Abstract
To determine whether the Beijing genotype of Mycobacterium tuberculosis is emerging in the Netherlands, we collected data on 6,829 patients during 1993 to 2000. Six percent had the Beijing genotype. This genotype was associated with diagnosis in recent years, young age, nationality, and multidrug resistance.
Keywords: Mycobacterium tuberculosis, epidemiology, multidrug resistance
The Beijing genotype is found frequently in Asia (1–3) but also in outbreaks of multidrug-resistant tuberculosis (MDRTB) in various parts of the world, including Cuba, Germany, Russia, and Estonia (4–7). The largest known epidemic of MDRTB in North America was caused by the W strain, a variant of the Beijing genotype (8,9). A recent study showed this strain’s emergence and association with drug resistance in Vietnam (10). The Beijing genotype was also responsible for a recent outbreak of tuberculosis (TB) on the Canary Islands (11).
The relatively high degree of genetic conservation of Beijing genotype strains found in a widespread area (12) suggests a recent dissemination and, hence, selective advantages associated with this genotype of Mycobacterium tuberculosis. Recently, Beijing genotype bacteria were reported to carry mutations in putative mutator genes, which may explain a higher adaptability of these bacteria under stress conditions such as exposure to the intracellular environment or anti-TB drugs (13). These mutations may also be the basis of differential interaction between Beijing genotype bacteria and the host immune defense system suggested in a recent study in Indonesia (14).
In the Netherlands, MDRTB is affecting <1% of new TB patients, in particular immigrants (15–17). The incidence of the Beijing genotype has not yet been described. The goal of our study was to determine whether the Beijing genotype is emerging in the Netherlands and whether this genotype is associated with multidrug resistance.
The Study
Patient data were obtained from the Netherlands Tuberculosis Register, which is maintained by the Royal Netherlands Tuberculosis Association and has been in place since 1993. Municipal health services, which are responsible for the followup of all TB patients, send information using standardized, precoded forms on all reported TB cases to the register. The register includes information on demographic characteristics, case detection, risk groups, type of disease, treatment, and treatment outcome.
Isolates obtained from all 8,210 culture-positive patients underwent restriction fragment length polymorphism (RFLP)–typing with IS6110 as a probe at the National Institute of Public Health and the Environment. The Beijing genotype was defined on the basis of spoligotype (no spacers 1–34; at least 4 of spacers 35–43) and a specific region A insertion (18). Certain IS6110 RFLP-genotype families (clades 47 and 61) were consistently found to be Beijing genotype (18). Isolates were assigned to the Beijing genotype on the basis of the IS6110 RFLP pattern (18). If any doubt about clade membership existed, we used spoligotyping for the final allocation.
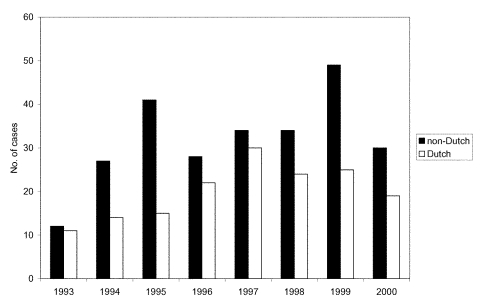
The RFLP results for the period 1993–2000 were matched to patient data on the basis of date of birth, postal area code, and sex, resulting in a perfect match for 5,994 (73%) patients and a near-perfect match for 835 (10%) patients; overall, 83% of the samples matched (6,829 patients). During this period, the Beijing genotype was observed in 516 cultures, representing 6% of the 8,210 patients. In the matched dataset, 415 (6%) of 6,829 were of the Beijing genotype. The number of TB cases attributable to the Beijing genotype tended to increase over time (Figure; p>0.05). This tendency was observed both among Dutch patients (r=0.66, 95% confidence interval [CI] –0.08 to 0.93) and non-Dutch patients (r=0.59, 95% CI –0.20 to 0.91) (Figure). While the increase in the number of cases among immigrants may be associated with an overall increase of immigrants to the Netherlands during the study period, the increasing numbers among Dutch patients likely reflect an increasing incidence rate.
Figure.
Number of tuberculosis cases with the Beijing genotype, Netherlands, 1993–2000.
The proportion of TB patients who had the Beijing genotype was significantly associated in univariate analysis with a later year of diagnosis, young age, nationality (increased among immigrants from Asia and decreased among those from Morocco, Turkey, Somalia, and other African countries, compared with Dutch citizens), and multidrug resistance (Table). These associations persisted in multivariate analysis (Table).
