivers of brain sexual differentiation in mammals (testosterone) and birds (testosterone and estradiol) has been the prevailing view until recent evidence of a role for centrally derived steroids (14) and potential genetic contributions, that is the direct effects of genes encoded on the sex chromosomes in differentiation of brain cells (15).
To gain a fuller understanding of the steroid hormone profile in discrete subregions of the developing mammalian brain, we conducted an exhaustive survey to quantify steroid content in multiple brain regions beginning embryonically and extending to adulthood. We reliably quantified estradiol, testosterone, and dihydrotestosterone (DHT) levels by RIA after C18 column purification, which reduced the steroid concentration selectively in the telencephalon, but substantially increased confidence in the identity and concentrations of the measured hormones. We also employed liquid chromatography followed by tandem mass spectrometry (LC/MS/MS) and determined that tissue concentrations of all three steroids were too low to be detected by this technique. The developmental profile of each steroid varied across brain regions, and levels were not reduced by prior adrenalectomy and gonadectomy. Regional differences in aromatase activity correlated with estradiol levels detected in each brain region.
Materials and Methods
All experiments were conducted in accordance with standards approved by the University of Maryland, Baltimore School of Medicine Institutional Animal Care and Use Committee.
Tissue and circulating (estradiol only) steroids were first measured from brain tissue of intact animals in experiment 1. To determine whether these were synthesized from a peripheral source, the gonads and adrenal glands were removed and tissue levels of the steroids assessed anew in experiment 2. Verification of the potential for local estradiol synthesis was then achieved by assessing activity of the enzyme aromatase in experiment 3.
Experiment 1. Steroid hormone profile in the developing rat brain
Subjects and sample collection
Subjects were 144 male and female Sprague Dawley rats born in the University of Maryland Baltimore vivarium. Brain tissue was collected from animals at embryonic d (E)19 and E21 and at birth [postnatal d (PN)0], PN2, PN4, PN6, PN8, PN10, PN15, PN20, PN30, and PN60. Pups collected on PN0 were killed between 0 and 12 h after birth. Six males and six females contributed to each time point and were randomly chosen from multiple litters (>15 litters). Note that intrauterine position was not considered in the case of embryonic animals. Blood was collected from an additional six male and six female pups on PN0, PN2, PN4, PN6, PN8, and PN10.
Sample collection
At the time of decapitation, trunk blood was collected and plasma extracted, then flash frozen on dry ice; further ether extraction and purification of plasma is described below. The brain was removed from the skull, and three brain areas were dissected out, the hippocampus, frontal cortex, and hypothalamus and quickly frozen in 2,methyl-butane on dry ice. All tissue and plasma were kept at −80 C until further processing. Hippocampus and preoptic area were dissected from an additional six males and six females, 0–2 h postpartum, and the tissue processed as described below for estradiol detection. Animals were not exsanguinated before tissue collection, because we had previously demonstrated this had no impact on steroid detection in perinatal brain tissue (7).
Measurement of estradiol, testosterone, and DHT levels by LC/MS/MS
After verifying the ability to detect estradiol, testosterone, and DHT in spiked samples via LC/MS/MS, we attempted to quantify the levels of each steroid in tissue samples from the developing brain. With the methods employed, it was concluded that the limits of sensitivity of LC/MS are above the threshold for detection of the very low quantities of steroid present in the developing brain. Details of the methods employed and the analyses can be found as Supplemental Data, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
Tissue processing
The tissue was mechanically homogenized using a tissue grinder consisting of a pestle with a polytetrafluoroethylene (Teflon)-coated tip and microcentrifuge tube. The homogenate was prepared in cold lysis buffer containing 0.1% protease inhibitor cocktail (P8340; Sigma, St. Louis, MO) and protein content determined by the Bradford assay.
Ether extraction
Four milliliters of diethyl ether was added to 325 μl of tissue homogenate in a Teflon-capped glass extraction tube, stirred horizontally for 1 h, then placed vertically to separate the organic and aqueous phases. The aqueous phase was frozen and the organic phase decanted into fresh glass tubes and the ether evaporated overnight.
C18 chromatography
The next day, the sample was reconstituted in 2 ml of methanol:water (5:95) before being loaded onto and pushed through conditioned solid phase octadyl C18 columns (Amersham RPN1910) (16). A fresh methanol:water (40:60) mixture was pushed through the column, and this fraction containing the steroid sulfate esters discarded. The final elution consisted of a methanol:water (85:15) mixture containing the nonconjugated steroids, which was collected in fresh tubes. The methanol was evaporated under medium heat (45–50 C). The extracts were reconstituted in 500 μl of estradiol calibrator (DSL 4401).
Radioimmunoassay
A 25 μl aliquot of each sample was diluted in 475 μl of 0.1M PBS and sent to the Ligand Assay and Analysis Core Facility at the University of Virginia Center for Research in Reproduction (University of Virginia, Charlottesville, VA) for RIA using commercially available kits for estradiol (sensitive estradiol DSL-39100), testosterone (sensitive testosterone, DPC-TKTT2), and DHT (DSL-96100). Control and spiked samples were also sent for analysis. Controls consisted of 475 μl of 0.1 m PBS with 25 μl of estradiol calibrator. Spiked samples consisted of adding synthetic 17β-estradiol (400 pg/ml, E8875; Sigma) before C18 column chromatography. An additional set of spiked samples consisting of 17β-estradiol (5.0, 20.0, and 33.0 pg/ml, E8875; Sigma), 17α-estradiol (5.0, 20.0, and 33.0 pg/ml, E0870-000; Steraloids, Newport, RI), testosterone (100, 500, and 1000 pg/ml, T1500; Sigma), DHT (20, 50, and 125 pg/ml, A8380; Sigma), and steroid-hormone binding globulin (SHBG) (5.0, 33.0, and 125.0 pg/ml, S1437; Sigma) were also submitted for assay. Intraassay variability for estradiol was approximately 5.6%, and interassay variability was approximately 13.1%; for testosterone assays, these were approximately 4.4 and approximately 12.5%, respectively, and for DHT assays, 8.25 and 9.8%, respectively.
Experiment 2. Effect of adrenalectomy and gonadectomy on estradiol, testosterone, and DHT concentration
Procedure
Male and female pups (six of each sex per group) were adrenalectomized and gonadectomized 0–12 h after birth (PN0) under cryoanethesia; sham operations, consisting of all the same manipulations as the experimental animals except removal of organs, served as controls. Survival rate from this combined surgery in males was at most 20%, whereas in females, it was approximately 80%. The source of this sex difference in survival rate is unknown. Note that males and females from 10 litters underwent surgery to prevent any bias related to a particular litter or maternal care. Incisions were sealed with cyanoacrylate Vetbond Surgical Adhesive (3M Animal Care Products, St. Paul, MN). Pups were placed under a heat lamp until they awoke, at which time they were promptly returned to the dams. On PN3, the animals were decapitated and the brain dissected as detailed above. Procedures for steroid extraction from plasma, processing of brains, and steroid measurement were identical to those previously described.
