Abstract
From May 1990 to March 1993, 38 patients (21 adults and 17 children) received 40 allografts that included the small bowel (14 isolated small bowel, 21 small bowel and liver, and 5 multivisceral transplantations). Fifteen patients (39%) had 26 episodes of CMV disease: 7 with one episode, 6 with two, and 1 each with three and four. CMV enteritis accounted for 21 (81%) of the episodes, hepatitis and pneumonitis for 2 each, and a viral syndrome for 1. Cox's proportional hazards univariate and multivariate analyses showed that significant first-episode risk factors were: CMV seropositive donors for negative recipients (relative risk [RR], 3.86; P=0.02), the average daily plasma trough level of tacrolimus (RR, 2.15; P=0.04), and total amount of steroid boluses (RR, 2.90; P=0.02). CMV disease recurrence factors were: CMV seronegative recipients (RR, 8.60; P=0.02) and total amount of steroid bolus pulses (RR, 12.39; P=0.004). Because long courses of ganciclovir prophylaxis could not prevent the development of CMV disease, avoidance of CMV seropositive grafts in seronegative recipients and new strategies to prevent heavy immunosuppression without the penalty of rejection will be necessary to ameliorate this problem in intestinal transplant recipients.
Cytomegalovirus is the most common infectious complication after organ transplantation, occurring in 60% to 70% of kidney, liver, bone marrow, heart, and lung transplant recipients (1–4). While the prevalence and timing of CMV infection are remarkably similar for all the transplanted organs, disease entrance is significantly different in the various transplant populations (1–4), reflecting variable factors in each rather than the selective virulence of endemic CMV strains.
In the last few years, intestinal transplantation has become a feasible therapeutic option for patients with short bowel syndrome. Consequently, we have looked for host factors in our intestinal transplant recipients who have been prone to infection generally (5, 6), and who have a particular susceptibility to CMV of the allografts. We present here an analysis of the incidence, timing, clinical outcome, and factors associated with CMV disease in the first 38 intestinal transplant recipients at our institution.
Materials and Methods
Patient population, immunosuppressive treatment, and CMV prophylaxis
From May 1990 to March 1993, 20 adults and 18 children (mean age 20±17 years) received 40 intestinal allografts at our institution. Fourteen underwent isolated small bowel transplantations, 21 had small bowel and liver transplantations, and 5 had multivisceral transplantations. The surgical procedure for each of these transplantations has been described previously (7). Immunosuppressive therapy included tacrolimus (FK506), steroids, and, in selected cases, low dose AZA (1–2 mg/kg/day). Tacrolimus was given initially as a continuous infusion at a dose of 0.15 mg/kg/day and converted to an oral dose of 0.3 mg/kg/day in divided doses, 1 to 2 weeks after transplantation, when the patient became tolerant to enteral feeding. The oral and intravenous routes were overlapped for several days at the transition. Trough plasma levels of tacrolimus were monitored daily with a target therapeutic level between 1 and 3 ng/ml. In adult patients, methylprednisolone was started intraoperatively with a 1-g bolus followed by a steroid taper from 200 mg to 20 mg over 5 days, and maintained at or weaned thereafter from the dose of 20 mg/day. Children were given 1 g of hydrocortisone intraoperatively, followed by methylprednisolone, which was tapered over 5 days from 100 to 10 mg/day. Prostaglandin E1 was started intraoperatively at a dose of 0.2–0.6 mg/kg/hr and continued for 7 to 14 days. Surveillance endoscopy with multiple mucosal biopsies was performed once or twice per week for the first 3 months, and whenever it was clinically indicated thereafter. The most severe acute graft rejections were treated either by augmenting tacrolimus therapy, 1-g steroid bolus of methylprednisolone in adults (or hydrocortisone in children), a 5-day steroid burst, or a 5-day course of OKT3 mAb (8). However, not all patients required drastic treatment, and the response to rejection was commensurate with its severity.
Thirteen patients (34%) received prophylaxis with ganciclovir 10 mg/kg/day for 2 weeks, followed by acyclovir 3200 mg/day (adults) or 800 mg/m2 3 times a day (children) for 6 months. In 8 patients (21%), ganciclovir prophylaxis was maintained for 3 months and followed by high dose acyclovir until 6 months. Eleven children and 2 adults who were CMV seronegative received only CMV-seronegative blood products in addition to their CMV-negative liver.