Table. Risk factors for the Beijing genotype of Mycobacterium tuberculosis, the Netherlands, 1993–2000.
| Risk factor | Beijing genotype | Total | (%) | Odds ratio |
|
|---|---|---|---|---|---|
| Crude | Adjustedb (95% confidence intervals) | ||||
|
Y of diagnosis
| |||||
| 1993 |
23 |
669 |
3 |
1.09 |
1.08 (1.03 to 1.13) |
| 1994 |
41 |
941 |
4 |
(per year) |
|
| 1995 |
56 |
863 |
6 |
|
|
| 1996 |
50 |
946 |
5 |
|
|
| 1997 |
64 |
872 |
7 |
|
|
| 1998 |
58 |
804 |
7 |
|
|
| 1999 |
74 |
879 |
8 |
|
|
| 2000 |
49 |
855 |
6 |
|
|
|
Sex
| |||||
| Male |
254 |
4,135 |
6 |
1 |
|
| Female |
161 |
2,694 |
6 |
0.97 |
|
|
Age group
| |||||
| <25 |
120 |
1,522 |
8 |
1 |
1 |
| 25–34 |
123 |
1,925 |
6 |
0.80 |
0.69 (0.53 to 0.91) |
| 35–44 |
64 |
1,080 |
6 |
0.74 |
0.60 (0.43 to 0.83) |
| 45–54 |
23 |
614 |
4 |
0.45 |
0.36 (0.23 to 0.57) |
| 55–64 |
24 |
493 |
5 |
0.60 |
0.55 (0.35 to 0.88) |
| 65–74 |
40 |
517 |
8 |
0.98 |
0.78 (0.52 to 1.15) |
| 75+ |
21 |
678 |
3 |
0.37 |
0.30 (0.18 to 0.50) |
|
Nationality
| |||||
| Netherlands |
160 |
2,825 |
6 |
1 |
1 |
| Europe (central and eastern) |
20 |
231 |
9 |
1.58 |
1.27 (0.77 to 2.09) |
| Turkey |
3 |
339 |
1 |
0.15 |
0.12 (0.04 to 0.38) |
| Morocco |
19 |
650 |
3 |
0.50 |
0.41 (0.25 to 0.68) |
| Somalia |
21 |
957 |
2 |
0.37 |
0.27 (0.17 to 0.44) |
| Africa (other) |
28 |
594 |
5 |
0.82 |
0.63 (0.41 to 0.96) |
| Asia |
140 |
786 |
18 |
3.61 |
3.01 (2.32 to 3.91) |
| Other |
19 |
345 |
6 |
0.97 |
0.86 (0.53 to 1.42) |
| Unknown |
5 |
102 |
5 |
0.86 |
0.73 (0.29 to 1.84) |
|
RFLP clusteringa
| |||||
| No |
212 |
3,227 |
7 |
1 |
|
| Yes, first case |
57 |
1,052 |
5 |
0.81 |
|
| Yes, later case |
146 |
2,550 |
6 |
0.86 |
|
|
Localization
| |||||
| Pulmonary |
268 |
4,064 |
7 |
1 |
|
| Extrapulmonary |
113 |
2,122 |
5 |
0.80 |
|
| Pulmonary and extrapulmonary |
34 |
643 |
5 |
0.79 |
|
|
Residing in Netherlands
| |||||
| <6 months |
46 |
641 |
7 |
1 |
|
| 6–11 mo |
12 |
301 |
4 |
0.54 |
|
| 12–23 mo |
28 |
358 |
8 |
1.10 |
|
| 2–4 y |
53 |
767 |
7 |
0.96 |
|
|
>5 y |
122 |
1,710 |
7 |
0.99 |
|
| Born in Netherlands |
117 |
2,437 |
5 |
0.65 |
|
| No information |
37 |
615 |
6 |
0.83 |
|
|
Drug resistance
| |||||
| Susceptible |
332 |
5,910 |
6 |
1 |
|
| H only |
11 |
227 |
5 |
0.86 |
|
| S only |
34 |
358 |
9 |
1.76 |
|
| Other patterns |
26 |
221 |
12 |
2.24 |
|
| Multidrug-resistant |
9 |
53 |
17 |
3.44 |
|
| Unknown |
3 |
60 |
5 |
0.88 |
|
|
Multidrug resistance
| |||||
| Yes |
9 |
53 |
17 |
3.21 |
2.64 (1.22 to 5.74) |
| No |
406 |
6,776 |
6 |
1 |
1 |
|
HIV infection
| |||||
| Yes |
14 |
294 |
5 |
0.76 |
|
| No | 401 | 6,535 | 6 | 1 | |
aRFLP, restriction fragment length polymorphism.