Experiment 3. Aromatase activity in the newborn rat brain
Sample collection
Tissue was collected from six newborn male and six female pups; tissue from two animals was pooled per sample (n = 3/sex). Hippocampus, frontal cortex, and hypothalamus tissue was collected as described above.
Aromatase activity
Aromatase activity was measured by a radiometric assay that quantifies the stereospecific production of 3H2O from [1β-3H] androstenedione being aromatized to estrone as an index of estrogen formation (17). Briefly, tissue samples were homogenized in sucrose phosphate buffer and assayed for protein content by Bradford assay. Triplicate aliquots of the supernatant from hypothalamus, hippocampus, and frontal cortex were incubated for 24 h at 37 C with 200 nm [1β-3H] androstenedione in the presence of a reduced nicotinamide adenine dinucleotide phosphate-generating system. Supernatant from the same samples were separately incubated with the aromatase inhibitors Letrozol (1 μm; Novartis, Basel, Switzerland) or 4-androsten-4-01 3,17-dione (4-OH) (10 μm; Sigma). The reaction was stopped by snap freezing the samples in a dry ice/2-methylbutane bath. The 3H2O generated during the incubation was purified through steroid extraction with chloroform, stripped of remaining steroids with activated charcoal/dextran, and finally quantified in the aqueous phase by liquid scintillation spectrophotometry.
Statistical analyses
Steroid concentrations for experiment 1 were analyzed via a mixed three-way factorial ANOVA design, with brain region (three levels: hippocampus, cortex, and hypothalamus) as the repeated factor, time (two or six levels; E19 and E21 or PN0–PN10) and sex (male vs. female) as the two independent factors; only the time points corresponding to dynamic changes in steroid levels, and also known to be a sensitive period in development of the rat brain because it pertains to cell proliferation, migration, and differentiation (18), were included in the neonatal period analysis, that is PN0–PN10. A significant regional difference was followed up by pair-wise comparisons between regions. As per our a priori objective, steroid levels in the three brain areas were also analyzed independently to properly ascertain differences across sex and time for each region. Statistically significant interactions were followed by appropriate simple effects analyses. Given that steroid levels are much higher at the embryonic time points, these data were analyzed separately using a similar design with time having only two levels for analysis. Additionally, due to heterogeneous variances associated with tissue steroid content, statistical analyses were conducted on Log transformed data; this excluded embryonic testosterone and estradiol in cortex and embryonic testosterone in hypothalamus. The data presented in the graphs and tables is the untransformed data. Due to the smaller sample size and heterogeneous variances in the estradiol levels in tissues collected within 2 h of birth, these were analyzed via a Mann-Whitney U test. Levels for each steroid from experiment 2 were analyzed as described for experiment 1, with surgical procedure replacing time as an independent factor.
Data on aromatase activity between brain regions was analyzed via a simple ANOVA design (hippocampus, frontal cortex, and hypothalamus). The effect of in vitro treatment with aromatase inhibitors was assessed separately for each brain region using a repeated measures ANOVA with one repeated factor (untreated, 4-OH, letrozol). Significant effects were further analyzed via Dunnett’s post hoc test, thus allowing a separate comparison of each group mean to the untreated mean while maintaining a family wise error rate α = 0.05.
Results
Experiment 1. Steroid hormone profile in the developing rat brain
Control and spiked samples
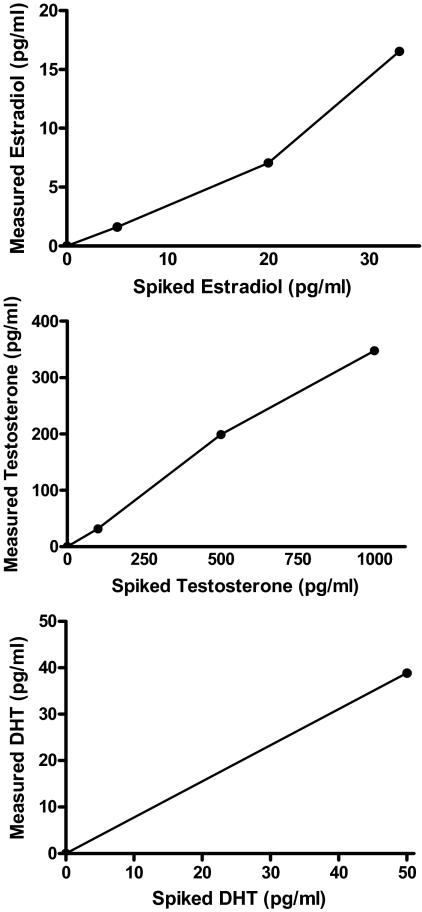
A slight reduction in steroid hormone levels from C18 column purification was evidenced by the detection of only 365 pg of 17β-estradiol/mg protein in brain homogenates spiked with 400 pg/ml of synthetic 17β-estradiol. RIA of the additional samples spiked with varying levels of 17β-estradiol after the purification step detected a linearly increasing concentration of the steroid (Fig. 1). Assay of samples spiked with the same concentration of 17α-estradiol reported the steroid as undetectable, and the same was true for samples spiked with SHBG; RIA for testosterone detected the testosterone spiked samples with a linear increase (Fig. 1), and there was no reported detection of testosterone in samples spiked with DHT or SHBG. Samples spiked with DHT are shown in Fig. 1, bottom, and show agreement between spiked and detected levels.
Figure 1.
RIA detection of 17β-estradiol (top), testosterone (middle), and dihydrotestosterone (DHT) (bottom) from samples spiked with 17β-estradiol (5, 20, and 33 pg/ml), testosterone (100, 500, and 1000 pg/ml), and DHT (0 and 50 pg/ml). There is a clear positive and linear relationship between the measured levels and spiked levels of each steroid. Note that the spiked samples were not extracted or purified in any way.
Plasma
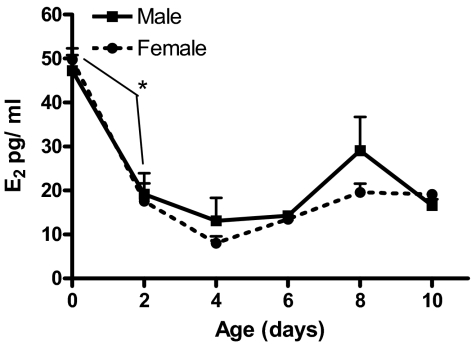
Plasma estradiol levels were elevated in both male and female rats at birth and declined rapidly, reaching a nadir at PN4 followed by a gradual rise until the last day of assay, PN10 (Fig. 2). Statistical analysis revealed a main effect of age (F5,53 = 36.95; P < 0.001) with Tukey’s post hoc analysis supporting an age difference between PN0 and PN2 (P < 0.001) with levels rising again after PN4. Due to the small volume of blood obtained from neonatal rats, we were unable to assess plasma testosterone levels in the same samples.
