Abstract
Testosterone (T) supplementation increases skeletal muscle mass, circulating GH, IGF-I, and im IGF-I expression, but the role of GH and IGF-I in mediating T’s effects on the skeletal muscle remains poorly understood. Here, we show that T administration increased body weight and the mass of the androgen-dependent levator ani muscle in hypophysectomized as well as castrated plus hypophysectomized adult male rats. T stimulated the proliferation of primary human skeletal muscle cells (hSKMCs) in vitro, an effect blocked by transfecting hSKMCs with small interference RNA targeting human IGF-I receptor (IGF-IR). In differentiation conditions, T promoted the fusion of hSKMCs into larger myotubes, an effect attenuated by small interference RNA targeting human IGF-IR. Notably, MKR mice, which express a dominant negative form of the IGF-IR in skeletal muscle fibers, treated with a GnRH antagonist (acyline) to suppress endogenous T, responded to T administration by an attenuated increase in the levator ani muscle mass. In conclusion, circulating GH and IGF-I are not essential for mediating T’s effects on an androgen-responsive skeletal muscle. IGF-I signaling plays an important role in mediating T’s effects on skeletal muscle progenitor cell growth and differentiation in vitro. However, IGF-IR signaling in skeletal muscle fibers does not appear to be obligatory for mediating the anabolic effects of T on the mass of androgen-responsive skeletal muscles in mice.
IGF-I signaling plays an important role in mediating the effect of testosterone on skeletal muscle progenitor cell growth and differentiation in vitro.
Testosterone (T) regulates skeletal muscle mass during puberty and sustains muscle mass in adult life, as shown by the deficits of muscle mass and strength in androgen-deficient men (1). Clinical trials data are in agreement that T administration increases skeletal muscle mass and strength in both young and older adults (2,3,4,5). T-induced increase in skeletal muscle mass in men is associated with skeletal muscle fiber hypertrophy and with an increase in the number of satellite cells (6). In addition, it has been shown that T regulates muscle mass during development and regeneration in animal models (7,8,9,10). However, the mechanisms by which T regulates skeletal muscle mass remain poorly understood; previous studies have reported inconsistent data on the effects of T on proliferation and differentiation of myogenic cells (11,12,13,14,15,16).
One possible explanation for the promyogenic effect of androgens is that T activates or synergizes with other effectors of muscle growth, such as GH and IGF-I. T administration in humans and rodents increases circulating GH and IGF-I levels, and T deficiency is associated with reduced levels of IGF-I in humans (17,18,19). However, the role of circulating GH and IGF-I in mediating the effects of T on the muscle is incompletely understood and was the subject of this investigation.
GH is the main regulator of IGF-I release from the liver, the principal source of circulating IGF-I. However, the autocrine and paracrine actions of the IGF-I produced locally in muscles and bones are equally important contributors to the IGF-I’s effects on postnatal growth (20). Skeletal muscle expresses two principal splicing variants of IGF-I, IGF-IEa and IGF-IEb [also known as mechano growth factor (MGF)]. IGF-IEb has been found to regulate satellite cell proliferation in response to mechanical stimuli and IGF-IEa the fusion and differentiation of myoblasts (21,22,23). A large body of data, including studies performed in mice expressing the dominant negative form of the IGF-I receptor (IGF-IR) in muscle fibers (MKR mice) and in the IGF-I transgenic mice, are in agreement that IGF-I regulates skeletal muscle growth and muscle protein synthesis (24,25,26,27). In addition, IGF-I has been shown to stimulate satellite cell proliferation and differentiation in vitro (28,29,30,31). Although T stimulates im expression of IGF-I (18,19), little is known about the role of im IGF-I in mediating T’s effects on muscle mass and myoblast proliferation and differentiation.
Levels of GH, IGF-I and T decline with human aging in association with the decreased muscle mass and strength and the reduced regeneration potential of skeletal muscle (1,32). Because of the growing interest in the potential application of androgens alone and in combination with recombinant human GH as function promoting therapy, a better understanding of the molecular action of T and GH/IGF-I on the skeletal muscle is desirable. Here, we investigated whether the anabolic effects of T on androgen-responsive skeletal muscle are mediated by circulating GH and IGF-I. We characterized the effects of T on the proliferation and myogenic differentiation of primary human skeletal muscle cells (hSKMCs). We further used a combination of in vivo and in vitro models to elucidate the role of im IGF-I in mediating the effects of T on androgen-responsive skeletal muscle and on hSKMCs proliferation and differentiation.
Materials and Methods
Animals
Sexually mature 2-month-old Sprague Dawley male rats were purchased from Charles River Laboratories (Boston, MA). Two-month-old male MKR mice, expressing a dominant negative form of the IGF-IR under the control of the muscle creatine kinase promoter, were generated in the laboratory of Derek Le Roith (24) and backcrossed onto the C57 strain before use in this study. Two-month-old male C57Bl/6J wild-type mice (Charles River Laboratories) were used as controls for MKR mice. At the end of the treatment, the animals were killed to collect blood and skeletal muscles. Extensor digitorum longus (EDL) and levator ani (LA) muscles were isolated, freed of visible connective tissue, and weighed. All animals were acclimatized before starting treatment. The animals were kept on a 12-h light, 12-h dark cycle and given food and water ad libitum. The animals were handled according to the National Institutes of Health guidelines for the care and use of laboratory animals, using protocols approved by the Committee for the Ethical Care and Use of Laboratory Animals of Boston University and University of Pennsylvania.
Antibodies
Primary antibodies were mouse monoclonal to myosin heavy chain (MyHC) (MF-20) (1:20 for immunofluorescence and 1:10,000 for Western blotting), mouse monoclonal to myogenin (F5D) (1:20), rabbit polyclonal to androgen receptor (1:20) (N-20) and mouse monoclonal to α-tubulin (TU-02) (1:4000) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit polyclonal to IGF-IRβ (1:500) (Cell Signaling Technology, Danvers, MA), and goat antihuman IGF-IR (R&D Systems, Inc., Minneapolis, MN). MF-20 and F5D hybridomas were developed by Fischman and Wright, respectively, and were obtained from the Developmental Studies Hybridoma Bank of the University of Iowa (Iowa City, IA). Secondary antibodies were goat antimouse Cy3-conjugated (1:400) (Jackson ImmunoResearch, West Grove, PA) and goat antimouse or rabbit IgG HRP-conjugated (1:2000) (Santa Cruz Biotechnology, Inc.).