bAdjusted for year of diagnosis, age, nationality, and multidrug resistance
The association between the proportion of TB cases with the Beijing genotype and age was observed in particular in Dutch patients (chi squaretrend 15.5; p<0.0001), and was not clear among non-Dutch patients (chi squaretrend 0.8; p>0.2). Among Dutch patients, the Beijing genotype was more commonly found in new cases (6%, 133/2,130) than among retreated cases (4%, 18/498; p<0.05). Among non-Dutch patients, the Beijing genotype was slightly more common in retreated cases (8%, 19/229) than in new cases (6%, 204/3,218); however, this occurrence was not significant (p>0.2). The incidence pattern by person and time appears consistent with transmission of the Beijing genotype from immigrants to the Dutch population (19).
The proportion of TB cases with the Beijing genotype was not substantially associated with HIV infection, pulmonary localization, RFLP clustering, or duration of stay in the Netherlands (Table). The lack of association with RFLP-clustering suggests that the Beijing genotype is not spreading more quickly than other M. tuberculosis strains.
Of the nine patients with the Beijing genotype and multidrug resistance, four were from central and eastern Europe, three from Asia, one from Africa, and one from the Netherlands. One of these nine patients was RFLP-clustered; the other patient in that RFLP cluster had been diagnosed previously and had isolated isoniazid resistance.
During 1993 to 2000 in the Netherlands, the Beijing genotype represented 6% of TB cases. The Beijing genotype was associated with recent diagnoses, young age (in particular among Dutch citizens), nationality (Eastern Europe and Asia), and multidrug resistance. Although the Beijing genotype was associated with multidrug resistance, the number of MDRTB cases was small. No secondary cases were observed with the Beijing genotype and MDRTB. Of the nine cases of MDRTB, only one case may have been caused by transmission within the Netherlands from a case-patient who had isoniazid resistance at the start of treatment and may have acquired rifampicin resistance during treatment. However, the spread of the Beijing genotype among young people suggests the emergence of multidrug resistance and emphasizes the need for continued surveillance.
Acknowledgments
We thank all municipal health services in the Netherlands for their contribution to the Netherlands Tuberculosis Register.
This study was performed within the framework of Concerted Action project QLK2-2000-00630 on Molecular Epidemiology of Tuberculosis.
Biography
Dr. Borgdorff is the chief epidemiologist of the Royal Netherlands Tuberculosis Association (KNCV) and professor of international health with special reference to tuberculosis at the University of Amsterdam. His areas of interest include the molecular epidemiology of tuberculosis and other communicable diseases.
Footnotes
Suggested citation for this article: de Thoisy B, Gardon J, Salas RA, Morvan J, Kazanji M. Mayaro virus in wild mammals, French Guiana. Emerg Infect Dis [serial online] 2003 Oct [date cited]. Available from: URL: http://www.cdc.gov/ncidod/EID/vol9no10/02-0743.htm
References
- 1.van Soolingen D, Qian L, de Haas PE, Douglas JT, Traore H, Portaels F, et al. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J Clin Microbiol. 1995;33:3234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan MY, Borgdorff M, Yip CW, de Haas PE, Wong WS, Kam KM, et al. Seventy percent of the Mycobacterium tuberculosis isolates in Hong Kong represent the Beijing genotype. Epidemiol Infect. 2001;127:169–71. 10.1017/S0950268801005659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bifani PJ, Mathema B, Kurepina NE, Kreiswirth BN. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 2002;10:45–52. 10.1016/S0966-842X(01)02277-6 [DOI] [PubMed] [Google Scholar]
- 4.Diaz R, Kremer K, de Haas PE, Gomez RI, Marrero A, Valdivia JA, et al. Molecular epidemiology of tuberculosis in Cuba outside of Havana, July 1994–June 1995: utility of spoligotyping versus IS6110 restriction fragment length polymorphism. Int J Tuberc Lung Dis. 1998;2:743–50. [PubMed] [Google Scholar]