Figure 2.
Postnatal developmental plasma estradiol (E2) content in males and females (mean pg/ml ± sem; n = 5–6 at each time point for each sex).
Embryonic brain
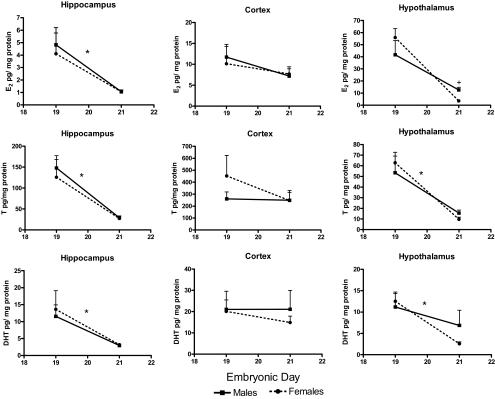
Estradiol levels were much higher in the embryonic vs. postnatal brain in all areas surveyed (see Figs. 3 and 4). Results of the three-way ANOVA indicate a regional difference in estradiol levels (F2,28 = 51.93; P < 0.001), with similar levels in the hypothalamus and cortex that were elevated compared with those in the hippocampus (F1,14 = 116.20 and F1,14 = 67.94, respectively; P < 0.001). The analysis also revealed an interaction of age x sex x region (F2,28 = 4.71; P < 0.05) on embryonic estradiol levels. Further analysis showed that in the hippocampus, estradiol was higher on E19 compared with E21 (F1,19 = 33.39; P < 0.001). The estradiol content was similar in males and females with the exception of the hypothalamus on E21, when males had three times higher estradiol content than females (F1,17 = 15.17; P < 0.05). Testosterone levels were also higher in the embryonic brain compared with postnatal (see Figs. 3 and 4). A regional difference in testosterone levels was also evident from the omnibus analysis (F2,28 = 68.70; P < 0.001) with levels higher in the cortex than the hippocampus (F1,14= 44.84; P < 0.001) and the hypothalamus (F1,14 = 124.78; P < 0.001) and surprisingly higher in the hippocampus vs. hypothalamus (F1,14 = 24.99; P < 0.001). Age differences in testosterone levels were specific by brain region (F2,28 = 7.44; P < 0.005). Note that in the hippocampus and hypothalamus, testosterone content is greatest at E19 (F1,18 = 64.78; P < 0.001 and F1,17 = 51.19; P < 0.001, respectively), whereas testosterone in the frontal cortex remains elevated at E21. Although visual inspection suggests a sex difference in hypothalamic testosterone content at E21, there was no significant effect. Similarly to the two previous steroids, the three-way factorial ANOVA revealed regional differences in DHT levels (F2,34 = 19.57; P < 0.001). Cortical levels were higher than those in either the hypothalamus (F1,17 = 21.30; P < 0.001) or hippocampus (F1,17 = 34.43; P < 0.001), whereas levels in the latter two regions did not differ from each other. Age differences were also found to be region specific (F2,34 = 4.70; P < 0.05). DHT content was more than twice as high on E19 vs. E21 in both the hippocampus (F1,19 = 28.00; P < 0.001) and hypothalamus (F1,19 = 22.31; P < 0.001) with no significant changes found in the cortex (Fig. 3).
Figure 3.
Embryonic (E19 and E21) tissue levels of estradiol (E2) (top), testosterone (T) (middle), and dihydrotestosterone (DHT) (bottom) in males and females (mean pg/mg protein ± sem; n = 5–6 at each time point for each sex. ANOVA* between E19 and E21, A significant age difference P < 0.05; +, a sex difference at that time point P < 0.05.
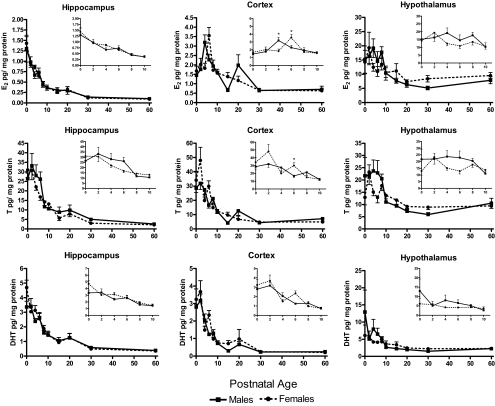
Figure 4.
Postnatal tissue levels of estradiol (E2) (top), testosterone (T) (middle), and dihydrotestosterone (DHT) (bottom) in males and females (mean pg/mg protein ± sem; n = 5–6 at each time point for each sex). Inset graphs magnify the dynamic portion (PN0–PN10) of the developmental profile of each steroid. ANOVA*, A sex difference at that time point P < 0.05).
Postnatal brain
Estradiol
Estradiol levels determined in brain tissue collected over the first 60 d of life for male and female rats are presented in Fig. 4, top; for clarity of presentation, the inset figures show an enlarged view of PN0–PN10. The omnibus analysis indicates a clear difference in estradiol levels between the brain regions (F2,106 = 1048.37; P < 0.001). Levels are highest in the hypothalamus, differing from the hippocampus (F1,53 = 1674.66; P < 0.001) and cortex (F1,53 = 742.91; P < 0.001). Levels were also higher in the cortex vs. the hippocampus (F1,53 = 397.54; P < 0.01). Estradiol varied differentially over the first 10 d of life according to each brain region (F10,106 = 7.71; P < 0.001). In the hippocampus, estradiol levels were elevated at birth and gradually declined thereafter (F5,56 = 26.13; P < 0.001), reaching asymptotically low levels by PN10 in both males and females. In the frontal cortex, age and sex interacted to influence estradiol content (F5,60 = 3.27; P < 0.05); levels peaked on PN4 in males (F1,60 = 9.75; P < 0.005) and PN6 in females (F1,60 = 6.08; P < 0.05) and then decreased similarly in males and females. In the hypothalamus, estradiol was high in both males and females at birth and decreased slowly with development in both sexes.