Muscle cell cultures
Primary hSKMCs were isolated from the vastus lateralis muscle of a young male donor. For the experiments, cells were grown in growth medium (GM) (Lonza, Walkersville, MD) plus 10% fetal bovine serum charcoal stripped (Gemini Bio-Products, Sacramento, CA). To differentiate, cells were shifted in differentiation medium (DM) composed by DMEM plus penicillin/streptomycin (Invitrogen, Carlsbad, CA) and 2% charcoal-stripped horse serum (Bioreclamation, Inc., Westbury, NY).
Transfections
Human SKMCs were transfected using DharmaFECT3 (Dharmacon, Inc., Lafayette, CO) with 10 nm each of two small interference oligonucleotides for the human IGF-IR messenger (s-7211 and s-7213; Ambion, Inc., Austin, TX) or with scrambled small interference RNAs (siRNAs) (Scra.) (4390843; Ambion, Inc.).
In vivo and in vitro treatments
T enanthate (TE) and T were purchased from Sigma-Aldrich (St. Louis, MO). TE was dissolved in sesame oil and was injected im three times per week at the indicated dosage. Acyline (A) was dissolved in saline solution and injected sc three times per week at a dose of 0.1 mg/d. Untreated animals were injected with equal volumes of sesame oil and saline solution as vehicle alone. T was dissolved in 100% ethanol, diluted in DMEM and added to hSKMCs; control dishes were treated with vehicle alone containing an equal volume of ethanol. Bicalutamide (Sigma-Aldrich) was added in GM to hSKMCs at 1 μm. Ly-294,002 (Ly) (Enzo Life Science, Plymouth Meeting, PA) was added in DM to hSKMCs. Antihuman IGF-IR antibody was supplemented at 2 μg/ml to hSKMCs cultured in DM.
T assay
Serum total T was measured by using liquid chromatography-tandem mass spectrometry as described (33). Briefly, samples, calibrators, and quality controls (60 μl) were added with 20 μl internal standard, containing 200 pg T-d3, in a 96-well plate. Proteins were precipitated with acetonitrile, and T and internal standard were isolated by an on line extraction. Cohesive Technologies Aria TX series high-turbulence liquid chromatography (HTLC) system (Thermo Scientific, San Jose, CA) was used for analysis. Samples were first separated by HTLC column, then the fraction containing analyte was loaded to a C18 analytical column and separated. Binary gradients consisting of water and methanol were used for HTLC column and of water and acetonitrile/water (50/50) for C18 column. Mass spectrometry was performed using TSQ quantum ultra (Thermo Scientific). The sensitivity of the assay was 2 ng/dl.
Cell proliferation assay
Cells were plated at low confluence in 96-well plates and grown in GM with or without 100 nm T or 1 μm bicalutamide for 3 or 7 d. Cell number was quantified by using the CyQUANT kit (Invitrogen).
RNA isolation and quantitative PCR (qPCR) analysis
Total RNA was isolated from LA muscles using the TRIzol plus RNA Purification kit (Invitrogen) and from hSKMCs using the SV Total RNA Isolation System (Promega Corp., Madison, WI); 500 ng of total RNA were used to synthesize c-DNA with the Accuscript HF 1st Strand c-DNA kit (Stratagene, La Jolla, CA). For mouse and rat IGF-I qPCR, 0.5 μm of the primer 5′-TTGCAGGTTGCT-3′ (34) was added to the c-DNA reaction. Quantitative PCR was carried out in triplicates in reactions consisting of 2× Power SYBR Green PCR Master mix (Applied Biosystems, Foster City, CA), 1 μl c-DNA, ribonuclease-free water and 200 nm of each primer. For human IGF-IR knockdown test, 1 μl of c-DNA was added to a mixture composed of 2× TaqMan Universal PCR Master mix supplemented with specific primers plus a MGB probe 6-FAM dye labeled (Hs00609566_m1; Applied Biosystems). Amplifications were performed on a 7500 Fast Real-Time PCR System (Applied Biosystems) with the following parameters: 2 min at 50 C, activation at 95 C for 10 min, 40 cycles of denaturation at 95 C for 15 sec, and annealing/extension at 60 C for 1 min.
Primer sequences for rat: glyceraldehyde-3-phosphate dehydrogenase (Gapdh) forward 5′-GCTCACTGGCATGGCCTTCCG-3′ and reverse 5′-GTAGGCCATGAGGTCCACCAC-3′; IGF-IEa forward 5′-GCTTGCTCACCTTTACCAGC-3′ and reverse 5′-AAATGTACTTCCTTCTGGGTCT-3′ (34); IGF-IEb (MGF) forward, same as IGF-IEa, and reverse 5′-AAATGTACTTCCTTTCCTTCTC-3′ (34); MyoD forward 5′-GCCGCCTACTACAGTGAGG-3′ and reverse 5′-GGTCCCCTGTTCTGCATCG-3′; and myogenin forward 5′-CTCCCTCAACCAGGAGGAGC-3′ and reverse 5′-CGATGGACGTAAGGGAGTGC-3′.
Primer sequences for mouse: Gapdh, IGF-IEa, and IGF-IEb same as rats.
Primer sequences for human: GAPDH forward 5′-GGTGAAGGTCGGAGTCAACG-3′ and reverse 5′-CATCGCCCCACTTGATTTTGG-3′; and IGF-IEa forward 5′-CTTCCGGAGCTGTGATCTA-3′ and reverse 5′-TGCGTTCTTCAAATGTACTTC-3′ (19).
Immunofluorescence analysis
Human SKMCs were fixed in 4% paraformaldehyde 10 min, permeabilized with 0.15% Triton X-100 10 min, blocked with 1% BSA for 40 min at room temperature, incubated overnight at 4 C with MF-20 in 1% BSA, and then incubated with the goat antimouse Cy3-conjugated 1 h at room temperature. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Pictures were acquired using a Nikon Eclipse TE2000-E microscope (Nikon Instruments, Inc., Melville, NY).
Myotube area and fusion index calculation
After 3 d in DM, hSKMCs were immunostained for MyHC using the MF-20 antibody. The area of 60–140 MyHC+ myotubes in each experimental point was calculated with the assistance of the SPOT Image software (Diagnostic Instruments, Sterling Heights, MI) in three separate experiments. Fusion index (35) was determined by dividing the number of nuclei in myotubes (with ≥2 nuclei) for the total number of nuclei analyzed (800–1200/dish) in three separate experiments. The number of nuclei/myotube was determined in the fields analyzed, and the number of myotubes with two to four nuclei and those with more than or equal to five nuclei was calculated (35).