- 5.Niemann S, Rusch-Gerdes S, Richter E. IS6110 fingerprinting of drug-resistant Mycobacterium tuberculosis strains isolated in Germany during 1995. J Clin Microbiol. 1997;35:3015–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marttila HJ, Soini H, Eerola E, Vyshevskaya E, Vyshnevskyi BI, Otten TFA. Ser315Thr substitution in katG is predominant in genetically heterogeneous multi-drug resistant Mycobacterium tuberculosis isolates originating from the St. Petersburg area in Russia. Antimicrob Agents Chemother. 1998;42:2443–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruuner A, Hoffner SE, Sillastu H, Danilovits M, Levina K, Svenson SB, et al. Spread of drug-resistant pulmonary tuberculosis in Estonia. J Clin Microbiol. 2001;39:3339–45. 10.1128/JCM.39.9.3339-3345.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bifani PJ, Plikaytis BB, Kapur V, Stockbauer K, Pan X, Lutfey ML. Origin and interstate spread of a New York City multidrug-resistant Mycobacterium tuberculosis clone family. JAMA. 1996;275:452–7. 10.1001/jama.275.6.452 [DOI] [PubMed] [Google Scholar]
- 9.Frieden TR, Sherman LF, Maw KL, Fujiwara PI, Crawford JT, Nivin B, et al. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA. 1996;276:1229–35. 10.1001/jama.276.15.1229 [DOI] [PubMed] [Google Scholar]
- 10.Anh DD, Borgdorff MW, Van LN, Lan NT, van Gorkom T, Kremer K, et al. Mycobacterium tuberculosis Beijing genotype emerging in Vietnam. Emerg Infect Dis. 2000;6:302–5. 10.3201/eid0603.000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caminero JA, Pena MJ, Campos-Herrero MI, Rodriguez JC, Garcia I, Cabrera P, et al. Epidemiological evidence of the spread of a Mycobacterium tuberculosis strain of the Beijing genotype on Gran Canaria Island. Am J Respir Crit Care Med. 2001;164:1165–70. [DOI] [PubMed] [Google Scholar]
- 12.Glynn JR, Whiteley J, Bifani PJ, Kremer K, van Soolingen D. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg Infect Dis. 2002;8:843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rad ME, Bifani P, Martin C, Kremer K, Samper S, Rauzier J, et al. Mycobacterium tuberculosis strains with a Beijing genotype carry mutations in putative mutator genes ogt and mutT. Emerg Infect Dis. 2003;7:838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Crevel R, Nelwan RH, de Lenne W, Veeraga Y, van der Zanden AG, Amin Z, et al. Mycobacterium tuberculosis Beijing genotype strains associated with febrile response to treatment. Emerg Infect Dis. 2001;7:880–3. 10.3201/eid0705.010518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinal MA, Laszlo A, Simonsen L, Boulahbal F, Kim SJ, Reniero A, et al. Global trends in resistance to antituberculosis drugs. World Health Organization—International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 2001;344:1294–303. 10.1056/NEJM200104263441706 [DOI] [PubMed] [Google Scholar]
- 16.Lambregts-van Weezenbeek CS, Jansen HM, Nagelkerke NJ, van Klingeren B, Veen J. Nationwide surveillance of drug-resistant tuberculosis in the Netherlands: rates, risk factors and treatment outcome. Int J Tuberc Lung Dis. 1998;2:288–95. [PubMed] [Google Scholar]
- 17.Lambregts-van Weezenbeek CS, Jansen HM, Veen J, Nagelkerke NJ, Sebek MM, van Soolingen D. Origin and management of primary and acquired drug-resistant tuberculosis in the Netherlands: the truth behind the rates. Int J Tuberc Lung Dis. 1998;2:296–302. [PubMed] [Google Scholar]
- 18.Kremer K, van den Brandt A, Kurepina NE, Glynn J, Bifani PJ, van Soolingen D. Definition of the Mycobacterium tuberculosis Beijing genotype. European Union Concerted Action project meeting, Cascais, May 27–30, 2002. Abstract 019. [Google Scholar]
- 19.Borgdorff MW, Nagelkerke NJ, De Haas PE, Van Soolingen D. Transmission of tuberculosis depending on the age and sex of source cases. Am J Epidemiol. 2001;154:934–43. 10.1093/aje/154.10.934 [DOI] [PubMed] [Google Scholar]