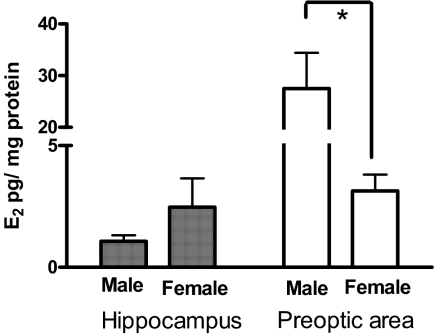
When tissue was collected within 2 h of birth (Fig. 5), we found similar estradiol levels in the male and female hippocampus (1.07 ± 0.24 and 2.47 ± 1.18 pg/mg protein, respectively), but in the preoptic area, males had over eight times the amount of estradiol as females (males = 27.52 ± 6.91 vs. females = 3.14 ± 0.67 pg/mg protein; Mann-Whitney U = 0; P < 0.05), consistent with previous reports (7).
Figure 5.
Tissue estradiol (E2) levels in hippocampus and preoptic area of males and females, collected within 2 h of birth. Mann-Whitney U test*, A sex difference P < 0.05.
Testosterone
Testosterone levels determined in brain tissue collected over the first 60 d of life for male and female rats are presented in Fig. 4, middle. Regional differences in testosterone were detected by the omnibus analysis (F2,100 = 9.84; P < 0.001), although a pair-wise comparison of regions only detected significantly higher levels in the cortex vs. hypothalamus (F1,50 = 19.55; P < 0.001). Similar to estradiol, changes in testosterone levels across the different ages are regionally specific (F10,100 = 4.10; P < 0.001) as are differences between the sexes (F2,100 = 4.84; P < 0.01). In hippocampus, there was initially high testosterone at birth that declines to near adult levels by PN6 (F5,54 = 14.05; P < 0.001). In the frontal cortex, testosterone content was similarly elevated in males and females at birth; however, the pattern of testosterone levels differed between the sexes over development (F5,59 = 2.34; P = 0.05); males showed a gradual decline, whereas female levels periodically increased and were significantly different from those of males at PN6 (P < 0.05). In the hypothalamus, males had relatively elevated levels of testosterone during the first week of life compared with females with the exception of PN2, where levels were similar. Testosterone declines in both sexes with increasing age (F5,57 = 2.91; P < 0.05). The amount of tissue from dissected preoptic area was too small to allow for multiple samples and, therefore, was not assayed for testosterone or DHT.
Dihydrotestosterone
DHT levels determined in brain tissue collected over the first 60 d of life for male and female rats are presented in Fig. 4, bottom. Overall, DHT levels differed by brain region (F2,112 = 99.24; P < 0.001) with levels being higher in the hypothalamus than the hippocampus (F1,56 = 64.19; P < 0.001) and cortex (F1,56 = 150.32; P < 0.001) and higher in the hippocampus vs. cortex (F1,56 = 52.24; P < 0.001). Results of the omnibus analysis also revealed a sex difference in DHT that is region specific (F2,112 = 3.22; P < 0.05). A precipitous drop in DHT levels across the ages was also found to be regionally specific (F10,112 = 2.86; P < 0.005). DHT content was elevated at birth and gradually decreased over the first 10 d of life in the hippocampus (F5,56 = 16.75; P < 0.001) and cortex (F5,60 = 26.84; P < 0.001).
Experiment 2. Effect of adrenalectomy and gonadectomy on estradiol, testosterone, and DHT concentration in plasma and brain
Estradiol
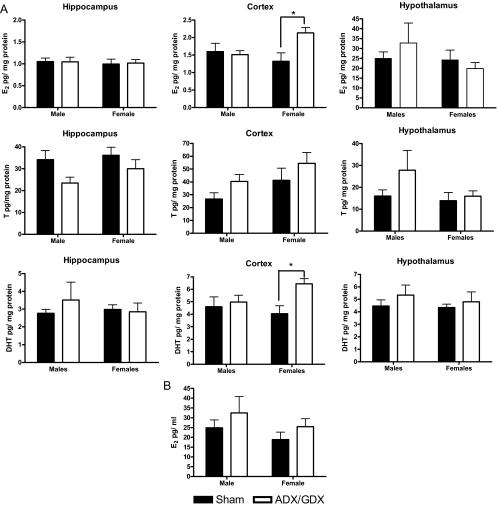
Mean estradiol levels in the hippocampus, frontal cortex, and hypothalamus of PN3 animals having undergone adrenalectomy and gonadectomy on the day of birth are presented in Fig. 6A. Similar to what was reported in experiment 1, there is an overall regional difference in estradiol levels (F2,30 = 18.52; P < 0.001). However, there was no effect of removal of peripheral steroidogenic organs in either males or females on estradiol in plasma (Fig. 6B), hippocampus, or hypothalamus. Analysis of the data from the frontal cortex revealed a significant sex by treatment interaction (F1,16 = 4.95; P < 0.05), and simple effects analysis showed a significant increase in estradiol only in female rats having undergone GDX/ADX at birth (F1,16 = 9.01; P < 0.01).
Figure 6.
A, Tissue estradiol (E2) (top), testosterone (T) (middle), and dihydrotestosterone (DHT) (bottom) levels (pg/mg protein) on PN3 from animals having undergone sham or adrenalectomy/gonadectomy (ADX/GDX) surgery on the day of birth (PN0). Filled bars represent intact animals, whereas open bars represent animals with organs removed. Data are mean ± sem; ANOVA*, P < 0.05, n = 6 per group. B, Plasma estradiol concentration on PN3 in animals having undergone sham or ADX/GDX surgery at birth.
Testosterone
Mean testosterone levels in the hippocampus, frontal cortex, and hypothalamus of PN3 animals having undergone adrenalectomy and gonadectomy on the day of birth are presented in Fig. 6A. No regional differences or effect of surgery were observed. Due to the small volume of blood obtained from rats at this age, we were unable to assess plasma testosterone.
Dihydrotestosterone
Mean DHT levels measured from the hippocampus, cortex, and hypothalamus after adrenalectomy and gonadectomy at birth are presented in Fig. 6A, bottom. Results of a three-way ANOVA confirm that DHT levels are regionally dependent (F2,30 = 10.67; P < 0.001). We also found an overall treatment effect (F1,15 = 8.12; P < 0.05), with surgery increasing DHT levels. Further evaluation revealed that although removal of peripheral steroidogenic organs did not affect DHT in the hippocampus and hypothalamus, levels were significantly increased in the cortex subsequent to adrenalectomy and gonadectomy (F1,15 = 5.12; P < 0.05). Further analysis revealed that this was most evident in females (F1,15 = 7.99; P < 0.05).