Western blotting
Human SKMCs were lysed in radioimmunoprecipitation assay buffer (10 mm Tris-Cl, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, 0.5 mm EDTA, 150 mm NaCl, 10% glycerol, 0.5% Na deoxycholate, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 10 μg/ml aprotinin, 1 mm phenylmethylsulfonylfluoride, 1 mm Na3VO4, and 5 mm NaF). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membrane (GE Healthcare, Piscataway, NJ). Membranes were blocked in 5% milk in Tris-buffered saline plus 0.1% Tween-20 (TBS-T) and incubated overnight at 4 C with primary antibodies in 5% milk TBS-T (5% BSA in TBS-T for IGF-IRβ). Secondary antibodies were incubated in 5% milk TBS-T. ECL (GE Healthcare) was used to impress X-Omat films (Kodak, Rochester, NY) that were scanned with the FluorChem-SP Imaging Analysis System (Alpha Innotech, San Leandro, CA) for densitometry.
Data analysis
Results are means ± sem. One-way ANOVA was used in experiments with more than two independent groups. If overall ANOVA revealed significant difference, Student’s t test was used to analyze differences between the groups of rats or mice and treatments in vitro. P ≤ 0.05 was considered statistically significant.
Results
T rescues LA muscle mass in castrated (Cast) rats in the absence of circulating GH and IGF-I
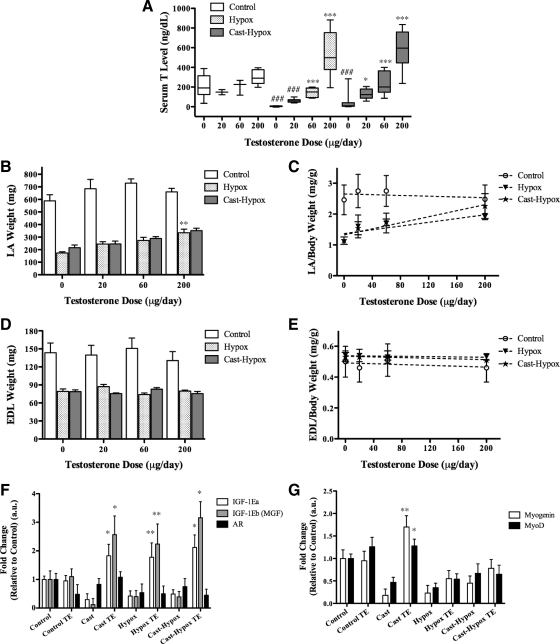
To determine whether circulating GH and IGF-I are essential for mediating the anabolic effect of T on androgen-responsive muscle, we used the Hershberger Cast rat bioassay for androgens. Rats were weighed and assigned randomly to one of four groups: intact (Control), Cast, hypophysectomized (Hypox), and Cast plus Hypox (Cast-Hypox) (n = 12 per group). Within each group, the rats received either im injections of TE [200 μg/d (n = 6)] or sesame oil (Oil) (n = 6). After 2 wk of treatment, the rats were weighed and killed. The Hypox and Cast-Hypox rats had lower body weight (Fig. 1A) and lower body weight gain during treatment compared with Control and Cast rats (Fig. 1B). The body weight gain in Hypox and Cast-Hypox rats treated with TE was significantly greater than in the sesame oil-treated controls (Fig. 1B). LA weight was lower in Cast, Hypox, and Cast-Hypox rats than in Control rats. TE administration increased LA weight in Cast rats, as expected, but also in Hypox and Cast-Hypox rats (Fig. 1C). Notably, TE-treated Cast, Hypox, and Cast-Hypox rats had higher LA muscle mass than oil-treated rats even after adjusting for body weight (Fig. 1D). In contrast, EDL muscle mass, although increased by TE treatment in the Cast-Hypox rats, was not significantly different between oil and TE-treated rats after adjustment for body weight (Fig. 1, E and F).
Figure 1.
Effect of T in Cast and Hypox rats. Intact (Control), Cast, Hypox, and Cast-Hypox 2-month-old male rats were treated with 200 μg/d TE in sesame oil three times each week for 2 wk. Untreated rats were injected with sesame oil alone (Oil). A, The effect of TE or sesame oil administration on body weight in Control, Cast, Hypox, and Cast-Hypox rats. B, The effect of treatments on body weight gain in Control, Cast, Hypox, and Cast-Hypox rats. C, The effect of treatments on LA weight in Control, Cast, Hypox, and Cast-Hypox rats. LA muscle mass is lower in the absence of endogenous T or GH and is rescued by TE treatment. D, LA muscle mass in relation to body weight in Cast, Hypox, and Cast-Hypox TE-treated rats. EDL muscle mass [absolute (E) and relative to body weight (F)] in Control, Cast, Hypox, and Cast-Hypox rats treated with sesame oil or TE. Results are means ± sem (n = 6 per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001 when values are compared with oil-treated rats within each experimental group; ###, P < 0.001 when values are compared with control oil-treated rats. Representative critical values for t test statistical analysis: Control Oil vs. Hypox Oil, t = 7.481, df = 10 (A); Cast-Hypox Oil vs. Cast-Hypox TE, t = 7.408, df = 12 (C); and Cast-Hypox Oil vs. Cast-Hypox TE, t = 2.23, df = 12 (E).
We next compared the responsiveness of Hypox and Cast-Hypox rats to graded doses of TE. Rats were assigned randomly to three experimental groups (n = 24 per group) and treated with 0, 20, 60, or 200 μg/d of TE (n = 6 in each dose group). Serum T levels were lower in the Hypox and Cast-Hypox rats than in controls (Fig. 2A). TE administration was associated with dose-dependent increments in serum T level (Fig. 2A) and LA mass (Fig. 2, B and C) but not EDL mass (Fig. 2, D and E). These data demonstrate that the LA muscle responds in a dose-dependent manner to T even in the absence of GH secretion from the pituitary gland. Serum IGF-I levels in the Hypox and Cast-Hypox rats were less than 10% of Control and Cast rats (data not shown), indicating that T is able to rescue LA mass in a circulating IGF-I-deficient state.
Figure 2.