CMV infection: surveillance cultures and definition of CMV enteritis
Specimens for CMV cultures and serology were obtained whenever infection was suspected clinically. CMV cultures were performed by the shell vial assay and standard culture technique. Serological tests for CMV were performed by a semiautomated immunofluorescence (FIAX) test in those patients who were CMV seronegative. In 4 patients, 2 with and 2 without CMV infection, CMV-DNA was monitored in blood buffy coats using the polymerase chain reaction (PCR)* technique.
Asymptomatic CMV infection was defined by seroconversion or positive culture without evidence of clinical symptoms. Symptomatic infection included CMV viral syndrome and invasive CMV disease. The diagnosis of CMV viral syndrome required laboratory documentation of CMV infection along with fever >38°C for 2 or more days in the absence of other causes, combined with any of the following findings: atypical lymphocytosis >3%, white blood cell count <4,000/mm3, or platelets <100,000/mm3. Invasive disease was defined as tissue invasion with histopathologic confirmation and/or by isolation of virus from a tissue specimen. CMV enteritis was defined by either detection of typical CMV inclusion bodies, unequivocal immunoperoxidase staining of the virus, and/or positive culture by either shell vial technique or standard culture, along with a mononuclear infiltrate in intestinal biopsy. Recurrence of CMV disease was defined as a new episode of disease after at least 1 month of negative histopathology and/or virology.
Factors associated with CMV disease and recurrent disease
Thirteen clinical variables were analyzed in patients who did and did not develop CMV disease: age, type of intestinal transplantation, donor and recipient serological status, use of ganciclovir prophylaxis, use of seronegative blood products, number of units of blood products transfused (packed red cells, platelets, and fresh frozen plasma), treatment with OKT3, average blood level of tacrolimus per day, average daily dose of maintenance steroids (adjusted to weight), AZA/kg/day, and the total amount of steroid boluses given adjusted to weight.
Statistical analysis
Univariate and multivariate analysis of risk factors for the first episode and recurrent CMV disease were done using the Cox proportional hazards model. Pretransplant donor-recipient serological status was analyzed as indicator variable using negative donor/negative recipient as the reference category. The likelihood ratio chi-square test was used to assess the risk of each factor. Based on the results of these univariate analysis, a multivariate analysis was performed. Variables were chosen to be included in the multivariate analyses if they had a P<0.25. The backward elimination method was used as a variable selection technique using the likelihood ratio chi-square test as means to assess each factor. Variables were entered or excluded from the model based on a P-value for entry of 0.10 and a P-value for exclusion of 0.15. Approximate 95% confidence intervals (CI) were generated for each relative risk (RR). The cumulative 1-year mortality was estimated using the Kaplan-Maier method.
Results
Incidence, timing, and treatment of CMV disease
Fifteen patients (39%) developed 26 episodes of CMV disease (1.7 episodes per patient): 7 had 1 episode, 6 had 2, and 1 each had 3 and 4. The first episode occurred at a median of 54 days (range 21–274), the second at 116 days (range 70–277), the third at 173 days (159 and 186) and the fourth at 238 days after transplantation. CMV enteritis was the most frequent type of disease accounting for 21 (81%) episodes. There were also 2 episodes each of hepatitis and pneumonitis, and 1 of viral syndrome. Notably, CMV viremia was present in only 10 (48%) of the episodes of enteritis, despite apparent clinical, histopathologic, and/or virologic signs of infection in the intestinal allograft. Of the 4 patients studied with PCR, 2 remained CMV negative throughout and without any other diagnostic criteria of CMV infection. The other 2 developed CMV enteritis, but in one, viremia could not be detected contemporaneously with PCR testing.
Table 1 shows the donor/recipient CMV serologic status, incidence, and number of episodes of CMV disease in the 40 intestinal transplantations. None of the 16 seronegative recipients who received seronegative grafts developed CMV disease. Only one of these patients seroconverted asymptomatically 316 days after transplantation. In contrast, the incidence of CMV disease among CMV-seropositive recipients was 50%, regardless of whether the donor's serologic status was negative (4/8) or positive (2/4). Two (17%) of these 12 seropositive recipients had CMV disease recurrence after their first postoperative bout of disease.