Experiment 3. Aromatase activity in the newborn rat brain
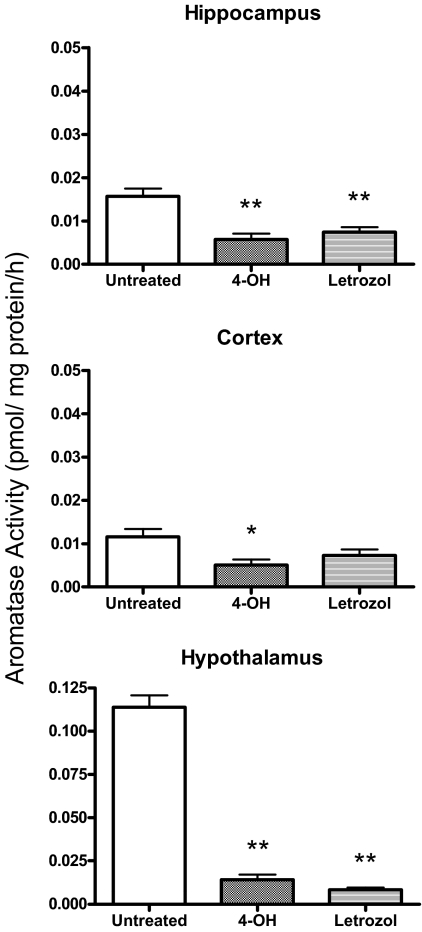
We detected significant differences in aromatase activity between brain regions (F2,15 = 179.81; P < 0.0001) with activity highest in the hypothalamus and low but detectable in the hippocampus and frontal cortex (P < 0.001) (Fig. 7). Because of the large difference in activity level between brain areas, the effect of aromatase inhibition was assessed separately for each area. There was a significant reduction in aromatase activity in all three brain areas after incubation with the aromatase inhibitors (hypothalamus: F2,10 = 239.70; P < 0.001, hippocampus: F2,10 = 13.99; P < 0.005, and cortex: F2,10 = 5.13; P < 0.05). Letrozol and 4-OH were similarly effective in reducing activity in the hippocampus (P < 0.01) and hypothalamus (P < 0.001), but only 4-OH incubation significantly reduced aromatase activity in the cortex (P < 0.05).
Figure 7.
Aromatase activity in the hippocampus, frontal cortex, and hypothalamus of the newborn rat, each sample consists of tissue dissected from two animals. Males and females are included together as no sex difference in aromatase activity was detected. ANOVA*, P < 0.05; **, P < 0.01; n = 6 per group, three of each sex.
Discussion
We present here a comprehensive developmental profile of estradiol, testosterone, and DHT levels in the hippocampus, frontal cortex, and hypothalamus of male and female Sprague Dawley rats. Brain steroid content was at its highest on E19, dropped precipitously by E21, and in most instances, reached nadir on PN10– PN12. A distinct profile of steroid concentration is observed in each brain region postnatally. No correlation in tissue and plasma estradiol levels was observed. Removal at birth of the peripheral steroidogenic organs, the adrenals and gonads, did not reduce estradiol, testosterone, or DHT content in any brain region examined on PN3. Aromatase activity was detected in all brain areas assayed and, although substantially lower in the hippocampus and cortex, was nonetheless reduced by aromatase inhibitors. Together with the historical literature, these data suggest that the classic view of testicularly derived androgens being the sole determinant of steroid-mediated sexual differentiation of the rodent brain warrants rethinking. We propose that early gonadal steroidogenesis is subsequently replaced by local steroidogenesis in the brain, without which the process of sexual differentiation would not be complete. The findings also suggest that intrinsic steroidogenesis may be a heretofore unappreciated component of normal brain development outside the context of sexual differentiation, particularly in the cortex and hippocampus. The data presented here do not directly test either of these hypotheses but instead provide the framework by which working models can be proposed and ultimately tested.
Measures of peripheral steroid levels during development are consistent with the view that the period during which sex differences can be observed is exceedingly short. Whole-body content of androgen (testosterone + DHT) is higher in males on both E18 and E19 but is equivalent in the two sexes on later gestational days leading up to birth (19). A second period of elevated androgens in males occurs immediately postnatal, although the magnitude of the sex difference is much smaller. Circulating testosterone is elevated in males 2 h after birth (4,5), and whole-body androgen content is higher in males at 1 and 3 h after delivery but not later (20), whereas plasma androgen is higher in males only at 1-h postpartum, not 24 h. Thus, the period of sexually dimorphic exposure to elevated circulating androgens appears to be largely prenatal, lasting only up to 3 h after birth by some accounts (20), although others report days of elevated circulating androgens (3,6). A temporary reduction in the capacity of the liver to metabolize steroids to inactive water-soluble byproducts is largely responsible for the elevated androgens in the circulation at birth. Within 24 h of birth the liver resumes metabolism and the sex difference in circulating and whole-body androgen is lost (20). This extremely brief period of sex differences in androgen exposure is consistent with a requirement for postnatal castration to occur immediately after birth, vs. later, to be effective at preventing masculinization of physiology and behavior. The importance of the postnatal surge in testosterone varies for different sexually dimorphic parameters. The positive feedback control of LH and defeminization of sexual behavior are both maximally affected by castration within minutes of birth, with declining effectiveness with as little as a 6-h delay (4,21,22). Conversely, the volume of the sexually dimorphic nucleus of the hypothalamus- preoptic area is equally reduced by castration at 0 h as at 24 h, suggesting that variables other than the immediate postnatal rise in testosterone are critical to its differentiation (22). We observed measurably high levels of estradiol in male hypothalamus for up to 6 d postnatal, corresponding to the sensitive period for sexually dimorphic nucleus off the hypothalamus differentiation. Moreover, the brain region controlling defeminization remains unknown, but we have recent evidence implicating estradiol action in the mediobasal hypothalamus as a critical component (23,24,25), and we also observed elevated estradiol in this brain region for 1 wk postnatal. Thus, there are at least three distinct critical periods for the organizational effects of steroid hormones on sexual differentiation, corresponding to behavioral masculinization, defeminization, and control of gonadotropin secretion (26). However, there has been no empirical explanation as to how a unitary source of steroid, the gonad, could direct these temporally distinct processes. However, if the prenatal surge in testosterone is instead differentially regulating local steroidogenesis in the brain, set to begin at discrete times, and occurring at different levels, this would provide the desired temporal variability necessary for regional differentiation. Emerging theories on highly localized steroid synthesis, in the brain and the immune system, and their paracrine action (27,28) may be fundamental to differences in steroid-induced differentiation in males and females.