Dose-dependent effects of T on LA and EDL muscle in vivo. Two-month-old Control, Hypox, and Cast-Hypox male rats were treated with 0 (sesame oil alone), 20, 60, or 200 μg/d of TE three times each week for 2 wk. A, Serum T levels. T level was lower in both Hypox and Cast-Hypox rats compared with controls. TE injections increased T levels dose dependently. B and C, LA muscle mass [absolute (B) or in relation to body weight (C)]. LA muscle mass was lower in Hypox and Cast-Hypox rats compared with controls and rescued by TE treatment in a dose-dependent manner. D and E, EDL muscle mass [absolute (D) or in relation to body weight (E)] was not affected by TE treatment. F, mRNA expression of IGF-IEa, IGF-IEb (MGF), and AR by qPCR of RNA extracted from LA muscle. G, Myogenin and MyoD expression in LA muscle. Cast, Hypox, and Cast-Hypox rats shown significant reduction of IGF-IEa, IGF-IEb, and myogenin expression. TE up-regulated IGF-IEa and IGF-IEb expression in Cast, Hypox, and Cast-Hypox rats and myogenin in Cast rats. Results are means ± sem (n = 6 per TE dosage). *, P < 0.05; **, P < 0.01; ***, P < 0.001 when values are compared with oil-treated rats inside each experimental group; and ###, P < 0.001 when compared with control oil-treated rats (A); **, P < 0.01 when values are compared with oil-treated rats (B); and *, P < 0.05; **, P < 0.01 when values are compared vs. oil-treated rats inside each experimental group (F and G). Representative critical values for t test statistical analysis: Control 0 vs. Hypox 0, t = 7.638, df = 19; Hypox 0 vs. Hypox 200, t = 9.061, df = 25 (A).
We used qPCR to assess the effect of TE administration on the expression of IGF-I splice variants in the LA muscle. Muscle IGF-IEa and IGF-IEb mRNA expression was lower in Cast, Hypox, and Cast-Hypox rats compared with Control rats (Fig. 2F). TE up-regulated IGF-IEa and IGF-IEb mRNA expression in these groups of rats, but not in Control rats (Fig. 2F). Androgen receptor (AR) mRNA expression was somewhat decreased in Cast and Hypox rats but not affected by TE treatment (Fig. 2F). In addition, TE rescued the mRNA expression of two muscle-specific transcription factors, MyoD and myogenin, in Cast rats (Fig. 2G). As IGF-I has been implicated in satellite cell proliferation in vivo (22,23), these data led us to hypothesize that T might expand myoblasts, by inducing the local release of IGF-I.
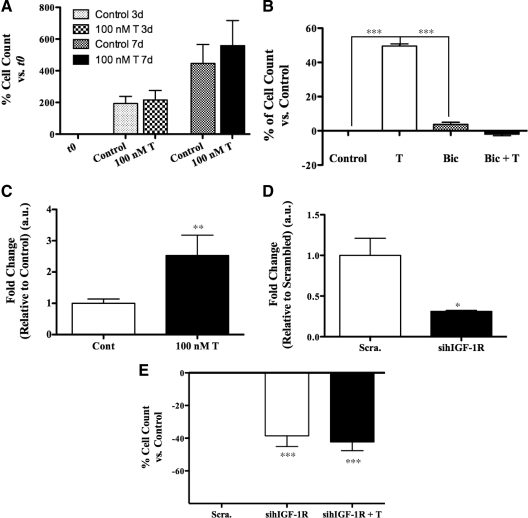
T increases the proliferation of primary hSKMCs in vitro
We determined the effects of T on the proliferation of hSKMCs. We reasoned that T supplementation would increase the total number of cells as a result of cell cycle induction. After 3 and 7 d of treatment, T-treated wells had higher cell number compared with control wells (Fig. 3A). The addition of bicalutamide blocked this response (Fig. 3B), suggesting that T’s effect on myoblast proliferation is AR-mediated. Moreover, T induced IGF-IEa expression in the hSKMCs after 5 d of treatment (Fig. 3C).
Figure 3.
Effect of T on the proliferation of primary hSKMCs in vitro. A, Effect of T on the number of cells after 3 and 7 d of coincubation of hSKMCs with 100 nm T. Cells were plated at very low confluence and grown in GM with and without 100 nm T supplemented every 2 d. The number of cells was quantified by using the CyQUANT kit. Data are expressed as the percent of increments in the number of cells of the various experimental points vs. initial counts at t 0. T increased the number of cells after 3 and 7 d of treatment. B, Bicalutamide (Bic) supplementation blocked T effect on cell proliferation. Data are expressed as the percent of increments in the number of cells in T-treated wells vs. medium alone control wells (Control). C, qPCR analysis for IGF-IEa mRNA expression in total RNA extracted from hSKMCs grown as in A and treated with 100 nm T for 5 d. T treatment induces IGF-IEa expression. D and E, CyQUANT analysis on hSKMCs transfected with 10 nm each of two separate sihIGF-IR or with 10 nm of Scra. and treated with and without 100 nm T supplemented every 2 d for 7 d. D, qPCR to show IGF-IR knockdown after 4 d. E, IGF-IR knockdown reduced the number of cells, and T supplementation was unable to rescue their proliferation. Results are means ± sem of five separate experiments (A) and of three separate experiments (B–E). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To determine whether IGF-I plays a role in mediating T’s effect on myoblast proliferation, we transfected proliferating hSKMCs with two siRNA for the human IGF-IR (sihIGF-IR) or with Scra. IGF-IR knockdown reduced the expression of endogenous IGF-IR (Fig. 3D) and reduced the number of cells compared with Scra. (Fig. 3E). Treatment with T failed to rescue the inhibition of proliferation in the cells transfected with the sihIGF-IR (Fig. 3E), indicating that T requires IGF-I signaling to induce myoblast proliferation in this in vitro system.
T increases the fusion of primary hSKMCs in vitro
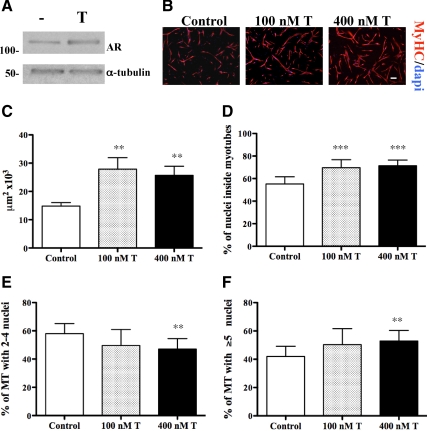
The effect of T on muscle differentiation in vitro has been inconsistent across studies (11,13,14,15,16). We investigated the effect of T on myogenic differentiation by treating hSKMCs with or without T for 3 d in low-serum conditions. Both T-treated and control cells formed myotubes when shifted to DM at low confluence, but cells supplemented with T showed increased levels of AR (Fig. 4A) and formed larger myotubes (Fig. 4, B and C), as tested by immunostaining for MyHC, a marker of muscle terminal differentiation widely used to evaluate myotube morphology. T-treated cells had a higher fusion index and myonuclear number compared with controls (Fig. 4, D–F) and showed a dose-dependent increase in myotube area (Fig. 5A). Consistent with the observed myotube hypertrophy, T up-regulated the expression of MyHC and myogenin, two markers of myogenic differentiation (Fig. 5, B and C). To evaluate whether T’s effects under proliferation conditions are additive to those observed during differentiation conditions, we determined the effect on myogenic differentiation by treating hSKMCs with T either in GM, DM, or both. The hSKMCs treated with T in both GM and DM generated the largest myotubes (Fig. 5, D and E). These results demonstrate that T enhances terminal differentiation of hSKMCs in vitro.
