Abstract
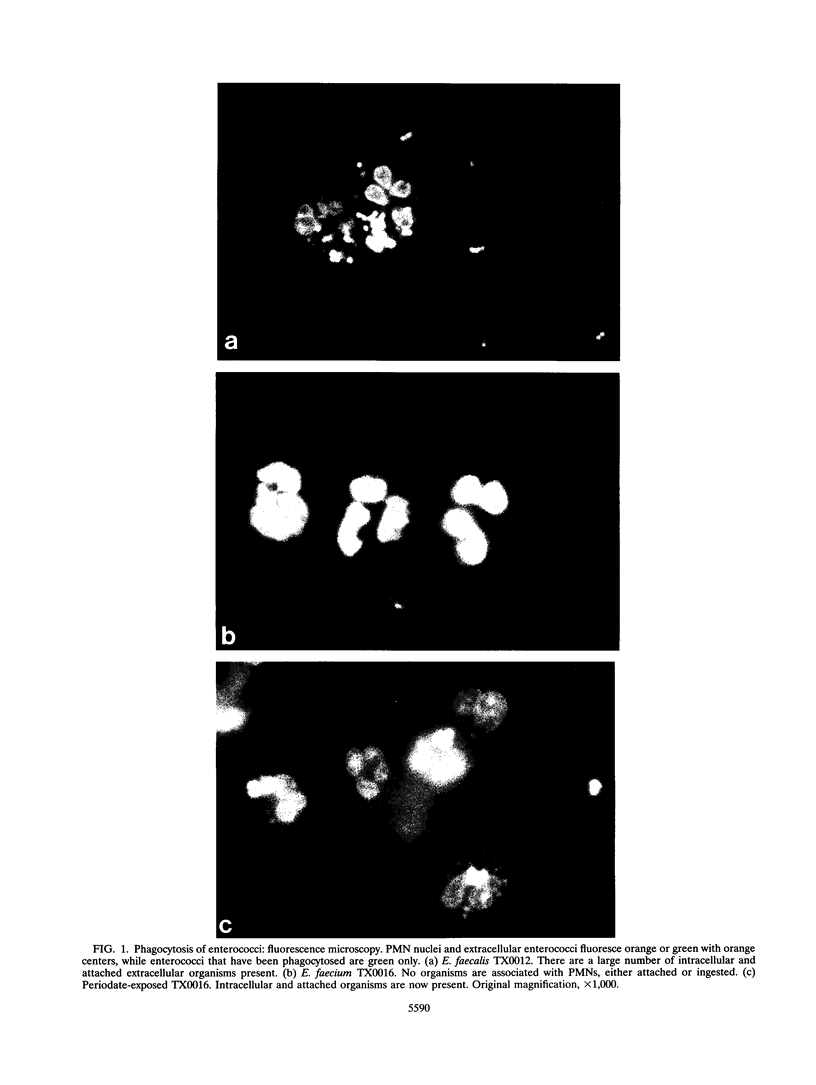
During a previous study of the opsonic requirements for neutrophil (polymorphonuclear leukocyte [PMN])-mediated killing of enterococci, we identified two strains of Enterococcus faecium (TX0015 and TX0016) that were resistant to PMN-mediated killing. To better define the mechanism of this resistance, we examined phagocytosis with a fluorescence assay and found that TX0016 was completely resistant to phagocytosis by PMNs; this finding was confirmed by electron microscopy. Examination of multiple strains of enterococci revealed that all 20 strains of Enterococcus faecalis tested were readily phagocytosed (mean, 18 intracellular organisms per PMN; range, 7 to 28). In contrast, only 13 (50%) of 26 strains of E. faecium tested were susceptible to phagocytosis (> or = 7 organisms per PMN); the other 13 strains showed < or = 3 organisms per PMN. Enterococcus casseliflavus ATCC 25788 and one strain of Enterococcus hirae were also resistant to phagocytosis, while two strains of Enterococcus durans, Enterococcus mundtii ATCC 43186, and one strain each of Enterococcus raffinosus and Enterococcus solitarius were readily phagocytosed. Exposure of E. faecium TX0016 to sodium periodate, but not to the protease trypsin or pronase or to phospholipase C, eliminated resistance to phagocytosis. Sialic acid, a common periodate-sensitive structure used by microorganisms to resist opsonization, could not be demonstrated in E. faecium TX0016 by the thiobarbituric acid method, nor was phagocytosis of TX0016 altered by neuraminidase treatment. This study suggests that there is a difference in susceptibility to phagocytosis by PMNs between different species of enterococci and that a carbohydrate-containing moiety which is not sialic acid may be involved in the resistance of E. faecium TX0016 to phagocytosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arduino R. C., Murray B. E., Rakita R. M. Roles of antibodies and complement in phagocytic killing of enterococci. Infect Immun. 1994 Mar;62(3):987–993. doi: 10.1128/iai.62.3.987-993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993 Aug;37(8):1563–1571. doi: 10.1128/aac.37.8.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Pei Z. Pathogenesis of Campylobacter fetus infections: critical role of high-molecular-weight S-layer proteins in virulence. J Infect Dis. 1993 Feb;167(2):372–377. doi: 10.1093/infdis/167.2.372. [DOI] [PubMed] [Google Scholar]
- Drevets D. A., Campbell P. A. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J Immunol Methods. 1991 Aug 28;142(1):31–38. doi: 10.1016/0022-1759(91)90289-r. [DOI] [PubMed] [Google Scholar]
- Dubreuil D., Lallier R., Jacques M. Immunoelectron microscopic demonstration that Renibacterium salmoninarum is encapsulated. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):313–315. doi: 10.1016/0378-1097(90)90304-9. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Kasper D. L., Jennings H. J., Baker C. J., Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982 Mar;128(3):1278–1283. [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M. Aminoglycoside resistant enterococcal endocarditis. Infect Dis Clin North Am. 1993 Mar;7(1):117–133. [PubMed] [Google Scholar]
- Facklam R. R., Collins M. D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989 Apr;27(4):731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo M. E., Singh K. V., Baker C. J., Murray B. E. Typing of group B streptococci: comparison of pulsed-field gel electrophoresis and conventional electrophoresis. J Clin Microbiol. 1993 Jun;31(6):1430–1434. doi: 10.1128/jcm.31.6.1430-1434.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallén Sandgren C., Mamo W., Larsson I., Lindahl M., Björk I. A periodate-sensitive anti-phagocytic surface structure, induced by growth in milk whey, on Staphylococcus aureus isolated from bovine mastitis. Microb Pathog. 1991 Sep;11(3):211–220. doi: 10.1016/0882-4010(91)90051-b. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Raucher B., Altarac D., Monka J., Marchione S., Singh K. V., Murray B. E., Wolff J., Walters B. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin Infect Dis. 1993 Jun;16(6):750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- Harvey B. S., Baker C. J., Edwards M. S. Contributions of complement and immunoglobulin to neutrophil-mediated killing of enterococci. Infect Immun. 1992 Sep;60(9):3635–3640. doi: 10.1128/iai.60.9.3635-3640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hed J., Stendahl O. Differences in the ingestion mechanisms of IgG and C3b particles in phagocytosis by neutrophils. Immunology. 1982 Apr;45(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Hermann M., Jaconi M. E., Dahlgren C., Waldvogel F. A., Stendahl O., Lew D. P. Neutrophil bactericidal activity against Staphylococcus aureus adherent on biological surfaces. Surface-bound extracellular matrix proteins activate intracellular killing by oxygen-dependent and -independent mechanisms. J Clin Invest. 1990 Sep;86(3):942–951. doi: 10.1172/JCI114796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann R. D., Sievertsen H. J., Knobloch J., Fischetti V. A. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1657–1661. doi: 10.1073/pnas.85.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann R. D. Target recognition failure by the nonspecific defense system: surface constituents of pathogens interfere with the alternative pathway of complement activation. Infect Immun. 1992 Mar;60(3):721–727. doi: 10.1128/iai.60.3.721-727.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Michalek M. T., Mold C., Bremer E. G. Inhibition of the alternative pathway of human complement by structural analogues of sialic acid. J Immunol. 1988 Mar 1;140(5):1588–1594. [PubMed] [Google Scholar]
- Miranda A. G., Singh K. V., Murray B. E. DNA fingerprinting of Enterococcus faecium by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J Clin Microbiol. 1991 Dec;29(12):2752–2757. doi: 10.1128/jcm.29.12.2752-2757.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992 Jun;14(6):1173–1176. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Lopardo H. A., Rubeglio E. A., Frosolono M., Singh K. V. Intrahospital spread of a single gentamicin-resistant, beta-lactamase-producing strain of Enterococcus faecalis in Argentina. Antimicrob Agents Chemother. 1992 Jan;36(1):230–232. doi: 10.1128/aac.36.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samaroj B. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest. 1983 Sep;72(3):1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Markowitz S. M., Lopardo H. A., Patterson J. E., Zervos M. J., Rubeglio E., Eliopoulos G. M., Rice L. B., Goldstein F. W. Evidence for clonal spread of a single strain of beta-lactamase-producing Enterococcus (Streptococcus) faecalis to six hospitals in five states. J Infect Dis. 1991 Apr;163(4):780–785. doi: 10.1093/infdis/163.4.780. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Ross R. P., Heath J. D., Dunny G. M., Weinstock G. M. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J Bacteriol. 1993 Aug;175(16):5216–5223. doi: 10.1128/jb.175.16.5216-5223.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990 Jan;3(1):46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazur J. H., Cepure A., Kane J. A., Hellerquist C. G. Glycans from streptococcal cell walls. The molecular structure of an antigenic diheteroglycan of glucose and galactose from Streptococcus faecalis. J Biol Chem. 1973 Jan 10;248(1):279–284. [PubMed] [Google Scholar]
- Pazur J. H. beta-D-Glucose 1-phosphate. A structural unit and an immunological determinant of a glycan from streptococcal cell walls. J Biol Chem. 1982 Jan 25;257(2):589–591. [PubMed] [Google Scholar]
- Rosen H., Michel B. R., Chait A. Phagocytosis of opsonized oil droplets by neutrophils. Adaptation to a microtiter plate format. J Immunol Methods. 1991 Nov 5;144(1):117–125. doi: 10.1016/0022-1759(91)90237-a. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Culver D. H., Gaynes R. P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991 Sep 16;91(3B):72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wells V. D., Wong E. S., Murray B. E., Coudron P. E., Williams D. S., Markowitz S. M. Infections due to beta-lactamase-producing, high-level gentamicin-resistant Enterococcus faecalis. Ann Intern Med. 1992 Feb 15;116(4):285–292. doi: 10.7326/0003-4819-116-4-285. [DOI] [PubMed] [Google Scholar]