The study of tissue content and plasma levels of estrogens and androgens in developing rats has been vexed by the relative lack of sensitivity of the assays and thus the need for pooling samples from multiple individuals. Recent advances in the sensitivity of RIA for estradiol allowed us to measure estradiol in the plasma of individual pups on various days postpartum and in discrete brain regions of individual animals. In initial experiments, plasma samples were not extracted before assay for estradiol, and levels were extremely high, in the 55–380 pg/ml range, but when an another set of plasma samples were extracted with diethyl ether and purified through C18 column chromatography, thereby separating conjugated and nonconjugated steroids, and only the latter were quantified, estradiol levels were very high at birth and declined precipitously, being reduced 5-fold by the second day of life in both males and females. Serum levels of the steroid binding globulin α fetoprotein remain constant until PN10 (29), suggesting that the elevated serum estradiol at birth was of maternal origin and is then quickly cleared from the newborns’ circulation. Interestingly, at later time points, we recorded a noticeable rise in plasma estradiol at the end of the first postnatal week in both sexes. Neither the origin nor functional significance of this late rise in estradiol is known nor does it correlate with the pattern of estradiol content in the assayed brain regions. Recent evidence suggests a later developmental period of active brain feminization by estradiol (30), and this late rise in estradiol may contribute to that process.
An additional unexpected and unexplained pattern of changes in steroid hormone levels was that seen in the developing frontal cortex. Although male testosterone gradually declined over the first 10 d of life, there were two distinct and significant peaks in testosterone in females at PN2 and again at PN6. The profile of cortical estradiol was similar between the sexes with the exception of levels peaking at PN4 in males and PN6 in females and decreasing thereafter. This dynamic pattern suggests a regulated process, but what that process is remains unclear. In many instances, it is the ratio of androgens to estrogens that is important to a particular physiological response (31). We found a strong correlation (r > 0.75) between testosterone and DHT levels in all three brain areas assayed; however, the relationship between testosterone and estradiol was more variable, with a tight correlation in the hypothalamus (r = 0.92), a moderate one in the hippocampus (r = 0.67), and a poor relationship between levels of these two steroids in the cortex (r = 0.39). A closer look at the cortex suggests that the relationship between estradiol and testosterone is strong for the first 6 d of life (r > 0.67), whereas none exists thereafter. These differing relationships between testosterone and its metabolites indicate that either steroidogenesis or steroid metabolism in the hippocampus and cortex is very different from the hypothalamus. The notion that only testosterone, as opposed to any C19 steroid, serves as a substrate for aromatase and therefore precursor to estradiol has recently been challenged (32), and these data support the view that steroidogenesis in vivo is more complex and nuanced than the currently constructed “network-like frame” that Lieberman calls into question.
Confidence that the current results reflect an accurate and reliable quantification of tissue steroid levels rests in part on the ability to compare them with previous findings. We previously reported estradiol levels of 16–18 pg/mg protein in the rat telencephalon 2 h after birth, declining to 10–14 pg/mg protein 30 h later (7). By contrast, the values in the current study are 10-fold lower. This was not true for the hypothalamus, however, where we previously reported levels of 10–15 pg/mg protein and find the same here, thus ruling out a general loss in the sensitivity of the assay as an explanation. An important concern in the use of RIA to quantify steroid concentration is the potential for nonspecific antibody binding producing a false positive. In the current study, we employed the additional step of C18 column purification after ether extraction to remove conjugated steroids, which includes the glucuronide and sulfate moieties of estrogen and testosterone, as well as the methylated catechol estrogens. Steroids are conjugated by the liver to increase water solubility and promote excretion (33). It is unknown whether a similar process occurs in brain. The reduction in estradiol concentration that occurred specifically in the hippocampus and cortex after removal of conjugated steroids in this study compared with our previous report (7) raises the intriguing possibility that there is regionally specific metabolism of these steroids. Although this suggests differential actions of the parent compound, such variation in metabolism might also allow for local actions of these metabolites. There is increasing evidence that steroid metabolites may be biologically active, such as decreasing plasma levels of cholesterol and triglycerides (34), altering the preovulatory prolactin surge (35), and disrupting the cell cycle in cancer cell lines (36). Alternatively, the column purification could have removed related estrogens, such as estrone, which has a double bond at position 17, and shows a 6.9% cross-reactivity in this RIA. Finally, there is the potential for reduced recovery of steroid after column purification. Use of a similar method of extraction and purification to measure pregnenolone reported recovery of the nonconjugated steroid was less than 75% (16), but the current findings cannot be due to a simple loss of estradiol given that estradiol content in the hypothalamus did not change between studies. Furthermore, the regional differences in aromatase activity observed here directly parallel estradiol in the corresponding brain areas, with high aromatase activity in the hypothalamus correlated with high estradiol and low aromatase activity in the hippocampus and cortex correlated with low estradiol.
Technical limitations in measuring brain steroid content
There are inherent limitations to the use of RIA in the quantification of low level steroids. One is the detection limit of the assay, although development of ultrasensitive assays now allows detection of much lower steroid levels, i.e. 3–5 pmol of estradiol in human serum (37). Of greater concern is nonspecific antibody interaction with steroid binding proteins (38). This can be particularly problematic in the case of steroid hormone binding globulin, where individual variation in this protein could influence steroid measurement. In the current study, samples were ether extracted to eliminate the binding proteins attached to steroids (39) followed by separation to allow for assay of only the unconjugated steroids. Because RIA is antibody based, there is also the potential for cross-reactivity with other steroids (40). The analysis of samples spiked with 17α-estradiol, a naturally occurring optical isomer of estradiol that has recently been detected in certain brain regions of the neonatal mouse (41), had no cross-reactivity with the currently employed antibody for 17β-estradiol detection. Taken together, these steps lend further credence to our finding of low but measurable levels of neonatal estradiol in the brain.
In contrast to RIA, the specificity of steroid detection by LC/MS/MS assay is undisputed (42). One of the main drawbacks is the low sensitivity of this assay vs. the ultra sensitive RIA, as carried out in the current studies. The calibration curves for the LC/MS/MS, presented as a Supplemental Fig. 1, speak to the low sensitivity of this assay. Caruso et al. (43) have also attempted to quantify estradiol via LC/MS/MS, and their endeavors proved unsuccessful, with levels below the limit of detection in comparable brain areas. The limit of detection for the current assay was approximately 350 pg/mg protein, a value much higher than the 15–20 pg/mg protein of estradiol that is detected in the hypothalamus or the 1–2 pg/mg protein detected in the telencephalic areas by RIA. Pooling of multiple samples (12–32 samples) was still not sufficient to provide a reading for any of the brain areas evaluated due to the much lower sensitivity of LC/MS/MS vs. RIA. This, combined with the expense associated with each sample quantified by LC/MS/MS makes this approach suboptimal for use in the detection of brain steroid content.