Figure 4.
Effect of T on the myogenic differentiation of hSKMCs in vitro. Human SKMCs were expanded then shifted at low confluence to DM with and without T, which was supplemented daily for 3 d. Control cells were treated with vehicle alone. A, T supplementation increased AR expression levels, as shown by Western blotting. B, hSKMCs cultured with 0, 100, or 400 nm T and immunostained for MyHC; nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar, 200 μm. C, Mean myotube area was calculated for samples showed in B and expressed in μm2. Fusion index and nuclear number assays were determined in the same samples, by considering two classes of myotubes, with two to four nuclei and with more than or equal to five nuclei (D–F). T supplementation increased myotube area and resulted in formation of bigger myotubes with greater number of myonuclei. Results are means ± sem of three independent experiments. **, P < 0.01; ***, P < 0.001.
Figure 5.
Effect of T on the myogenic differentiation of hSKMCs in vitro. A, hSKMCs were shifted at high confluence in DM with and without T, supplemented daily for 3 d, and immunostained for MyHC. Myotube area was calculated as in Fig. 4. B, Western blotting of protein extracted from samples as in A; proteins were run in two separate gels; molecular mass expressed in kDa. C, Densitometry of films developed in B. T supplementation increases myotube area and the expression level of MyHC and myogenin. D and E, hSKMCs were grown in GM with and without 100 nm T, supplemented every 2 d for 4 d, then shifted to DM with and without 400 nm T, supplemented daily for 3 d. Myotube area (D) and fusion index (E) were calculated as in Fig. 4. T supplementation in both GM and DM increased myotube area and formed bigger myotubes. Results in A, D, and E are means ± sem of three independent experiments. **, P < 0.01; ***, P < 0.001. Results in B and C represent one of three independent experiments.
The role of IGF-I signaling in regulating T’s effect on muscle differentiation in vitro
To determine whether IGF-I mediates T’s effect on hSKMCs differentiation, we transfected hSKMCs either with two sihIGF-IR constructs or with Scra. IGF-IR knockdown by sihIGF-IR was associated with reduced expression of endogenous IGF-IR and MyHC, as determined by qPCR (Fig. 6A) and Western blotting (Fig. 6B), respectively. Human SKMCs treated with sihIGF-IR formed smaller myotubes compared with control (Scra.). However, T treatment partially rescued the inhibitory effect of sihIGF-IR on hSKMCs myotubes size (Fig. 6C). We considered the possibility that the knockdown of IGF-IR by sihIGF-IR might have been incomplete. Accordingly, we used two additional approaches, described below, to block IGF-IR signaling.
Figure 6.
Effect of T on hSKMCs in culture conditions in which the IGF-I signaling is down-regulated. A–C, hSKMCs were transfected in GM with 10 nm each of two separate sihIGF-IR or with Scra. After 36 h, the cells were shifted at high confluence to DM with and without 400 nm T supplemented daily for 3 d. The cells were lysed to harvest proteins and total RNA or fixed to assess muscle terminal differentiation by immunofluorescence. A, qPCR for the hIGF-IR showing marked reduction in IGF-IR mRNA expression in wells transfected with sihIGF-IR. B, sihIGF-IR reduced the levels of the β-subunit of the human IGF-IR and of MyHC, as shown by Western blotting. C, Myotube area was calculated as in Fig. 4. T supplementation increased myotube area in cells transfected with sihIGF-IR. D and E, hSKMCs were shifted at low confluence to DM with or without 8 or 16 μm Ly and 400 nm T supplemented daily for 3 d, then fixed for immunofluorescence. Myotube area, fusion index, and the number of nuclei were calculated as in Fig. 4; Ly inhibited myotube formation, and T rescued this effect only at low dose of Ly. F, hSKMCs were shifted at low confluence to DM with and without 400 nm T and 2 μg/ml of a blocking antibody against hIGF-IR (Ab). Human IGF-IR antibody and T were supplemented daily for 3 d. T did not increase myotube area in the presence of the antibody. Results are mean ± sem of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, and in A is shown vs. scrambled-treated cells. #, P < 0.05; ##, P < 0.01; ###, P < 0.001 when compared with control, untreated cells.
Because IGF-I signals mainly through phosphatidylinositol 3 kinase (PI3K) (28,36,37,38), we repeated the differentiation experiment by treating hSKMCs with Ly, a PI3K inhibitor. As expected, Ly-treated hSKMCs formed smaller myotubes with fewer myonuclei than those treated with medium alone. T rescued partially the inhibitory effect of Ly on hSKMCs differentiation at the lower (8 μm) but not at the higher (16 μm) Ly concentration (Fig. 6, D and E). Additionally, we repeated the differentiation experiment using a blocking antibody for IGF-IR. Human SKMCs treated with anti-IGF-IR antibody formed smaller myotubes compared with control, but T was not able to rescue this inhibitory effect (Fig. 6F). Taken together, these data suggest that T’s effects on myogenic differentiation are attenuated when the IGF-IR-signaling is inhibited.
T increases body weight and LA muscle mass in MKR mice
To evaluate in vivo whether the local IGF-I signaling is required for mediating T’s effect on the LA, we used MKR mice that express a dominant-negative mutant of the IGF-IR in muscle fibers (24). This mutant IGF-IR has been reported to block the early IGF-I-dependent signaling in MKR mice (24). MKR mice are characterized by reduced skeletal muscle mass (24) and by the failure to increase muscle mass in response to GH treatment (25). A, a GnRH antagonist, was used to induce T deficiency. Two-month-old male MKR mice were assigned randomly to one of three experimental groups (n = 6 per group): vehicle-treated controls (MKR), A-treated (MKR-A), and A plus TE (AT)-treated (MKR-AT). Three additional groups (n = 6 per group) of wild-type (Control) male mice were treated similarly with vehicle (Control), A (Control-A), or AT (Control-AT). A was administered in saline solution for 2 wk before initiating T treatment and was continued during TE administration. TE was supplemented in sesame oil at a dose of 200 μg/d for 2 wk; corresponding controls received injections of sesame oil alone. A treatment significantly reduced body weight only in wild-type mice, whereas the LA muscle mass decreased in both wild-type and MKR mice (Fig. 7, A and B). TE rescued the LA weight in A-treated wild-type as well as MKR mice, even after adjusting for body weight (Fig. 7, B–E). The EDL muscle mass was not affected by treatments (data not shown). Serum T levels were lower in untreated MKR mice. T levels were reduced by A treatment, whereas TE administration increased T levels in both wild-type and MKR mice (Fig. 7F). Using qPCR analysis of RNA from the LA of MKR mice, we found that TE up-regulated IGF-IEa and IGF-IEb mRNA expression in A-treated animals (Fig. 7G), similarly to what we observed in Cast rats (Fig. 2F). These data suggest that functional IGF-IR-dependent signaling inside mature muscle fibers does not appear to be obligatory in mediating T’s anabolic effects on the LA.
