Abstract
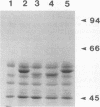
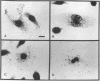
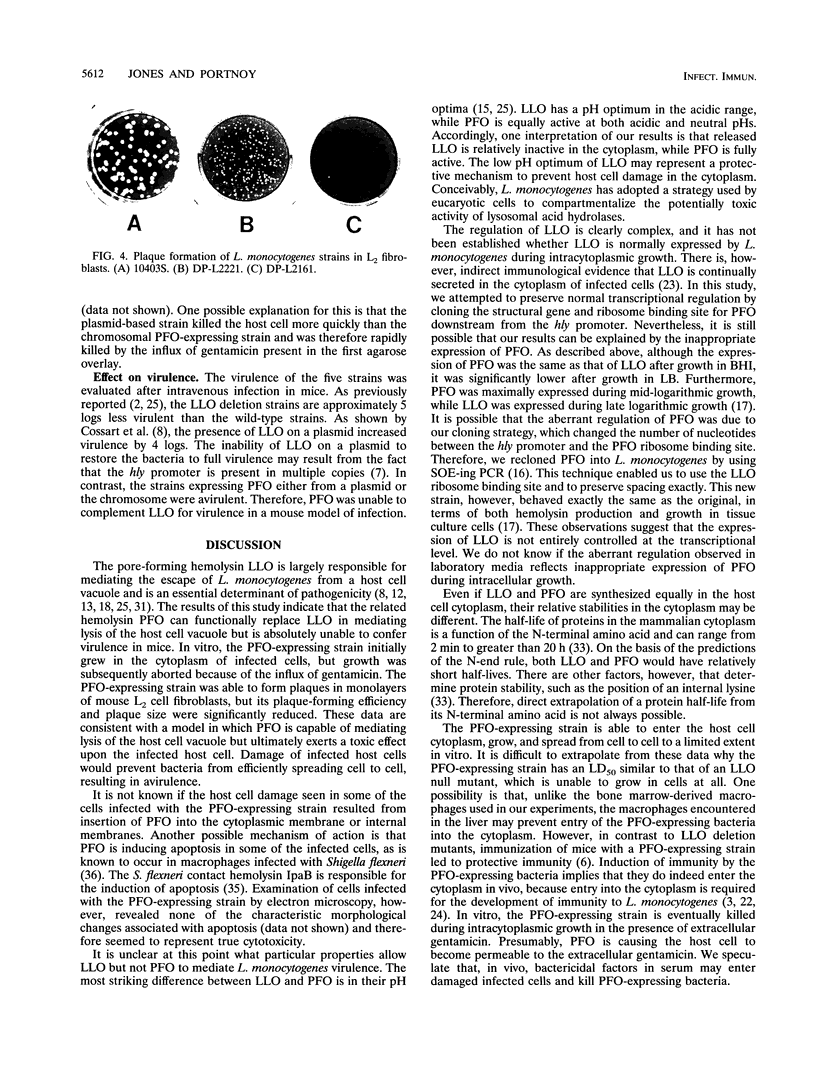
Listeriolysin O (LLO) is a pore-forming cytolysin that enables Listeria monocytogenes to escape from a host cell vacuole. The structural gene for the related cytolysin perfringolysin O (pfo) was cloned downstream from the promoter for hly, the gene encoding LLO, both on a plasmid and on the L. monocytogenes chromosome. Both strains secreted active PFO, although regulation was not identical to that of LLO. The chromosomal PFO-expressing strain was characterized for intracellular growth and cell-to-cell spread. It escaped from a host cell vacuole with 64% efficiency compared with the wild type as determined by immunofluorescent staining of bacteria for F-actin, a marker for entry into the cytoplasm. In addition, it replicated intracellularly with a doubling time similar to that of the wild type for 5 h, after which growth was aborted because of a cytotoxic effect on the host cell and influx of extracellular gentamicin. The chromosomal PFO strain was able to plaque in mouse L2 fibroblasts, but it did so at 20% efficiency compared with the wild type and the plaques were significantly smaller. Both strains expressing PFO were completely avirulent in mice. These results indicate that PFO can mediate escape from a host cell vacuole but cannot complement an hly deletion strain for virulence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. A., Bouwer H. G., Portnoy D. A., Hinrichs D. J. Pathogenicity and immunogenicity of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1992 Apr;60(4):1625–1632. doi: 10.1128/iai.60.4.1625-1632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Sansonetti P. J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987 Apr 1;138(7):2266–2271. [PubMed] [Google Scholar]
- Bielecki J., Youngman P., Connelly P., Portnoy D. A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990 May 10;345(6271):175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- Bishop D. K., Hinrichs D. J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987 Sep 15;139(6):2005–2009. [PubMed] [Google Scholar]
- Bouwer H. G., Gibbins B. L., Jones S., Hinrichs D. J. Antilisterial immunity includes specificity to listeriolysin O (LLO) and non-LLO-derived determinants. Infect Immun. 1994 Mar;62(3):1039–1045. doi: 10.1128/iai.62.3.1039-1045.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli A., Tilney L. G., Portnoy D. A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993 Apr;8(1):143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P., Vicente M. F., Mengaud J., Baquero F., Perez-Diaz J. C., Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989 Nov;57(11):3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri G. A., Sanger J. M., Portnoy D. A., Southwick F. S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets D. A., Canono B. P., Campbell P. A. Listericidal and nonlistericidal mouse macrophages differ in complement receptor type 3-mediated phagocytosis of L. monocytogenes and in preventing escape of the bacteria into the cytoplasm. J Leukoc Biol. 1992 Jul;52(1):70–79. doi: 10.1002/jlb.52.1.70. [DOI] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellin B. G., Broome C. V. Listeriosis. JAMA. 1989 Mar 3;261(9):1313–1320. [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987 Jul;55(7):1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990 May;8(5):528–535. [PubMed] [Google Scholar]