Table 1. Donor/recipient CMV serological status and number of episodes of CMV disease.
| D/R | No. of transplants | CMV disease | Episodes |
|---|---|---|---|
| −/− | 16 | 0 | 0 |
| −/+ | 8 | 4 (50%) | 5 |
| +/+ | 4 | 2 (50%) | 3 |
| +/− | 12 | 9 (75%) | 18 |
| Total | 40a | 15 (38%) | 26 |
Two patients had two intestinal transplantations.
Nine (75%) of the high risk group of 12 seronegative recipients who received seropositive grafts developed 18 episodes of CMV disease. Their 9 primary infections and 9 recurrences accounted for 69% of the 26 disease episodes in the study, with an overwhelming incidence of intestinal graft involvement. Of the 3 exceptional patients who escaped this complication, 2 died after 23 and 49 posttransplantation days, and the third was diagnosed of asymptomatic infection 103 days after transplantation.
When CMV disease was diagnosed, the first episode was treated with ganciclovir 10 mg/kg/day for 21 days. The second episode was treated with either ganciclovir 10 mg/kg/day or foscarnet 180 mg/kg/day for 1 month. Recurrence after the second episode was treated with either ganciclovir 10 mg/kg/day or foscarnet 180 mg/kg/day for 3 months and a maintenance dose of 5 mg/kg/day of ganciclovir or 90–120 mg/kg/day of foscarnet thereafter. All dosages of ganciclovir and foscarnet were adjusted for renal function. Four patients developed CMV disease recurrence during maintenance ganciclovir therapy and were converted to foscarnet. When 2 of them recurred during maintenance Foscarnet, induction doses of ganciclovir and CMV-specific hyperimmunglobulin (100 mg/kg/week) were added to the foscarnet therapy, despite which 1 of the patients developed a new recurrence during the triple therapy. This last patient was then given induction doses of ganciclovir and foscarnet for 3 months, followed by maintenance doses of both antivirals. No further episodes occurred during the subsequent 4 months.
Twenty-six (68%) of the 38 patients survived >1 year. Of the 12 deaths, 7 were among the 15 patients (47%) in whom CMV disease developed, occurring 253±131 days after transplantation. However, evidence of active CMV disease at death and/or autopsy was present in only 1 patient. Five patients (22%) who did not develop CMV disease died at 105±141 days after transplantation (Fig. 1). Using the Kaplan-Meier analysis, 1-year survival did not show a statistical difference between the two groups (P=0.3). The principal causes of death were technical complications (n=3), infectious complications (n=5), rejection (n=2), and disseminated posttransplant lymphoproliferative disorders (PTLD) (n=2).
Figure 1.
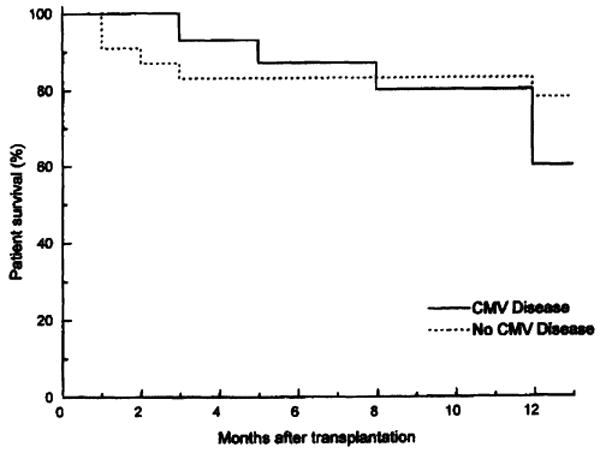
One-year patient survival in intestinal transplant recipients in whom CMV disease did and did not develop.
CMV vs. rejection or lymphoproliferative disease
A mean 4 episodes of histopathologic acute allograft rejection occurred per patient. Beyond the 3 posttransplant months, 73% of patients who developed CMV disease experienced rejection of intestinal grafts. In contrast, the incidence was 39% in those patients who did not develop CMV disease. Four patients (11%) developed PTLD at 243±141 days after transplantation. Two of these patients experienced CMV disease before the development of PTLD.