Figure 7.
Effect of T on the body weight, LA mass, and T levels in MKR mice. Three-month-old wild-type (Control) and MKR male mice were treated with a GnRH antagonist, A, or AT. A was delivered three times per week at a dose of 0.1 mg/d in saline, whereas TE was administered three times per week at a dose of 200 μg/d in sesame oil. Untreated mice were injected with vehicle alone. A, A reduced body weight of control mice that was rescued by T. B–E, LA muscle mass [absolute (B) or in relation to body weight (C–E)] was reduced by A treatment in both control and MKR mice and was rescued by T. F, Serum T levels were reduced by A treatment in control mice. TE increased serum T level in both control and MKR mice. G, IGF-IEa and IGF-IEb mRNA expression assessed by qPCR in LA of MKR mice. TE induced IGF-IEa and IGF-IEb expression. Results are means ± sem (n = 6 per each group). *, P < 0.05; **, P < 0.01, ***, P < 0.001. Representative critical values for t test statistical analysis: MKR-A vs. MKR-AT, t = 3.76, df = 10 (B).
Discussion
We investigated the role of circulating GH and IGF-I and im IGF-I in mediating the anabolic effects of T in skeletal muscle using in vitro and in vivo models. Here, we demonstrate that T increases body weight and the weight of the androgen-responsive LA muscle even in GH-deficient rats that had markedly suppressed IGF-I levels. T administration only partially restored body weight in Hypox rats, consistent with the important role of pituitary hormones, other than GH, in regulating body mass. In contrast, T supplementation restored the LA weight in Hypox rats to that observed in Cast rats with intact pituitary, even after adjustment for body weight. Thus, circulating GH and IGF-I are not essential for mediating T’s effects on androgen-responsive muscle.
T stimulates pulsatile GH secretion, and its administration increases circulating GH and IGF-I levels in boys with constitutional delay of puberty and community-dwelling men (2,39,40). Also, GH and IGF-I have been shown to modulate Leydig cell sensitivity to LH. These positive and cooperative interactions between the T and GH/IGF-I axes are important in augmenting pubertal growth in mammals. It is notable that Hypox rats displayed an increase in whole body and EDL weight, whereas intact rats did not, even though the ratio of EDL to body weight did not change, in response to T administration. The mechanisms that render EDL muscle responsive to androgen administration after hypophysectomy merit further investigation. Collectively, our results indicate that circulating GH and IGF-I are dispensable for mediating T’s action on body weight and LA mass, similarly to what has been observed in Cast GH-receptor null mice (41).
Rodents express two main IGF-I isoforms, IGF-IEa and IGF-IEb (21,23,42). Although IGF-IEb has been implicated in satellite cell expansion, IGF-IEa is thought to enhance myoblast differentiation (21,23). In our in vivo experiments, T administration up-regulated the expression of both IGF-IEa and IGF-IEb in the LA of Cast, as well as Hypox, rats. Our data are consistent with reports showing that androgens stimulate the expression of IGF-I in the skeletal muscle (18,19) and show that T induces im IGF-I expression even in the absence of circulating GH/IGF-I.
We show that T stimulates the proliferation of primary hSKMCs consistent with previous data (6,10,43,44,45). T’s effects on muscle progenitor cell proliferation were associated with the up-regulation of IGF-IEa expression and blocked by the knockdown of IGF-IR, consistent with the crucial role of IGF-I signaling in muscle progenitor cell proliferation (29,31,43). These data suggest that IGF-I signaling plays an important role in mediating T’s effects on the muscle progenitor cell proliferation in vitro.
When hSKMCs were maintained in differentiation conditions, T promoted fusion and differentiation, leading to the formation of larger myotubes with a greater number of myonuclei, and up-regulated the expression of MyHC and myogenin. These novel observations are consistent with reports of muscle fiber hypertrophy observed in men treated with T (3,6,46). When IGF-I signaling was blocked using either IGF-IR knockdown or pharmacological blockade of PI3K, the effects of T on myogenic differentiation were attenuated. Taken together, these data suggest that IGF-I signaling plays an important role in mediating T’s effects on terminal differentiation of hSKMCs in vitro.
The in vitro data on the effects of T in hSKMCs differ somewhat from those observed in vivo in the MKR mice, in which LA mass responded to T by an attenuated, but still significant, increase. The IGF-IR-dependent signaling is partially, but not completely, lost in the MKR mice, because the dominant negative mutant form of the IGF-IR is expressed only in mature muscle fibers (24); for this, it is possible that the reduced level of IGF-R signaling in the skeletal muscle of MKR mice is sufficient to mediate T’s anabolic effect. Although IGF-IR signaling is reduced in the skeletal muscle fibers in the MKR mice, this signaling is still intact in other cell types, such as motor neurons, fibroblasts, and muscle progenitor cells; it is then plausible that the observed anabolic effects of T in the MKR mice might reflect its action at these other cell types in which IGF-IR signaling is still preserved. Indeed, neural input has been shown to play an important role in mediating the response of the LA muscle to androgen (47,48,49), and it has been reported that the MKR mice compensate the marked muscle hypoplasia present from birth to 3 wk of age with an increase in the number of nuclei per myofiber as they grow to adulthood, indicating compensatory proliferation and differentiation of satellite cells (24). In addition, the MKR mice have been reported to undergo muscle hypertrophy after mechanical loading and to activate Akt through IGF-IR-independent pathways (50). Therefore, it is also possible that T might increase the mass of the LA in MKR mice by an IGF-IR-independent mechanism. Regardless of the underlying mechanism, IGF-IR signaling does not seem obligatory for mediating the responsiveness of the LA muscle to T in the MKR mice.