Effect of removing peripheral steroidogenic organs on brain steroid levels
Characterizing the developmental time course of estradiol, testosterone, and DHT in brain tissue is a first step in understanding the role of steroids in brain development. However, a persistent question remains: What is the source of these steroids, particularly in the neonatal female brain? In males, it has been shown that gonadectomizing the animals at birth significantly reduces hypothalamic estradiol 2 h later (5). However, the dynamic profile of the steroids in brain tissue that we observed does not correspond with reports on plasma testosterone levels in the neonatal rat (6,40). Furthermore, the inactive ovary of females at this age (44,45) suggests another source for steroids measured in the brain. To assess the contribution of peripheral steroidogenic organs to brain levels of steroid, we surgically removed the gonads and adrenal glands at birth and quantified steroids in blood and brain tissue 3 d later. There was no reduction in any of the steroids in tissue from both males and females, but instead, we observed a significant increase in DHT and estradiol in the frontal cortex of animals whose gonads and adrenals were removed compared with those receiving sham surgery. Weisz and Gunsalus (46) found reduced but detectable levels of circulating estradiol 4 d after adrenalectomy, but not ovariectomy alone, in 6-, 14-, and 20-d-old animals. Note that the measured estradiol levels include an estradiol-immunoreactive substance (46). When the same surgery was performed in adults, estradiol was undetectable (46). This suggests slower metabolic clearance of steroids in the immature animal and may be the basis of why we continued to detect steroids in the neonatal brain 3 d after surgery. However, the results of a recent study suggest that low levels, similar to those found in intact animals, are detectable in the frontal cortex and amygdala but not the hippocampus of adult female gonadectomized rats (47).
Aromatase activity in the newborn rat brain
Aromatase activity is detectable in the neonatal preoptic area, hypothalamus, amygdala, hippocampus, and cortex (48,49,50,51). We replicate these findings by showing aromatase activity in the hypothalamus, hippocampus, and frontal cortex. At birth, estradiol levels are 10-fold higher in the hypothalamus than the hippocampus and cortex. Aromatase activity is similarly elevated in the hypothalamus vs. the telencephalon, but the telencephalic activity is nonetheless functional as evidenced by significant inhibition by Letrozol and reliable estradiol detection in these brain regions. Taken together, these findings further support the contention that the neonatal telencephalon of both males and females contains some androgen and synthesizes estradiol. The functional significance of this local steroid synthesis remains to be determined but may contribute to sex differences in glial and neurogenesis observed in the developing hippocampus (52,53).
In summary, the data presented here confirm and extend the notion that sex differences in brain steroid levels occur transiently during a restricted developmental period closely associated with birth. However, levels of androgens and estrogens are elevated in the brains of both sexes for an extended period, enduring through the first week of life. The amount of steroid in well-established sexually dimorphic regions is typically higher than that in the hippocampus and cortex and may be due to a combination of regionally specific uptake, synthesis, and metabolism. Dynamic changes in steroid levels in male and female cortex hint at a regulated process, but the basis of this regulation is unknown. Elucidating the profile of brain steroid content across development contributes to our understanding of both the etiology of sex differences and normal brain development.
Supplementary Material
Acknowledgments
We thank R.L. Foltz and D. Andrenyak from the University of Utah for their assistance.
Footnotes
This work was supported by National Institute of Neurological Disorder and Stroke Grant NS050525–01A (to M.M.M.) and Natural Sciences and Engineering Research Council of Canada, Postdoctoral Fellowship (A.T.M.K.) and National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant U54-HD28934, University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations: DHT, Dihydrotestosterone; E, embryonic day; LC/MS/MS, liquid chromatography followed by tandem mass spectrometry; 4-OH, 4-androsten-4-01 3,17-dione; PN, postnatal day; SHBG, steroid-hormone binding globulin.
First Published Online November 10, 2010
References
- Phoenix CH, Goy RW, Gerall AA, Young WC 1959 Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382 [DOI] [PubMed] [Google Scholar]
- Whalen RE, Edwards DA 1967 Hormonal determinants of the development of masculine and feminine behavior in male and female rats. Anat Rec 157:173–180 [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL 1980 Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology 106:306–316 [DOI] [PubMed] [Google Scholar]
- Corbier P, Edwards DA, Roffi J 1992 The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys 100:127–131 [DOI] [PubMed] [Google Scholar]
- Rhoda J, Corbier P, Roffi J 1984 Gonadal steroid concentrations in serum and hypothalamus of the rat at birth: aromatization of testosterone to 17 β-estradiol. Endocrinology 114:1754–1760 [DOI] [PubMed] [Google Scholar]
- Lieberburg I, Krey LC, McEwen BS 1979 Sex differences in serum testosterone and in exchangeable brain cell nuclear estradiol during the neonatal period in rats. Brain Res 178:207–212 [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM 2004 Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology 145:2906–2917 [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L 1975 The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res 31:295–319 [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C 2006 α-Fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 9:220–226 [DOI] [PubMed] [Google Scholar]
- McCarthy MM 2008 Estradiol and the developing brain. Physiol Rev 88:91–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nunez JL, Perrot-Sinal TS 2004 GABA, estrogen, and sex differences in the brain. In: Smith SS, ed. Neurosteroid effects in the central nervous system. The role of the GABAA receptor. Washington, DC: CRC Press; 173–195 [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM 2004 Sexual differentiation of the vertebrate nervous system. Nat Neurosci 7:1034–1039 [DOI] [PubMed] [Google Scholar]
- Simerly RB 2002 Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536 [DOI] [PubMed] [Google Scholar]
- Holloway CC, Clayton DF 2001 Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat Neurosci 4:170–175 [DOI] [PubMed] [Google Scholar]
- Arnold AP 2004 Sex chromosomes and brain gender. Nat Rev Neurosci 5:701–708 [DOI] [PubMed] [Google Scholar]
- Wang MD, Wahlström G, Backström T 1997 The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. J Steroid Biochem Mol Biol 62:299–306 [DOI] [PubMed] [Google Scholar]
- Lephart ED, Simpson ER 1991 Assay of aromatase activity. Methods Enzymol 206:477–483 [DOI] [PubMed] [Google Scholar]