Factors associated with the first episode and recurrence of CMV disease
Variables found with univariate analysis to be significantly associated with the development of a first episode of CMV disease included: donor seropositive/recipient seronegative status, isolated small bowel transplantation, the average level per day of tacrolimus, the average dose of maintenance steroids per day adjusted to patients weight, and the amount of pulse steroids adjusted to weight (Table 2). Multivariate analysis (Table 2) showed that significant risk factors for the first episode of CMV disease were donor seropositive/recipient seronegative status (RR, 3.86; 95% CI, 1.21–12.36; P=0.02), the average level of tacrolimus per day (RR, 2.15; 95% CI, 1.02–4.52; P=0.04), and the amount of steroids given as pulses (RR, 2.90; 95% CI, 1.16–7.29; P=0.02). Factors associated with recurrence of CMV disease were the donor seropositive/recipient seronegative status (RR, 8.60; 95% CI, 1.46–50.53; P=0.02) and the amount of steroids given as pulses (RR, 12.39; 95% CI, 1.16–131.83; P=0.004).
Table 2. Univariate and multivariate analysis of risk factors for the first episode of CMV disease.
| Variable | CMV disease | No disease | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|---|
| Relative risk | 95% CI | P | Relative risk | 95% CI | P | |||
| Agea | 31 (2–50) | 10 (0.5–58) | 1.02 | (0.99–1.06) | 0.14 | |||
| Seronegative bloodb | 5 (33%) | 13 (57%) | 0.57 | (0.20–1.68) | 0.31 | |||
| D+/R−b, c | 9 (60%) | 3 (13%) | 5.89 | (2.00–17.27) | 0.001 | 3.86 | (1.21–12.36) | 0.02 |
| Isolated small bowel Txb | 9 (60%) | 5 (22%) | 0.33 | (0.12–0.94) | 0.04 | |||
| Ganciclovir prophylaxisb | 8 (53%) | 14 (61%) | 0.70 | (0.25–1.94) | 0.49 | |||
| FFPa | 4 (0–53) | 5 (0–100) | 1.00 | (0.98–1.03) | 0.95 | |||
| PLTa | 6 (0–196) | 8 (0–129) | 1.00 | (0.99–1.01) | 0.78 | |||
| PRCa | 23 (4–65) | 26 (7–128) | 0.99 | (0.97–1.02) | 0.46 | |||
| Tacrolimusa | 2.47 (1.23–4.01) | 1.67 (0.58–4.36) | 3.63 | (1.95–6.76) | 0.0001 | 2.15 | (1.02–4.52) | 0.04 |
| Steroid bolusa | 0.77 (0.11–2.72) | 0.34 (0.08–1.43) | 4.45 | (2.13–9.29) | 0.0001 | 2.90 | (1.16–7.29) | 0.02 |
| Steroid maintenancea | 0.54 (0.16–2.33) | 0.31 (0.07–1.82) | 2.95 | (1.16–7.51) | 0.02 | |||
| AZAb | 8 (53%) | 12 (54%) | 0.84 | (0.30–2.33) | 0.73 | |||
| OKT3b | 0 | 4 (18%) | ||||||
Median and range.
Number of patients and percentage.
D+/R−, donor positive/recipient negative.
Discussion
The hazard of CMV infection in recipients of whole organs and bone marrow has been noted almost from the beginning of successful clinical use of these procedures (9). Realization that the donor organ is a frequent inoculation source (10) as well as a vulnerable posttransplantation target, and recognition that CMV disease frequency is associated with the intensity of immunosuppression are common to all kinds of allograft recipients. The infectious syndromes resemble those found in patients with inherited (11) or virus-induced acquired immune deficiency disorders (12).
The strong association between the development and/or recurrence of CMV disease and the amount of both tacrolimus and steroid pulses was expected. Both drugs inhibit cell-mediated responses, through blockade of cytokine expression (13, 14), impeding the main host mechanism against CMV infections (15). Humoral immunity, which also is important in protection from progressive infection of the immunocompromised host (16), is suppressed by high dose steroid therapy, which decreases serum immunoglobulin levels with a delayed maximal effect 2–4 weeks after treatment (17). Gastrointestinal lesions that contain cytomegalic cells have been reported in association with steroid therapy (18, 19), prompting several authors to propose that steroid-associated peptic ulcer disease may be, in fact, a CMV disease (20, 21).