Our study has several strengths and some limitations due to the particular experimental models and protocols we used. For example, the range of T concentrations in in vitro and in vivo experiments extended from physiologic to supraphysiologic levels. However, the combined application of in vitro and in vivo approaches and the consistency of results across in vivo and in vitro experiments lend strength to the inferences. To minimize the risk of confounding due to nonspecific or off-target effects associated with any one strategy, we used multiple approaches to block IGF-IR-mediated signaling in vitro (sihIGF-IR, PI3K inhibition, and IGF-IR antibody), and we used primary SKMCs, rather than muscle cell lines, for their higher myogenic commitment. We recognize that no experimental model can faithfully replicate the complexity of the human organism. For example, the vascular adaptations that might occur in a live organism and contribute to the skeletal muscle growth upon T treatment are not reproduced in cell culture systems. In particular, hSKMCs maintained in culture lack motor innervation, which is known to be important in mediating the responsiveness of the LA muscle to changes in T concentrations in rodents (47,48,49). Human SKMCs cultures also lack interactions with other cell types, such as motor neurons and fibroblasts, which are present in the LA muscle and may modulate response to androgen administration in vivo (49,51). Moreover, because the hSKMCs are derived from the human vastus lateralis, they may differ in their responsiveness from cells derived from the rodent LA. In addition, the growth factors and other constituents of the culture medium may affect the response to T in in vitro experiments, and it is possible that the anti-IGF-IR antibody we used may affect muscle differentiation per se, by interfering with the activity of proteins present on the surface of hSKMCs, which are necessary for their fusion. Finally, we cannot exclude the possibility that blocking IGF-IR signaling may lead to abrogation of responsiveness to all anabolic stimuli, not just T, reflecting the pivotal role of IGF-IR-mediated signaling in the maintenance of skeletal muscle homeostasis.
In summary, circulating GH and IGF-I are not essential in the adult male rat for mediating T’s anabolic effects on body weight and androgen-responsive LA muscle. T stimulates the proliferation and differentiation of hSKMCs in vitro and induces im IGF-I expression that plays an important role in mediating T’s effects on myoblast proliferation as well as differentiation. However, in the MKR mice, IGF-IR signaling does not appear to be obligatory for mediating the anabolic effects of T. Further investigations are needed to determine whether the observed differences between in vitro and in vivo models are due to an incomplete abrogation of IGF-IR signaling in the MKR mice or to an important role of innervation or of other cell types in mediating the response to T. Our data do not preclude the involvement of other signaling pathways, which were not studied in this investigation, in mediating T’s effects on myoblast proliferation and differentiation.
Acknowledgments
We wish to thank Dr. Derek Le Roith (Mount Sinai School of Medicine, New York, NY) for providing the MKR mice.
Footnotes
This work was supported by grants from the Department of Medicine and Evans Medical Foundation of the Boston University (C.S.), the National Institutes of Health Grant 5R01DK070534-07 (to S.B.), the Boston Claude D. Pepper Older Americans Independence Center Grant 5P30AG031679 (to S.B.), and the Paul Wellstone Muscular Dystrophy Cooperative Research Center Grant U54 AR052646 (to E.R.B.).
Disclosure Summary: The authors have nothing to disclose. C.M. is an employee of Pfizer, Inc.
First Published Online November 17, 2010
Abbreviations: A, Acyline; AR, androgen receptor; AT, A plus TE; Cast, castrated; Cast-Hypox, Cast plus Hypox; DM, differentiation medium; EDL, extensor digitorum longus; Gapdh, glyceraldehyde-3-phosphate dehydrogenase; GM, growth medium; hSKMC, human skeletal muscle cell; HTLC, high-turbulence liquid chromatography; Hypox, hypophysectomized; IGF-IR, IGF-I receptor; LA, levator ani; Ly, Ly-294,002; MGF, mechano growth factor; MyHC, myosin heavy chain; PI3K, phosphatidylinositol 3 kinase; qPCR, quantitative PCR; Scra., scrambled siRNA; sihIGF-IR, siRNA for the human IGF-IR; siRNA, small interference RNA; T , testosterone; TBS-T, Tris-buffered saline plus 0.1% Tween-20; TE, T enanthate.
References
- Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, Montori VM, Gao W, Dalton JT 2006 Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab 2:146–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ 2002 Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282:601–607 [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S 2002 Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab 283:154–164 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW 2005 Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 90:678–688 [DOI] [PubMed] [Google Scholar]
- Storer TW, Woodhouse L, Magliano L, Singh AB, Dzekov C, Dzekov J, Bhasin S 2008 Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc 56:1991–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha-Hikim I, Roth SM, Lee MI, Bhasin S 2003 Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab 285:E197–E205 [DOI] [PubMed] [Google Scholar]
- MacLean HE, Chiu WS, Notini AJ, Axell AM, Davey RA, McManus JF, Ma C, Plant DR, Lynch GS, Zajac JD 2008 Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J 22:2676–2689 [DOI] [PubMed] [Google Scholar]
- Ophoff J, Van Proeyen K, Callewaert F, De Gendt K, De Bock K, Vanden Bosch A, Verhoeven G, Hespel P, Vanderschueren D 2009 Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology 150:3558–3566 [DOI] [PubMed] [Google Scholar]
- Joubert Y, Tobin C, Lebart MC 1994 Testosterone-induced masculinization of the rat levator ani muscle during puberty. Dev Biol. 162:104–110 [DOI] [PubMed] [Google Scholar]
- Nnodim JO 2001 Testosterone mediates satellite cells activation in denervated rat levator ani muscle. Anat Rec 263:19–24 [DOI] [PubMed] [Google Scholar]
- Lee DK 2002 Androgen receptor enhances myogenin expression and accelerates differentiation. Biochem Biophys Res Commun 294:408–413 [DOI] [PubMed] [Google Scholar]
- Benjamin CL, Jenster G, Piedrahita JA 2004 Use of artificial androgen receptor coactivators to alter myoblast proliferation. J Steroid Biochem Mol Biol 91:111–119 [DOI] [PubMed] [Google Scholar]
- Diel P, Baadners D, Schlüpmann K, Velders M, Schwarz JP 2008 C2C12 myoblastoma cell differentiation and proliferation is stimulated by androgens and associated with a modulation of myostatin and Pax7 expression. J Mol Endocrinol 40:231–241 [DOI] [PubMed] [Google Scholar]
- Doumit ME, Cook DR, Merkel RA 1996 Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology 137:1385–1394 [DOI] [PubMed] [Google Scholar]