- Kathariou S., Metz P., Hof H., Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987 Mar;169(3):1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Kathariou S., Goebel W. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect Immun. 1988 Jan;56(1):79–82. doi: 10.1128/iai.56.1.79-82.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J., Vicente M. F., Chenevert J., Pereira J. M., Geoffroy C., Gicquel-Sanzey B., Baquero F., Perez-Diaz J. C., Cossart P. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect Immun. 1988 Apr;56(4):766–772. doi: 10.1128/iai.56.4.766-772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer E. G. Cellular immunity to intracellular bacteria. Curr Opin Immunol. 1993 Aug;5(4):492–496. doi: 10.1016/0952-7915(93)90028-q. [DOI] [PubMed] [Google Scholar]
- Pamer E. G. Direct sequence identification and kinetic analysis of an MHC class I-restricted Listeria monocytogenes CTL epitope. J Immunol. 1994 Jan 15;152(2):686–694. [PubMed] [Google Scholar]
- Portnoy D. A. Innate immunity to a facultative intracellular bacterial pathogen. Curr Opin Immunol. 1992 Feb;4(1):20–24. doi: 10.1016/0952-7915(92)90118-x. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Tweten R. K., Kehoe M., Bielecki J. Capacity of listeriolysin O, streptolysin O, and perfringolysin O to mediate growth of Bacillus subtilis within mammalian cells. Infect Immun. 1992 Jul;60(7):2710–2717. doi: 10.1128/iai.60.7.2710-2717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K., Youngman P. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie. 1992 Jul-Aug;74(7-8):705–711. doi: 10.1016/0300-9084(92)90143-3. [DOI] [PubMed] [Google Scholar]
- Sun A. N., Camilli A., Portnoy D. A. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1990 Nov;58(11):3770–3778. doi: 10.1128/iai.58.11.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten R. K. Nucleotide sequence of the gene for perfringolysin O (theta-toxin) from Clostridium perfringens: significant homology with the genes for streptolysin O and pneumolysin. Infect Immun. 1988 Dec;56(12):3235–3240. doi: 10.1128/iai.56.12.3235-3240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. The N-end rule. Cell. 1992 May 29;69(5):725–735. doi: 10.1016/0092-8674(92)90285-k. [DOI] [PubMed] [Google Scholar]
- Wirth R., An F. Y., Clewell D. B. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J Bacteriol. 1986 Mar;165(3):831–836. doi: 10.1128/jb.165.3.831-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky A., Kenny B., Ménard R., Prévost M. C., Holland I. B., Sansonetti P. J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994 Feb;11(4):619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A., Prevost M. C., Sansonetti P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992 Jul 9;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]