While the types of CMV infections in our intestinal recipients have been similar to those receiving other organs, the epidemiologic pattern has been distinguished by a high incidence of infection and recurrence rates in the bowel allograft itself, a 50% incidence of CMV disease in patients who were seropositive before transplantation, less than 50% incidence of viremia, and relative intractibility of the disease. Although the 26 episodes of CMV disease in 15 patients did not significantly degrade 1-year patient survival in comparison to CMV-free patients, there was an increased trend to mortality as well as very difficult management problems.
The possibility that the gastrointestinal tract has a particular tropism for the virus has been suggested by the 30–50% incidence of CMV-positive cultures in ostensibly normal gastroduodenal biopsy of kidney and liver recipients (22–24), and in patients with acquired immune deficiency syndrome in whom the gastrointestinal tract and retina are the most common targets of CMV infection (12, 25). Although it has been shown recently that endothelial cells are a common target for CMV infection (26, 27), gastrointestinal epithelial cells can also harbor the virus (25, 28) and could be a cause of relapse. The presence in the intestine of large numbers of donor lymphocytes, monocytes, and polymorphonuclear leukocytes (29) could make the intestine a prime target for CMV infection because most of these leukocytes presumably contain latent virus (30). Added to this milieu, ischemic injury to the mucosa during preservation and the occurrence of rejection in the vast majority of cases (8) could make the intestinal graft an ideal sanctuary for the virus, which particularly infects inflamed or regenerating tissues (31, 32) without necessarily being reflected in viremia.
It was not possible to rule out in these patients that the unusual CMV profile herein reported was caused by ganciclovir- or foscarnet-resistant CMV strains (33). This was due to the difficulty in isolating the virus when the patients were receiving antiviral therapy (34). Although sporadic spot checks in other kinds of organ recipients failed to demonstrate antiviral drug resistance, this is something that needs to be considered, particularly in those patients who receive antiviral therapy for long periods of time.
Insight into the spectacular ability of organs from CMV carriers to infect recipients, or, conversely, of an infected recipient to infect a graft, has come from recent discovery of the ubiquitous migration of donor “passenger” leukocytes that begins within a few minutes after graft revascularization (35–38). The infectious potential of these long-surviving chimeric cells is obvious, and presumably related to the relative cell dose sprayed into recipient tissues from different transplanted organs procured from carriers of CMV (and presumably some other viruses). Because a long course of currently available prophylaxis cannot prevent life-threatening CMV disease in the unusually vulnerable CMV-negative intestinal recipient, avoidance of CMV-seropositive grafts has overriding importance. Approaches to prevent long-term, heavy immunosuppression are a possible future alternative. One such initiative may be to augment the spontaneous cell migration by administering CMV-negative donor bone marrow before surgery. Although this strategy would have been considered heretical until recently, it has proved to be safe and effective with all other organs, including the leukocyte-rich liver (38).
Table 3. Risk factors associated with recurrent CMV disease.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Relative risk | 95% CI | P | Relative risk | 95% CI | P | |
| Age | 1.08 | (1.03–1.14) | 0.003 | |||
| Seronegative blood | 0.13 | (0.02–1.05) | 0.06 | |||
| D+/R−a | 15.21 | (3.09–74.95) | 0.0008 | 8.60 | (1.46–50.53) | 0.02 |
| Isolated small bowel Tx | 0.13 | (0.27–0.63) | 0.01 | |||
| Tacrolimus | 4.65 | (1.70–12.68) | 0.003 | |||
| Steroid bolus | 18.68 | (3.55–98.26) | 0.0008 | 12.39 | (1.16–131.83) | 0.004 |
D+/R−, donor positive/recipient negative.
Footnotes
This work was supported by grants from the Veterans Administration and Project Grant DK-29961 from the National Institutes of Health.
Abbreviations: CI, confidence interval; PCR, polymerase chain reaction; PTLD, posttransplant lymphoproliferative disorder; RR, relative risk.