- Chen Y, Lee NK, Zajac JD, MacLean HE 2008 Generation and analysis of an androgen-responsive myoblast cell line indicates that androgens regulate myotube protein accretion. J Endocrinol Invest 31:910–918 [DOI] [PubMed] [Google Scholar]
- Vlahopoulos S, Zimmer WE, Jenster G, Belaguli NS, Balk SP, Brinkmann AO, Lanz RB, Zoumpourlis VC, Schwartz RJ 2005 Recruitment of the androgen receptor via serum response factor facilitates expression of a myogenic gene. J Biol Chem 280:7786–7792 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW 2001 Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 281:1172–1181 [DOI] [PubMed] [Google Scholar]
- Lewis MI, Horvitz GD, Clemmons DR, Fournier M 2002 Role of IGF-I and IGF-binding proteins within diaphragm muscle in modulating the effects of nandrolone. Am J Physiol Endocrinol Metab 282:483–490 [DOI] [PubMed] [Google Scholar]
- Lewis MI, Fournier M, Storer TW, Bhasin S, Porszasz J, Ren SG, Da X, Casaburi R 2007 Skeletal muscle adaptations to testosterone and resistance training in men with COPD. Appl Physiol 103:1299–1310 [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A 1993 Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59–72 [PubMed] [Google Scholar]
- Matheny Jr RW, Nindl BC, Adamo ML 2010 Minireview: mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology 151:865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Goldspink G 2002 Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 522:156–160 [DOI] [PubMed] [Google Scholar]
- Hill M, Goldspink G 2003 Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández AM, Dupont J, Farrar RP, Lee S, Stannard B, Le Roith D 2002 Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J Clin Invest 109:347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Barton E, Muja N, Yakar S, Pennisi P, Leroith D 2005 Intact insulin and insulin-like growth factor-I receptor signaling is required for growth hormone effects on skeletal muscle growth and function in vivo. Endocrinology 146:1772–1779 [DOI] [PubMed] [Google Scholar]
- Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz R 1995 Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270:12109–12116 [DOI] [PubMed] [Google Scholar]
- Musarò A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N 2001 Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27:195–200 [DOI] [PubMed] [Google Scholar]
- Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR 1997 The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 272:6653–6662 [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ 2001 Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3:1009–1013 [DOI] [PubMed] [Google Scholar]
- Jacquemin V, Butler-Browne GS, Furling D, Mouly V 2007 IL-13 mediates the recruitment of reserve cells for fusion during IGF-1-induced hypertrophy of human myotubes. J Cell Sci 120:670–681 [DOI] [PubMed] [Google Scholar]
- Foulstone EJ, Huser C, Crown AL, Holly JM, Stewart CE 2004 Differential signalling mechanisms predisposing primary human skeletal muscle cells to altered proliferation and differentiation: roles of IGF-I and TNFα. Exp Cell Res 294:223–235 [DOI] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD 2003 Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 547:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir-Petermann T, Codner E, Pérez V, Echiburú B, Maliqueo M, Ladrón de Guevara A, Preisler J, Crisosto N, Sánchez F, Cassorla F, Bhasin S 2009 J Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. Clin Endocrinol Metab 94:1923–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema U, Brown R, Mudera V, Yang SY, McGrouther G, Goldspink G 2005 Mechanical signals and IGF-I gene splicing in vitro in relation to development of skeletal muscle. J Cell Physiol 202:67–75 [DOI] [PubMed] [Google Scholar]
- Horsley V, Friday BB, Matteson S, Kegley KM, Gephart J, Pavlath GK 2001 Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J Cell Biol 153:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musarò A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N 1999 IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 400:581–585 [DOI] [PubMed] [Google Scholar]
- Galvin CD, Hardiman O, Nolan CM 2003 IGF-1 receptor mediates differentiation of primary cultures of mouse skeletal myoblasts. Mol Cell Endocrinol 200:19–29 [DOI] [PubMed] [Google Scholar]
- Kaliman P, Viñals F, Testar X, Palacín M, Zorzano A 1996 Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J Biol Chem 271:19146–19151 [DOI] [PubMed] [Google Scholar]
- Devesa J, Lois N, Arce V, Diaz MJ, Lima L, Tresguerres JA 1991 The role of sexual steroids in the modulation of growth hormone (GH) secretion in humans. J Steroid Biochem Mol Biol 40:165–173 [DOI] [PubMed] [Google Scholar]
- Keenan BS, Richards GE, Ponder SW, Dallas JS, Nagamani M, Smith ER 1993 Androgen-stimulated pubertal growth: the effects of testosterone and dihydrotestosterone on growth hormone and insulin-like growth factor-I in the treatment of short stature and delayed puberty. J Clin Endocrinol Metab 76:996–1001 [DOI] [PubMed] [Google Scholar]
- Venken K, Movérare-Skrtic S, Kopchick JJ, Coschigano KT, Ohlsson C, Boonen S, Bouillon R, Vanderschueren D 2007 Impact of androgens, growth hormone, and IGF-I on bone and muscle in male mice during puberty. J Bone Miner Res 22:72–82 [DOI] [PubMed] [Google Scholar]
- Barton ER 2006 The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab 31:791–797 [DOI] [PubMed] [Google Scholar]
- Powers ML, Florini JR 1975 A direct effect of testosterone on muscle cells in tissue culture. Endocrinology 97:1043–1047 [DOI] [PubMed] [Google Scholar]
- Joubert Y, Tobin C 1995 Testosterone treatment results in quiescent satellite cells being activated and recruited into cell cycle in rat levator ani muscle. Dev Biol 169:286–294 [DOI] [PubMed] [Google Scholar]
- Kamanga-Sollo E, Pampusch MS, Xi G, White ME, Hathaway MR, Dayton WR 2004 IGF-I mRNA levels in bovine satellite cell cultures: effects of fusion and anabolic steroid treatment. J Cell Physiol 201:181–189 [DOI] [PubMed] [Google Scholar]
- Eriksson A, Kadi F, Malm C, Thornell LE 2005 Skeletal muscle morphology in power-lifters with and without anabolic steroids. Histochem Cell Biol 124:167–175 [DOI] [PubMed] [Google Scholar]
- Buresová M, Gutmann E, Hanzlíková V 1972 Differential effects of castration and denervation on protein synthesis in the levator ani muscle of the rat. J Endocrinol 54:3–14 [DOI] [PubMed] [Google Scholar]
- Sar M, Stumpf WE 1977 Androgen concentration in motor neurons of cranial nerves and spinal cord. Science 197:77–79 [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP 1980 Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science 210:564–566 [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Le Roith D, Ward CW, Bodine SC 2008 A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol 586:283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JA, Breedlove SM, Jordan CL 2007 Androgen receptor expression in the levator ani muscle of male mice. J Neuroendocrinol 19:823–826 [DOI] [PubMed] [Google Scholar]