- Rice D, Barone Jr S 2000 Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Env Health Persp 108(Suppl 3):511–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ, Woutersen PJ, Slob AK 1991 Sex difference in whole-body androgen content in rats on fetal days 18 and 19 without evidence that androgen passes from males to females. Biol Reprod 44:747–751 [DOI] [PubMed] [Google Scholar]
- Baum MJ, Brand T, Ooms M, Vreeburg JT, Slob AK 1988 Immediate postnatal rise in whole body androgen content in male rats: correlation with increased testicular content and reduced body clearance of testosterone. Biol Reprod 38:980–986 [DOI] [PubMed] [Google Scholar]
- Corbier P, Roffi J, Rhoda J 1983 Female sexual behavior in male rats: effect of hour of castration at birth. Physiol Behav 30:613–616 [DOI] [PubMed] [Google Scholar]
- Handa RJ, Corbier P, Shryne JE, Schoonmaker JN, Gorski RA 1985 Differential effects of the perinatal steroid environment on three sexually dimorphic parameters of the rat brain. Biol Reprod 32:855–864 [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Liang SL, Thompson SM, McCarthy MM 2008 Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron 58:584–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, McCarthy MM 2008 The role of neonatal NMDA receptor activation in defeminization and masculinization of sex behavior in the rat. Horm Behav 54:662–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, Mong JA, McCarthy MM 2007 Glutamate AMPA/kainite receptors, not GABA(A) receptors, mediate estradiol-induced sex differences in the hypothalamus. Dev Neurobiol 67:304–315 [DOI] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA 1990 Termination of the hormone-sensitive period for differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res Dev Brain Res 52:17–23 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF 2006 Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci 29:241–249 [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Pradhan DS, Shah AH, Charlier TD, Chin EH, Soma KK 2008 Neurosteroids, immunosteroids, and the Balkanization of endocrinology. Gen Comp Endocrinol 157:266–274 [DOI] [PubMed] [Google Scholar]
- Piñeiro A, Calvo M, Iguaz F, Lampreave F, Naval J 1982 Characterization, origin and evolution of α-fetoprotein and albumin in postnatal rat brain. Int J Biochem 14:817–823 [DOI] [PubMed] [Google Scholar]
- Bakker J, Brock O 2010 Early oestrogens in shaping reproductive networks: evidence for a potential organizational role of oestradiol in female brain development. J Neuroendocrinol 22:728–735 [DOI] [PubMed] [Google Scholar]
- Li X, Rahman N 2008 Impact of androgen/estrogen ratio: lessons learned from the aromatase over-expression mice. Gen Comp Endocrinol 159:1–9 [DOI] [PubMed] [Google Scholar]
- Lieberman S 2008 The generally accepted version of steroidogenesis is not free of uncertainties: other tenable and possibly superior rendition may be invented. J Steroid Biochem Mol Biol 109:197–199 [DOI] [PubMed] [Google Scholar]
- Ebner T, Remmel RP, Burchell B 1993 Human bilirubin UDP-glucuronosyltransferase catalyzes the glucuronidation of ethinylestradiol. Mol Pharmacol 43:649–654 [PubMed] [Google Scholar]
- Gordon S, Cantrall EW, Cekleniak WP, Albers HJ, Mauer S, Stolar SM, Bernstein S 1964 Steroid and lipid metabolism. The hypocholesteremic effect of estrogen metabolites. Steroids 4:787–791 [Google Scholar]
- Katayama S, Fishman J 1982 2-Hydroxyestrone suppresses and 2-methoxyestrone augments the preovulatory prolactin surge in the cycling rat. Endocrinology 110:1448–1450 [DOI] [PubMed] [Google Scholar]
- Lottering ML, Haag M, Seegers JC 1992 Effects of 17β-estradiol metabolites on cell cycle events in MCF-7 cells. Cancer Res 52:5926–5932 [PubMed] [Google Scholar]
- Ikegami S, Moriwake T, Tanaka H, Inoue M, Kubo T, Suzuki S, Kanzakili S, Seino Y 2001 An ultrasensitive assay revealed age-related changes in serum oestradiol at low concentrations in both sexes from infancy to puberty. Clin Endocrinol (Oxf) 55:789–795 [DOI] [PubMed] [Google Scholar]
- Key TJ, Moore JW 1988 Interference of sex-hormone binding globulin in a no-extraction double-antibody radioimmunoassay for estradiol. Clin Chem 34:1357–1358 [PubMed] [Google Scholar]
- Stanczyk FZ, Cho MM, Endres DB, Morrison JL, Patel S, Paulson RJ 2003 Limitations of direct estradiol and testosterone immunoassay kits. Steroids 68:1173–1178 [DOI] [PubMed] [Google Scholar]
- Carlström K 1996 Low endogenous estrogen levels—analytical problems and tissue sensitivity. Acta Obstet Gynecol Scand Suppl 163:11–15 [PubMed] [Google Scholar]
- Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS 2005 17α-Estradiol: a brain-active estrogen? Endocrinology 146:3843–3850 [DOI] [PubMed] [Google Scholar]
- Guo T, Chan M, Soldin SJ 2004 Steroid profiles using liquid chromatography-tandem mass spectrometry with atmospheric pressure photoionization source. Arch Pathol Lab Med 128:469–475 [DOI] [PubMed] [Google Scholar]
- Caruso D, Scurati S, Maschi O, De Angelis L, Roglio I, Giatti S, Garcia-Segura LM, Melcangi RC 2008 Evaluation of neuroactive steroid levels by liquid chromatography-tandem mass spectrometry in central and peripheral nervous system: effect of diabetes. Neurochem Int 52:560–568 [DOI] [PubMed] [Google Scholar]
- Carson R, Smith J 1986 Development and steroidogenic activity of preantral follicles in the neonatal rat ovary. J Endocr 110:87–92 [DOI] [PubMed] [Google Scholar]
- Sokka TA, Huhtaniemi IT 1995 Functional maturation of the pituitary-gonadal axis in the neonatal female rat. Biol Reprod 52:1404–1409 [DOI] [PubMed] [Google Scholar]
- Weisz J, Gunsalus P 1973 Estrogen levels in immature female rats: true or spurious-ovarian or adrenal? Endocrinology 93:1057–1065 [DOI] [PubMed] [Google Scholar]
- Barker JM, Galea LAM 2009 Sex and regional differences in estradiol content in the prefrontal cortex, amugdala and hippocampus of adult male and female rats. Gen Comp Endocrinol 164:77–84 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Philip A, Hurlburt C, Naftolin F 1985 Estrogen formation in the developing rat brain: sex differences in aromatase activity during early post-natal life. Psychoneuroendocrinology 10:355–361 [DOI] [PubMed] [Google Scholar]
- Tobet SA, Baum MJ, Tang HB, Shim JH, Canick JA 1985 Aromatase activity in the perinatal rat forebrain: effects of age, sex and intrauterine position. Brain Res 355:171–178 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Walters MJ, Clark AS, Toran-Allerand CD 1994 Aromatase in the cerebral cortex, hippocampus, and mid-brain: ontogeny and developmental implications. Mol Cell Neurosci 5:691–698 [DOI] [PubMed] [Google Scholar]
- Jacobson NA, Ladle DR, Lephart ED 1997 Aromatase cytochrome P450 and 5 α-reductase in the amygdala and cortex of perinatal rats. Neuroreport 8:2529–2533 [DOI] [PubMed] [Google Scholar]
- Zhang JM, Konkle AT, Zup SL, McCarthy MM 2008 Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci 27:791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Tonelli L, Regenold WT, McCarthy MM 2010 Effects of neonatal flutamide treatment on hippocampal neurogenesis and synaptogenesis correlate with depression-like behaviors in preadolescent male rats. Neuroscience 169:544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.