References
- 1.Pouteil-Noble C, Ecochard R, Landrivon G, et al. Cytomegalovirus infection—an etiological factor for rejection? Transplantation. 1993;55:851. doi: 10.1097/00007890-199304000-00032. [DOI] [PubMed] [Google Scholar]
- 2.Dummer JS. Cytomegalovirus infection after liver transplantation: clinical manifestations and strategies for prevention. Rev Infect Dis. 1990;12:s767. doi: 10.1093/clinids/12.supplement_7.s767. [DOI] [PubMed] [Google Scholar]
- 3.Meyer JD, Ljngman P, Fisher LD. Cytomegalovirus excretion as a predictor of cytomegalovirus disease after bone marrow transplantation: importance of cytomegalovirus viremia. J Infect Dis. 1990;162:373. doi: 10.1093/infdis/162.2.373. [DOI] [PubMed] [Google Scholar]
- 4.Smyth RL, Scott JP, Borysiewicz LK, et al. Cytomegalovirus infection in heart-lung transplant recipients: risk factors, clinical associations, and response to treatment. J Infect Dis. 1991;164:1045. doi: 10.1093/infdis/164.6.1045. [DOI] [PubMed] [Google Scholar]
- 5.Kusne S, Manez R, Bonet H, et al. Infectious complications after small bowel transplantation in adults. Transplant Proc. 1994;26:1682. [PMC free article] [PubMed] [Google Scholar]
- 6.Green M, Reyes J, Nour B, Tzakis A, Todo S. Early infectious complications of liver-intestinal transplantation in children: preliminary analysis. Transplant Proc. 1994;26:1420. [PubMed] [Google Scholar]
- 7.Furukawa H, Abu-Elmagd K, Reyes J, et al. Technical aspects of intestinal transplantation. In: Braverman MH, Tawes RL, editors. Surgical Technology International II. San Francisco: Surgical Technology International; 1993. p. 165. [PubMed] [Google Scholar]
- 8.Abu-Elmagd K, Tzakis A, Todo S, et al. Monitoring and treatment of intestinal allograft rejection in humans. Transplant Proc. 1993;25:1202. [PMC free article] [PubMed] [Google Scholar]
- 9.Rifkind D. Cytomegalovirus infection after renal transplantation. Arch Intern Med. 1965;116:554. [PubMed] [Google Scholar]
- 10.Ho M, Suwansirikul S, Dowling JN, et al. The transplanted kidney as a source of cytomegalovirus infections. N Engl J Med. 1975;293:1109. doi: 10.1056/NEJM197511272932201. [DOI] [PubMed] [Google Scholar]
- 11.Buckley RH. Immunodeficiency diseases. JAMA. 1987;258:2841. [PubMed] [Google Scholar]
- 12.Tyms AS, Taylor DL, Parkin JM. Cytomegalovirus and the acquired immunodeficiency syndrome. J Antimicrob Chemother. 1989;23:89. doi: 10.1093/jac/23.suppl_a.89. [DOI] [PubMed] [Google Scholar]
- 13.Kay JE, Moore AL, Doe SEA, et al. The mechanism of action of FK506. Transplant Proc. 1990;22(suppl 1):96. [PubMed] [Google Scholar]
- 14.Almawi WY, Lipman ML, Stevens AC, Zanker B, Hadro ET, Strom TB. Abrogation of glucorticoid-mediated inhibition of T cell proliferation by the synergistic action of IL-1, IL-6, and IFN-gamma. J Immunol. 1991;146:3523. [PubMed] [Google Scholar]
- 15.Pasternack MS, Medearis DN, Jr, Rubin RH. Cell-mediated immunity in experimental cytomegalovirus infections: a perspective. Rev Infect Dis. 1990;12(suppl 1):S720. doi: 10.1093/clinids/12.supplement_7.s720. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen L, Matkin C, Spaete R, Pachl C, Merigan TC. Antibody response to human cytomegalovirus glyproteins gB and gH after natural infection in humans. J Infect Dis. 1991;164:835. doi: 10.1093/infdis/164.5.835. [DOI] [PubMed] [Google Scholar]
- 17.Butler WT, Rossen RD. Effects of corticosteroids on immunity in man: decreased serum IgG concentration caused by 3 or 5 days of high doses methylprednisolone. J Clin Invest. 1973;52:2629. doi: 10.1172/JCI107455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orloff JJ, Saito R, Lasky S, Dave H. Toxic megacolon in cytomegalovirus colitis. Am J Gastroenterol. 1989;84:794. [PubMed] [Google Scholar]
- 19.Henson D. Cytomegalovirus inclusion bodies in the gastrointestinal tract. Arch Pathol. 1972;93:477. [PubMed] [Google Scholar]
- 20.Ayulo M, Aisner SC, Maegolis K, Moravec C. Cytomegalovirus associated gastritis in a compromised host. JAMA. 1980;243:1364. [PubMed] [Google Scholar]
- 21.Campbell DA, Piercy JR, Shnitka TK, Goldsand G, Devine RD, Weinstein WM. Cytomegalovirus-associated gastric ulcer. Gastroenterology. 1977;72:533. [PubMed] [Google Scholar]
- 22.Spencer GD, Hackman RC, MacDonald GB, et al. A prospective study of unexplained nausea and vomiting after marrow transplantation. Transplantation. 1986;42:602. doi: 10.1097/00007890-198612000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Alexander JA, Cuellar RE, Fadden RJ, Genovese JJ, Gavaler JS, Van Thiel DH. Cytomegalovirus infection of the upper gastrointestinal tract before and after liver transplantation. Transplantation. 1988;46:378. doi: 10.1097/00007890-198809000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Franzin G, Muolo A, Griminelli T. Cytomegalovirus inclusions in the gastroduodenal mucosa of patients after renal transplantation. Gut. 1981;22:698. doi: 10.1136/gut.22.9.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis ND, Boylston AW, Roberts AH, Parkin J, Pinchin AJ. Cytomegalovirus infection in gastrointestinal tracts of patients infected with HIV-1 or AIDS. J Clin Pathol. 1989;42:1055. doi: 10.1136/jcp.42.10.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts WH, Sneddon JM, Waldman J, Stephens RE. Cytomegalovirus infection of gastrointestinal endothelium demonstrated by simultaneous nucleus acid hybridization and immunohistochemistry. Arch Pathol Lab Med. 1989;113:461. [PubMed] [Google Scholar]
- 27.Percivalle E, Revello MG, Vago L, Morini F, Gerna G. Circulating endothelial cells permissive for human cytomegalovirus (HCMV) are detected in disseminated HCMV infections with organ involvement. J Clin Invest. 1993;92:663. doi: 10.1172/JCI116635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myerson D, Hackman RC, Nelson JA, Ward DC, McDougall JK. Widespread presence of histologically occult cytomegalovirus. Hum Pathol. 1984;15:430. doi: 10.1016/s0046-8177(84)80076-3. [DOI] [PubMed] [Google Scholar]
- 29.Iwaki Y, Starzl TE, Yagihashi A, et al. Replacement of donor lymphoid tissue in small-bowel transplants. Lancet. 1991;337:818. doi: 10.1016/0140-6736(91)92517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche JK, Cheung KS, Boldogh I, Huang ES, Lang DJ. Cytomegalovirus: detection in human colonic and circulating mononuclear cells in association with gastrointestinal disease. Int J Cancer. 1981;27:659. doi: 10.1002/ijc.2910270513. [DOI] [PubMed] [Google Scholar]
- 31.Eyre-Brook IA, Dundas S. Incidence and clinical significance of colonic cytomegalovirus infection in idiopathic inflammatory bowel disease requiring colectomy. Gut. 1986;27:1419. doi: 10.1136/gut.27.12.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffiths PD, Grundy JE. The status of CMV as a human pathogen. Epidemiol Infect. 1988;100:1. doi: 10.1017/s095026880006550x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erice A, Chou S, Biron KK, Stanat SC, Balfour HH, Jr, Jordan MC. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised hosts. N Engl J Med. 1989;320:289. doi: 10.1056/NEJM198902023200505. [DOI] [PubMed] [Google Scholar]
- 34.Manez R, St George K, Linden P, et al. Diagnosis of cytomegalovirus infections by the shell vial assay and conventional culture during antiviral prophylaxis. J Clin Microbiol. doi: 10.1128/jcm.32.11.2655-2659.1994. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starzl TE, Demetris AJ, Murase N, Ildstat S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 37.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontes P, Rao AS, Demetris AJ, et al. Bone marrow augmentation of donor-cell chimerism in kidney, liver, heart and islet transplantation. Lancet. 1994;344:151. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


